Abstract
In the present work, we have synthesized water soluble Ag nanoclusters using PMAA as a template with different Ag+: COO-ratios, to optimize it for highest brightness using less UV exposure time. Fluorescence polarization was 0.30 for and was found to vary with excitation and emission wavelength with few hundred picoseconds average fluorescence lifetime. Fluorescence Correlation Spectroscopy data depicts slower diffusion at red excitation compared to blue excitation in confocal volume than conventionally synthesized colloids proving presence of multiple sizes. The optical properties of the particles are dependent upon the excitation wavelength used and the emission wavelength collected.
Keywords: Metal Nanoclusters, Fluorescent Ag Nanoclusters, Polymethylacrylic Acid, Polarization, FCS
1. Introduction
During the last decade, novel fluorescent probes such as quantum dots, nanodiamonds and metal nanoclusters have attracted a great deal of attention from the scientific community [1-3]. Among these, the silver and gold nanoclusters have been in the limelight recently since they have bridged the gap between the atomic and colloidal behavior of noble metals [4-7]. These novel fluorescent structures are photostable, non-toxic and easy to prepare. Bright, fluorescent Ag nanoclusters, being small in size (comparable size to proteins) offer a multitude of potential applications in single-molecule microscopy, biological labeling, and in optical sensing [8-10]. While the research efforts in this area are still in the early stages, the focus has been primarily on different synthetic strategies, analytical applications in sensing, and some biological applications. However, an in- depth understanding of the basic physico-chemical behavior of these fluorescent Ag nanoclusters is imperative for its efficient use. This study is a fundamental spectroscopic evaluation and provides novel insights about the interesting properties of these silver nanoclusters.
Recently, Shang and Dong reported a new approach for the synthesis of fluorescent and water-soluble Ag nanoclusters using a common polyelectrolyte, polymethylacrylic acid (PMAA), as the template. Another report showed their utility in Cu sensing [11, 12]. We undertook this work to report the findings of our spectroscopic assessment of the system developed by Shang and Dong. Herein, we have optimized the Ag+: COO− ratio and UV exposure time simultaneously for the first time to synthesize Ag nanoclusters, characterize them, and demonstrate their steady state and time resolved behavior. Characterizing the optical properties of these nanoclusters will facilitate the development of new technology by utilizing these nanoclusters in several biochemical applications.
In the present work, we have synthesized water soluble Ag nanoclusters using PMAA as a template with different Ag+: COO− ratios, to optimize it for highest brightness using less UV exposure time. Fluorescence polarization was found to be very high for this Ag nanocluster which was found to vary with excitation and emission wavelength with an average fluorescence lifetime of few hundred picoseconds. Fluorescence Correlation Spectroscopy (FCS) data depicts slower diffusion at red excitation compared to blue excitation in confocal volume than conventionally synthesized colloids. The optical properties of the particles are dependent upon the excitation wavelength used and the emission wavelength collected.
2. Experimental Section
2.1 Materials
Polymethylacrylic acid (PMAA, Mw=9500), Silver nitrate and Nitric acid 70 % ACS reagent were purchased from Sigma-Aldrich. All the reagents were used as received.
2.2 Synthesis of Ag Nanoclusters
We used the method of Shang and Dong as reported previously [11]. Water-soluble fluorescent Ag nanoclusters were synthesized as follows: PMAA_Ag_ solutions (2 mL) with different Ag+: COOH− ratios were prepared using 7 mg of AgNO3 in 1 mL of water, which was mixed with 1 mL PMAA solutions of different concentrations. The pH of the solution was adjusted to 4.5 with 0.1 M HNO3 (aq.). The mixture was then incubated in dark for 10 min. Irradiation time using an UV illuminator (FOTODYNE Inc. USA) was varied from 30 sec to 45 min. The samples were then dialyzed using a dialysis bag with Mw 10,000 cutoff followed by centrifugation.
