Abstract
Animal models are frequently used to assist in the determination of the long- and short-term effects of space flight. The space environment, including microgravity, can impact many physiological and immunological system parameters. It has been found that ground based models of microgravity produce changes in white blood cell counts, which negatively affects immunologic function. As part of the Center of Acute Radiation Research (CARR), we compared the acute effects on white blood cell parameters induced by the more traditionally used animal model of hindlimb unloading (HU) with a recently developed reduced weightbearing analog known as partial weight suspension (PWS). Female ICR mice were either hindlimb unloaded or placed in the PWS system at 16% quadrupedal weightbearing for 4 h, 1, 2, 7 or 10 days, at which point complete blood counts were obtained. Control animals (jacketed and non-jacketed) were exposed to identical conditions without reduced weightbearing. Results indicate that significant changes in total white blood cell (WBC), neutrophil, lymphocyte, monocyte and eosinophil counts were observed within the first 2 days of exposure to each system. These differences in blood cell counts normalized by day 7 in both systems. The results of these studies indicate that there are some statistically significant changes observed in the blood cell counts for animals exposed to both the PWS and HU simulated microgravity systems.
1. Introduction
With the desire to further increase exploration of the Moon and Mars, as well as a growing interest in space tourism and extended stays on the International Space Station, it is important to determine the long- and short-term effects of space flight. The space environment, specifically psychological stress, radiation exposure, and weightlessness, can impact many physiological systems. Immunologic effects, in particular, can pose serious problems for astronauts due to suboptimal response in the face of immunogenic challenge.
It has been reported that astronauts returning from spaceflights have neutrophilia, lymphopenia, and eosinopenia (Taylor and Dardano, 1983; Taylor et al., 1986). Rats have also flown on spaceflights and have returned with variable changes in leukocyte counts, but consistently have neutropenia, lymphopenia, eosinopenia, and monocytopenia immediately post-flight, which are resolved by three days postflight (Allebban et al., 1994; Pecaut et al., 2003; Lange et al., 1987; Ichiki et al., 1996). To determine whether these changes are clinically or medically significant and to develop appropriate countermeasures, more research is necessary. However, the ability to perform an actual spaceflight experiment is difficult and expensive, and opportunities are limited. Instead, ground-based models have been developed to simulate the space environment, but there is no perfect model. Hindlimb unloading (HU), which is also known as hindlimb suspension, antiorthostatic suspension, hypokinetic suspension and hypodynamic suspension, is most commonly used and is a widely accepted model system (Aviles et al., 2005; Chapes et al., 1993; Lee et al., 2005; Sonnenfeld, 2003; Milstead et al., 2004; Morey-Holton and Globus, 2002). HU shares many of the same physiological changes observed following spaceflight, including changes in bone structure and composition, fluid shifts, and muscle atrophy (Chapes et al., 1993). Many studies have been performed showing that the HU system results in modifications to the immune response, including changes in leukocyte subset distribution and neutrophil activity, as well as increased tumor growth (Sonnenfeld, 2003, 2005; Lee et al., 2005).
HU is a model of full weightlessness in the hindlimbs, while missions to the Moon and Mars will result in partial weightbearing. It should be noted that the extreme cephalic fluid shift associated with HU is non-physiologic for small quadrupeds. A new model system, referred to as partial weight suspension (PWS), has recently been developed to better simulate the physiologic effects of chronically reduced quadrupedal loading in mice. Thus far, this model has only been used to study the musculoskeletal effects of partial weightbearing (Wagner et al., 2010). The objective of this study was to compare the effect on white blood cell parameters induced by HU and PWS. We hypothesized that the PWS system would be an equivalent model to HU for studying the effects of simulated spaceflight on white blood cell parameters.