2.3 UV-Visible Spectroscopy and Fluorescence Lifetime Measurements
UV-Vis absorption and fluorescence spectra were obtained using a Cary 50 bio UV–visible Spectrophotometer (Varian Inc.) and Cary Eclipse spectrofluorometer (Varian Inc.) respectively. The quantum yield of fluorescent Ag nanoclusters was determined by measuring the integrated fluorescence intensities of the Ag nanoclusters. A solution of rhodamine B in ethanol (0.7) was used as a reference. The anisotropy and fluorescence lifetime measurements were performed on a sample with 0.05 optical densities. For anisotropy measurements, the sample was excited using 470 nm and scanned from 500 nm to 850 nm in parallel and perpendicular polarization orientations. The G factor was measured and incorporated in the anisotropy calculation using the following formula:
Fluorescence lifetime measurements were carried out on a FluoTime 300 fluorometer (PicoQuant, Inc.) using a fianium laser. The fluorometer is equipped with an ultrafast microchannel plate detector (MCP) from Hamamatsu, Inc. The fluorescence lifetimes were calculated using FluoFit4 program from PicoQuant, Inc (Germany) using multi-exponential fitting model.
TEM images were taken with a JEOL 2100 transmission electron microscope with an accelerating voltage of 80 kV. Samples were prepared by placing a drop of solution onto copper grids with ultrathin carbon film and dried at room temperature.
2.4 Fluorescence Correlation Spectroscopy
FCS measurements were collected on a Microtime 200 system from Picoquant GmbH (Berlin, Germany). Solution of clusters was diluted in water to the nanomolar level. Then 30 μL of solution was dropped onto a 20 mm × 20 mm No. 1 coverslip (Menzel-Gläser). The focal height was adjusted to 20 μm above this coverslip using an Olympus iX71 microscope and an Olympus 60× 1.2 NA objective. The detailed description of the measurement can be found in the supplementary information.
3. Results and Discussion
Photoreduction of Ag+ and generation of fluorescent clusters require the use of a template or capping agent to prevent the aggregation of Ag to large colloids. PMAA was an ideal choice as the capping agent for multiple reasons: 1) Its carboxylic acid groups have a strong affinity for silver ions [11]. 2) It serves as a OH• radical scavenger to prevent oxidation of small silver clusters into silver oxide [13]. 3) Secondary radicals formed from the reaction of PMAA with OH• exhibit strong reducing power and produce Ag atoms associated with the polymer chains and 4) PMAA is biocompatible and biodegradable[14].
3.1 Optimization Studies
3.1.1 Changes in Absorption Spectra
The changes in absorption of the Ag nanoclusters with different Ag+: COO− ratios and duration of UV exposure has proved interesting and has not been investigated simultaneously. The stoichiometry of the silver ions and carboxylate groups play an important role in producing Ag nanoclusters. Before UV irradiation, the solution showed no absorption in the range of 300-800 nm, as expected. After 2 minutes of UV irradiation, an absorption peak appeared around 440 nm or 510 nm, depending on the Ag+: COO− ratio used. Further irradiation increases the intensity of this peak (either 440 or 510 nm) but does not change the wavelength. Figure 1 (A) shows the absorption spectra of Ag nanoclusters with various Ag+: COO− ratios at 10 min UV exposure. Continued UV irradiation after this (up to 45 minutes) did not result in any changes in the shape of the absorption spectra. Using a sonochemical method, Xu and Suslick et al reported that a 440 nm peak gradually disappeared with the appearance of a 520 nm shoulder. With increased sonication time this 520 nm peak gradually shifted to 490 nm [13]. A similar phenomenon has been observed by Zhang et al in polymer micro gels [15]. In our study, when the ratio of Ag+ to COO− was less than 2, only the 440 nm plasmonic peak was observed. When the ratio was above 4 there was a 510 nm peak alone. For a 3:1 ratio, absorption spectra having a 440 nm peak with a shoulder around 510 nm emerged with increasing UV exposure time (not shown in the graph). The Ag nanoclusters prepared from 2 or smaller ratios were less fluorescent than the clusters prepared with 4 or larger ratio. This can be ascribed to the presence of larger non-fluorescent Ag nanoparticles in the sample prepared with 2:1 or smaller ratio, which shows a typical plasmonic peak around 440 nm [4]. In most conventional colloid synthesis, excess amount of stabilizing or capping agent is typically used to get smaller size particles [16-18]. However, according to the protocol used by Shang and Dong, in order to get smaller clusters, the molar amount of polymer used must be less than the molar amount of silver. The reasons for this are not very well understood and require further investigation. Since many silver ions assemble together to form a cluster, which is stabilized by an acid group on the polymer, it is conceivable that the amount of acid group required to stabilize all the clusters will be less than number of silver clusters.
Figure 1.