2. Materials and methods
2.1. Subjects and procedure
Female ICR mice (Taconic Farms, Inc., Hudson, NY) 6–8 weeks of age were acclimated in a University of Pennsylvania animal facility for at least 24 h before manipulation. All procedures for the animal care and treatment were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Sentinel mice were monitored for Mouse Hepatitis Virus, Sendai virus, Epizootic Diarrhea of Infant Mice, Theiler’s Mouse Encephalomyelitis Virus, Mice Minute Virus, Mouse Parvovirus, Murine norovirus, Ectromelia virus, Pneumonia virus of mice, Reovirus 3, Lymphocytic Choriomeningitis virus, Mouse adenovirus 1 and 2, Polyoma virus of mice, Mycoplasma spp., Cilia-associated respiratory (CAR) bacillus, pinworms and fur mites. All were negative during the experimentation period. PWS caging was built as previously described with very minor modifications to allow for the same cages to be used for both PWS and HU models. All animals were individually housed and acclimated in the caging 3 days prior to experimentation. One Nestlets™ (Ancare, Bellmore, NY) was placed in each cage to serve as nesting and enrichment material. Animals were divided into four groups: jacketed control, non-jacketed control, PWS, and HU. All groups started with 5 animals each, but due to self-inflicted tail injury or animals freeing themselves from the suspension system over the course of the study, the final sample size may have been reduced; however, the final sample sizes for each group consisted of a minimum of 3 animals. Experimental groups at early time points were repeated in either duplicates or triplicates, resulting in a larger sample size for some groups. The results from the repeated groups agreed with the previous experiments and all values from all experiments were used for analyses. Sample sizes for PWS and HU groups varied from 7 to 15 mice for the 4 h, 1 day and 2 day time points, and 3–5 mice for 7–10 day time points (Table 1). Animals undergoing PWS and the jacketed controls were anesthetized with inhaled isoflurane for less than 5 min and placed in forelimb jackets just prior to placement in the PWS cages. HU animals and their non-jacketed controls were placed directly in the specialized cages without further anesthesia or manipulation. After three days of acclimation in the custom-built cages, animals in the PWS and HU groups were suspended. Animals in the PWS system were suspended as described by Wagner et al. (2010). In brief, the base of the tail was swabbed with an alcohol swab and benzoin tincture was applied. A Steri-Strip™ (3M, St. Paul, MN) was then placed at the base of the tail. The PWS harness was attached by placing a narrow piece of athletic tape through a ring of adjustable chain beads, then around the tail. The mouse was suspended in lunar-analog fashion to allow 16% weightbearing, and ran freely along a rod that allowed full movement of the mouse along the central axis of the cage (Fig. 1).
Table 1.
Mice were divided into four groups: jacketed control, non-jacketed control, partial weight suspension (PWS), and hindlimb unloading (HU). Sample sizes are reported for each group at each individual time point. Experimental groups at earlier time points were repeated in duplicates and triplicates. The variation in the size of the groups occurred as some animals were eliminated from the study after freeing themselves from the suspension system or due to self inflicted tail wounds.
| 4 h | 1 day | 2 days | 7 days | 10 days | |
|---|---|---|---|---|---|
| Jacketed | 3 | 4 | 5 | 3 | 3 |
| Unjacketed | 5 | 5 | 5 | 4 | 3 |
| PWS | 7 | 15 | 10 | 5 | 3 |
| HU | 9 | 10 | 10 | 4 | 4 |
Fig. 1.
Partial weight suspension (PWS) apparatus.
For HU animals, the base of the mouse tail was prepared as with the PWS system, including the placement of a Steri-Strip™ (3M, St. Paul, MN) at the base of the tail. The adjustable bead chain was placed parallel to the tail and taped with athletic tape. The hindlimbs of the mouse were then elevated at a 30° angle from the floor of the cage by placing the adjustable bead chain in an end coupling that was allowed to freely move across the rod at the top of the cage (similar to the PWS system). Both controls and jacketed controls were placed directly into the cages. Each group was left in the system for 4 h, 1 day, 2 days, 7 days or 10 days. The 4 h, 1 day, and 2 day PWS and HU experiments were repeated, resulting in a larger sample size for those groups. Food and water were provided ad libitum to animals in the PWS and HU groups. Controls and jacketed controls were pair-fed according to the previous day’s average consumption of the PWS and HU mice, respectively. The jacketed animals served as controls to only the PWS groups, while the control animals did not receive a jacket and served as controls to only the HU groups. Animals were weighed daily and PWS harnesses were adjusted to ensure all animals in the PWS group were 16% weightbearing. At the appropriate timepoints, animals were euthanized by CO2 asphyxiation and cardiac blood was collected in an EDTA tube and kept refrigerated or on ice until complete blood count (CBC) analysis were performed by Antech Diagnostics (Lake Success, NY) using a Cell-Dyn 3500 Multiparameter Automated Analyzer within 24 h of euthanasia. An Antech technologist reviewed smears from samples where counts were reported outside the reference range. A previous study to compare Antech’s automated counts and manual counts performed in house immediately after blood collection were performed; CBC data from samples collected 0–24 h post blood collection revealed no statistically significant differences between the automated and manual blood cell counts (unpublished data from Romero-Weaver, A., Ware, J.H., Sanzari, J.S., and Kennedy, A.R.).