(A) Absorption spectra of different Ag: COO-ratio used in preparation of Ag nanoclusters. (B) Shows the corrected peak emission [black line] and UV exposure time to reach peak emission [blue line] as function of different Ag: COO-ratio (C) Shows the two TEM images of 6:1 Ag+: COO-ratio Ag nanoclusters from different parts of the TEM grid. Red square in the 1st image shows the approximate area from where the second TEM image was captured. (Scale bar 10 nm in 1st image and 2 nm in 2nd image)
3.1.2 Optimization for Highest Brightness
In order to obtain the highest emission brightness using the least amount of UV exposure time during synthesis, the Ag+: COO− ratio needs to be optimized. For all the samples, the emission band occurred at 620 nm when excited at 500 nm. Figure 1 (B) shows the emission intensity (corrected for differences in absorbance) and UV exposure time to reach peak emission as a function of Ag+: COO− ratios. We found that a 6:1 ratio gave the highest brightness and that this emission level was obtained after being irradiated for 4 min. Further decreasing the relative amount of carboxylate groups (higher Ag : COO− ratio) led to a decrease in corrected fluorescence intensity, which may be due to Ag nanocluster agglomeration from the lack of sufficient carboxylate groups available to stabilize the nanoclusters. Figure 1 (C) shows TEM images of the Ag nanoclusters prepared from a 6:1 Ag+: COO− ratio. We found polydisperse size distribution of the clusters. The smallest particles were about 2 nm along with larger nanoparticle of more than 50 nm. The emission quantum yield was 6.5 %. The fluorescence emission intensity from a sample prepared with a 6:1 Ag+: COO− ratio was stable even after 3 months of storage in the dark.
3.2 Spectral Properties of Silver Nanoclusters Fluorescence
3.2.1 Excitation and Emission Spectra
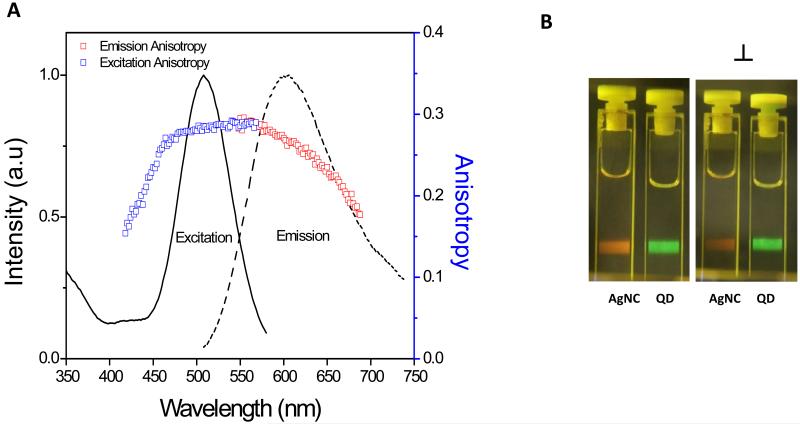
The emission spectrum depends on the excitation wavelength, which is due to the distribution of different association numbers in Ag nanoclusters [13]. Moreover, we observed also that excitation spectrum depends on the observation wavelength (see supplementary materials, figure 1A and figure 2A). This finding points towards the presence of different sizes distribution in the sample. We did not see changes in the excitation peak when we moved our emission observation beyond 700 nm.
Figure 2.
(A) Excitation and emission spectrum along with respective excitation and emission anisotropy using 470 nm excitation and 610 nm observation wavelength. (B) Shows the photograph comparing emission intensity of Ag clusters and 5 nm green emission quantum dots when excited with blue laser (470 nm) and observed through parallel (∥) and perpendicular (⊥) polarizer orientation.
3.2.2 Fluorescence Polarization and Lifetime
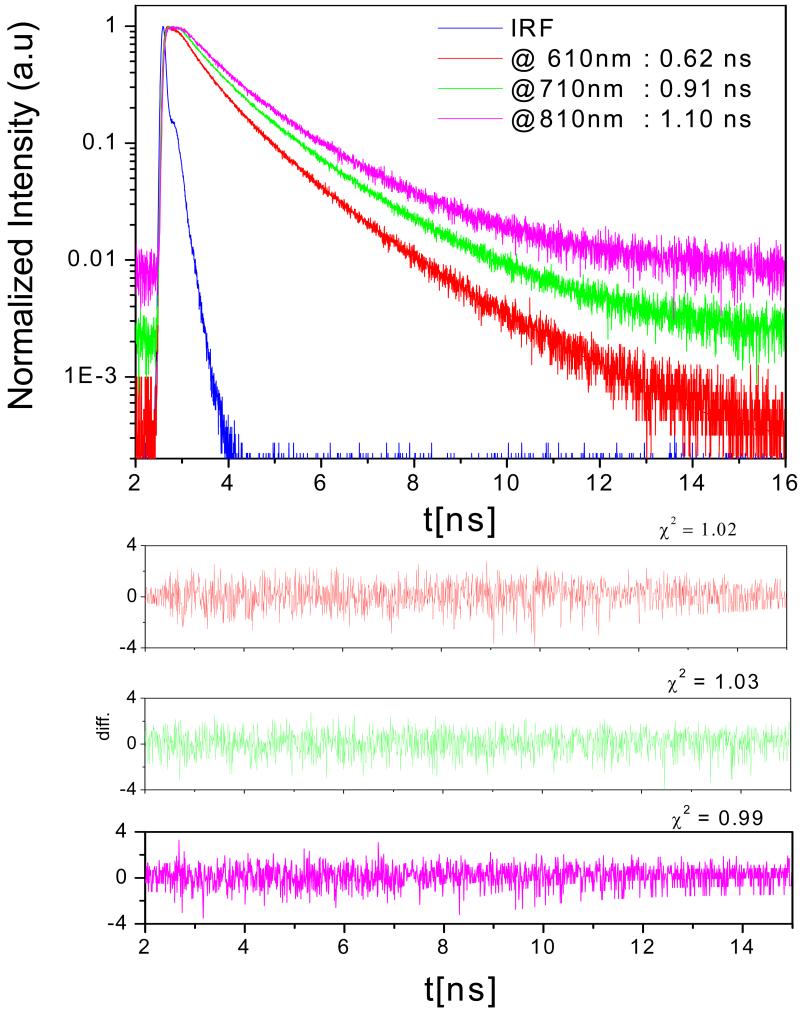
Considering the possible applications in bioimaging, chemical and biosensing, single-molecule studies, and possibly catalysis [5, 19-21], it is worth looking into some of the spectroscopic properties of the nanoclusters. Figure 2 (A) shows the excitation and emission spectra along with respective excitation and emission anisotropy data. Excitation anisotropy was surprisingly high (r = 0.30) for these Ag nanoclusters, thereby predicting non-spherical morphology of these clusters. This high anisotropy can also be partly attributed to the short fluorescence lifetime (avg. lifetime = 0.68 ns at 610 nm using 470 nm laser excitation). Furthermore, the polymer template makes the movement slower as several nanoclusters have coordinated with the carboxyl groups on polymer chain and thereby increases the molecular size and/or hydrodynamic radius. The large size (hydrodynamic radius) of this complex also contributes towards the high anisotropy value. Figure 3 shows the different lifetimes observed at different wavelengths when excited with 470 nm laser. Fluorescence lifetime increases with increasing wavelength of observation from 610 nm to 810 nm. Such unconventional observation could be due to the different microenvironments of the clusters or the different association numbers of silver atoms in clusters. Figure 2 (B) shows the photograph comparing emission intensity of Ag clusters and 5 nm green emission quantum dots (used as a reference) when excited with blue laser and observed through parallel ( ) and perpendicular (⊥) polarizer orientation. This qualitative information shows that the fluorescence intensity change for Ag clusters is visually evident when observed through parallel ( ) and perpendicular (⊥) polarizer and supports our quantitative anisotropy measurements. On the other hand, fluorescence intensity of quantum dots in both polarizer orientations is about the same, showing no polarization behavior. Anisotropy was found to be dependent on excitation and emission wavelength thus demonstrating the presence of multiple emitters in the solution (see supporting materials, figure 1(B) and 2(B)).
Figure 3.
Fluorescence intensity decays of Ag nanoclusters in water at room temperature when emission intensity was observed at 610nm (red), 710nm (green), and 810nm (magenta) at 480 nm excitation.