2.2. Statistical analysis
The goal of the statistical analysis was to compare hematologic outcomes (e.g., WBC, neutrophils, lymphocytes, monocytes and eosinophils) between the two types of suspension: HU versus PWS. There were four treatment groups: control (no jacket/no suspension), HU (no jacket/suspension), jacketed control (jacket/no suspension) and PWS (jacket/suspension). Placement of the animal in a jacket was a contributing factor both because of its known effect on the outcomes and because all animals in the PWS group while none of the HU group, were placed in a jacket. Therefore, a standard two group t-test ignoring placement in a jacket would not be optimal to compare the two types of suspension systems because it would not use data from all four treatment groups. Instead, regression analysis was used to determine if the two types of suspension systems were different, adjusting for the effect of the jacket. The general linear model included two independent factors: jacket (yes/no) and suspension (yes/no), in addition to a term for the jacket by suspension interaction
in which B0 is the Y-intercept and B1, B2 and B3 are regression coefficients (i.e., weights for each factor) of the linear equation. The jacket by suspension interaction term represents the incremental effect on a hematologic outcome for the treatment group with both jacket and suspension. To determine whether the effect of suspension differed between groups that had or did not have a jacket, a test of interaction (i.e., B3 = 0) was employed. This is essentially a comparison of differences (i.e., the difference defined by control versus HU compared to the difference defined by jacketed control versus PWS. Statistical analyses were performed in STATA 11.0 (StataCorp, College Station, TX). Statistical significance was set at p = 0.05.
To compare the results for each blood cell count between the suspended and non-suspended control groups in each system, at every time point, the data were analyzed using the Student’s t-test. The data were plotted and analyzed using GraphPad PRISM software (LaJolla, CA). Tables were generated, reporting differential counts as the percentage of WBC (absolute number of cells divided by absolute WBC count × 100) ± standard deviation (SD). A Student’s t-test was employed to determine changes in the percentage of cell type between suspended and non-suspended groups for both HU and PWS. Blood cell counts in all figures and tables are represented by the mean ± SD. Differences were considered significant when p < 0.05.
3. Results
3.1. Partial weight suspension vs. hindlimb unloading at designated time points
Fig. 2 illustrates the absolute blood cell counts from control animals (non-suspended) and from animals exposed to either the PWS or HU system for 4 h. By using linear regression analysis on WBC and lymphocyte counts (Fig. 2A and B), it was determined that the differences observed for the changes in these blood cell counts between the two systems were not statistically significant. By using linear regression analysis on the neutrophil, eosinophil and monocyte counts in the two different systems, it was determined that the changes in these blood cell counts between the two systems were statistically significant at the 4 h time point (Fig. 2C and E).
Fig. 2.
Linear regression analysis determines neutrophil, eosinophil, and monocyte counts are different between the hindlimb unloading (HU) and partial weight suspension (PWS) systems after a 4 h exposure. White blood cell (WBC) and lymphocyte counts (A and B) are not significantly different when comparing the two suspension systems after 4 h exposure. Animals subjected to the HU and PWS systems are observed to have statistically significant differences between the two groups with neutrophil, eosinophil, and monocyte counts (C–E, *). Data are shown as the average blood cell count ± SD, n ≥ 3.
After 1 day of exposure to each system, complete blood cell counts were determined in a separate cohort of animals. All blood cell types evaluated (WBC, lymphocytes, neutrophils, eosinophils and monocytes) were significantly different from the control, non-suspended animals in each system. Interestingly, exposure to the HU system resulted in an increase in all blood cell types, while exposure to the PWS system resulted in a decrease in all blood cell types at the 24 h time point (Fig. 3).