The emission spectra depend on the excitation wavelength used. Xu and Suslick also observed similar dependence with their Ag clusters synthesized using the sonication method [13], moreover; we observed that fluorescence lifetime of these Ag nanoclusters was also dependent on the excitation wavelength and increased with excitation wavelength (Table 1). Fluorescence intensity weighted average lifetime changed from 1 ns for blue excitation to 1.6 ns for red excitation. Figure 4 (A) shows the change in emission spectra with different excitations. The shift in the peak emission and fluorescence lifetime with excitation can be due to different size distributions of Ag cluster-polymer complex in the solution. Polydisperse distribution of the cluster-polymer complex is a disadvantage of this synthetic route and need further investigation on obtaining monodisperse size population.
Table 1.
shows the fluorescence lifetime data of Ag nanocluster at different excitation when observed at peak emission wavelength
| Excitation Wavelength (nm) |
Observation Wavelength (nm) |
τ1 (ns) |
τ2 (ns) |
τ3 (ns) |
Amplitude Weighted Average Lifetime (ns) < τ > |
Intensity Weighted Average Lifetime (ns) |
X2 |
|---|---|---|---|---|---|---|---|
| 480 | 605 | 0.05 | 0.5 | 1.5 | 0.4 | 1.0 | 1.14 |
| 525 | 635 | 0.4 | 1.5 | 0.6 | 1.0 | 1.51 | |
| 575 | 665 | 0.5 | 1.7 | 0.8 | 1.2 | 1.29 | |
| 600 | 690 | 0.6 | 1.8 | 1.1 | 1.4 | 1.16 | |
| 630 | 725 | 0.2 | 1 | 2.3 | 1.0 | 1.6 | 1.05 |
| 668 | 760 | 0.2 | 1 | 2.4 | 1.1 | 1.6 | 1.03 |
| 725 | 800 | 0.2 | 1 | 2.3 | 1.0 | 1.6 | 1.04 |
τ1, τ2 and τ3 are different components of fluorescence lifetime in nanoseconds.
, Where,
X2= Goodness of fit
Figure 4.
(A) Emission spectra of Ag nanocluster at different excitation (numbers on top of each spectra) (B) Shows the FCS pattern of Ag nanoclusters when excited with blue laser (470 nm) and red laser (633 nm).
3.2.3 Fluorescence Correlation Spectroscopy
Tao et al have discussed the formation of silver nanoclusters on poly acrylic acid polymer template and show the scheme that clusters are formed on the carboxylate groups of a polymer chain [22]. Furthermore, as mentioned previously, nanoclusters are formed on carboxylate groups of polymer and have polydisperse distribution. Thus, one would expect different diffusion times with different excitation. In order to examine the effect of the PMAA template, we decided to measure the diffusion coefficient of Ag nanocluster with blue and red excitation using fluorescence correlation spectroscopy (FCS). Figure 4 (B) shows the FCS pattern and diffusion coefficients of Ag clusters when excited using 470nm laser and 633 nm laser. It is evident from the plot that the diffusion of clusters is slower when excited with red light compared to its diffusion when excited with blue light. This reinforces the idea that the presence of different sizes and red excitation selectively excites larger-sized Ag cluster-polymer conjugate and hence demonstrates slower diffusion. Although the individual nanocluster is about 2.6 nm, their hydrodynamic radius is much larger since it is embedded in the polymer chains which causes their apparent slower diffusion when excited with red light.
4. Conclusion
In conclusion, we demonstrate that using the UV irradiation set up, a 6:1 Ag+: COOH− ratio was best for producing PMAA capped Ag nanoclusters. It is important to note that the ratio and the UV exposure time will vary depending upon the UV source used. We have decreased the UV irradiation time to 4 min which speeds up the process of synthesis; an essential requirement for scaling up the process. We believe such optimization studies will help researchers better understand and optimize the reaction conditions to produce highly fluorescent nanoclusters of noble metals like gold and platinum. In addition, our studies demonstrate high anisotropy values for these Ag nanoclusters in aqueous solution, and so these clusters potentially can be used as high polarization standards in microscopy studies. FCS revealed the slow diffusion of these clusters in aqueous solution owing to polymer template coating. Furthermore, fluorescence lifetime was found to be dependent on the observation wavelength. Finally, the spectral properties of the silver clusters did not change upon 3 month storage in laboratory conditions at room temperature.
Supplementary Material
Research Highlights.
Fluorescence anisotropy was high (0.30)
Fluorescence lifetime was short ( few hundred picoseconds)
Anisotropy and lifetimes were excitation wavelength dependant
Ag nanoclusters behaves as mixture of fluorophores
Two different diffusion coefficients when excited with blue and red laser
Acknowledgements
This work was supported by the NIH GRANT R01EB12003. We would like to thank Dr. Badri Maliwal for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Krauss TD, Peterson JJ. Nat. Mater. 2011;11:14–16. doi: 10.1038/nmat3206. [DOI] [PubMed] [Google Scholar]
- [2].Ho D. ACS Nano. 2009;3:3825–3829. doi: 10.1021/nn9016247. [DOI] [PubMed] [Google Scholar]
- [3].Shang L, Dong S, Nienhaus GU. Nano Today. 2011;6:401–418. [Google Scholar]
- [4].Xu H, Suslick KS. Adv Mater. 2010;22:1078–1082. doi: 10.1002/adma.200904199. [DOI] [PubMed] [Google Scholar]
- [5].Zhang M, Ye BC. Analyst. 2011;136:5139–5142. doi: 10.1039/c1an15891k. [DOI] [PubMed] [Google Scholar]
- [6].Yuan J, Guo W, Wang E. Anal. Chim. Acta. 2011;706:338–342. doi: 10.1016/j.aca.2011.08.043. [DOI] [PubMed] [Google Scholar]
- [7].Yin J, He X, Wang K, Qing Z, Wu X, Shi H, Yang X. Nanoscale. 2012;4:110–112. doi: 10.1039/c1nr11265a. [DOI] [PubMed] [Google Scholar]
- [8].Chen WY, Lan GY, Chang HT. Anal. Chem. 2011;83:9450–9455. doi: 10.1021/ac202162u. [DOI] [PubMed] [Google Scholar]
- [9].Deng L, Zhou Z, Li J, Li T, Dong S. Chem. Commun. (Camb) 2011;47:11065–11067. doi: 10.1039/c1cc14012d. [DOI] [PubMed] [Google Scholar]
- [10].Lan GY, Chen WY, Chang HT. Analyst. 2011;136:3623–3628. doi: 10.1039/c1an15258k. [DOI] [PubMed] [Google Scholar]
- [11].Shang L, Dong S. Chem. Commun. (Camb) 2008;(9):1088–1090. doi: 10.1039/b717728c. [DOI] [PubMed] [Google Scholar]
- [12].Li Shang SD. J. Mater. Chem. 2008;18:4636. [Google Scholar]
- [13].Xu H, Suslick KS. ACS Nano. 2010;4:3209–3214. doi: 10.1021/nn100987k. [DOI] [PubMed] [Google Scholar]
- [14].Acosta-Torres Laura S., Lopez-Marin Luz M., Nunez-Anita R. Elvira, Hernandez-Padron Genoveva, Castano Victor M. J. of Nanomater. 2011 [Google Scholar]
- [15].Zhang J, Xu S, Kumacheva E. Adv Mater. 2005;17:2336–2340. [Google Scholar]
- [16].Park KH, Im SH, Park OO. Nanotechnology. 2011;22:045602. doi: 10.1088/0957-4484/22/4/045602. [DOI] [PubMed] [Google Scholar]
- [17].Shon YS, Cutler E. Langmuir. 2004;20:6626–6630. doi: 10.1021/la049417z. [DOI] [PubMed] [Google Scholar]
- [18].Stevanovic M, Kovacevic B, Petkovic J, Filipic M, Uskokovic D. Int. J. Nanomedicine. 2011;6:2837–2847. doi: 10.2147/IJN.S24889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhou Z, Du Y, Dong S. Biosens. Bioelectron. 2011;28:33–37. doi: 10.1016/j.bios.2011.06.028. [DOI] [PubMed] [Google Scholar]
- [20].Diez I, Eronen P, Osterberg M, Linder MB, Ikkala O, Ras RH. Macromol. Biosci. 2011;11:1185–1191. doi: 10.1002/mabi.201100099. [DOI] [PubMed] [Google Scholar]
- [21].Guo W, Yuan J, Wang E. Chem. Commun. (Camb) 2009;23:3395–3397. doi: 10.1039/b821518a. [DOI] [PubMed] [Google Scholar]
- [22].Tao Y, Lin Y, Huang Z, Ren J, Qu X. Talanta. 2012;88:290–294. doi: 10.1016/j.talanta.2011.10.043. [DOI] [PubMed] [Google Scholar]
- [23].Kapusta P, Wahl M, Benda A, Hof M, Enderlein J. J. Fluoresc. 2007;17:43–48. doi: 10.1007/s10895-006-0145-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.