Fig. 3.
Diverging paths for HU and PWS systems was observed after 1 day exposure to suspension for all blood cell types. Linear regression analysis determined that each blood cell type (A–E) was statistically significantly different from their respective non-suspended control between the two suspension systems (*). Data are shown as the average blood cell count ± SD, n ≥ 3.
At the 2 day time point, there was a statistically significant difference between the results for the two systems when comparing WBC counts and neutrophil counts (Fig. 4A and C). The differences between the changes for the two different systems for lymphocyte, eosinophil and monocyte counts were not considered statistically significant.
Fig. 4.
Diverging paths for HU and PWS systems was observed after 2 day exposure to suspension for WBC and neutrophils. Linear regression analysis determines there are statistically significant suspension-induced differences between the two systems when comparing the absolute WBC and neutrophil count at the 2 day time point (*) (A and C). No differences are seen with HU and PWS systems when comparing the lymphocyte, eosinophil, and monocyte counts (B, D, E). Data are shown as the average blood cell count ± SD, n ≥ 3.
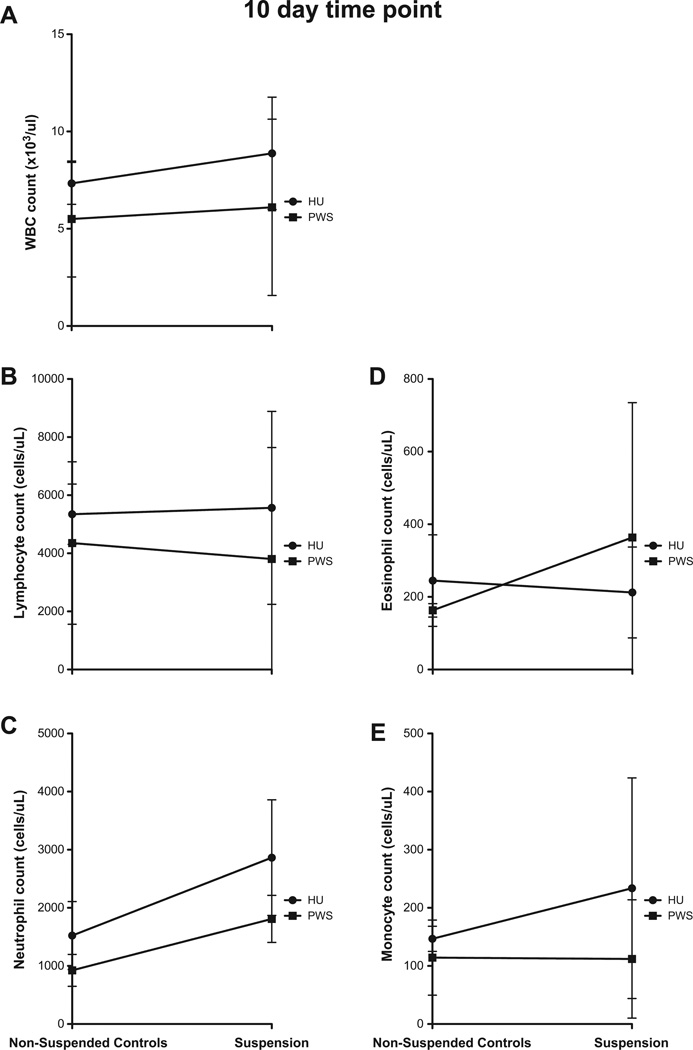
Although there were changes in cell counts at the 7 day time point, the differences in these changes were not statistically significant when the results from the two systems were compared (data not shown). At 10d after suspension, linear regression analysis determines both PWS vs. HU were not significant for all cell types (Fig. 5).
Fig. 5.
No changes are reported in cell counts between HU and PWS after 10 day exposure to suspension. Linear regression analysis determines the differences in WBC (A), lymphocyte (B), neutrophil (C), eosinophil (D) and monocyte (E) counts are not statistically significant between the two systems. Data are shown as the average blood cell count ± SD, n ≥ 3.
3.2. Blood cell count changes induced by the hindlimb unloading system
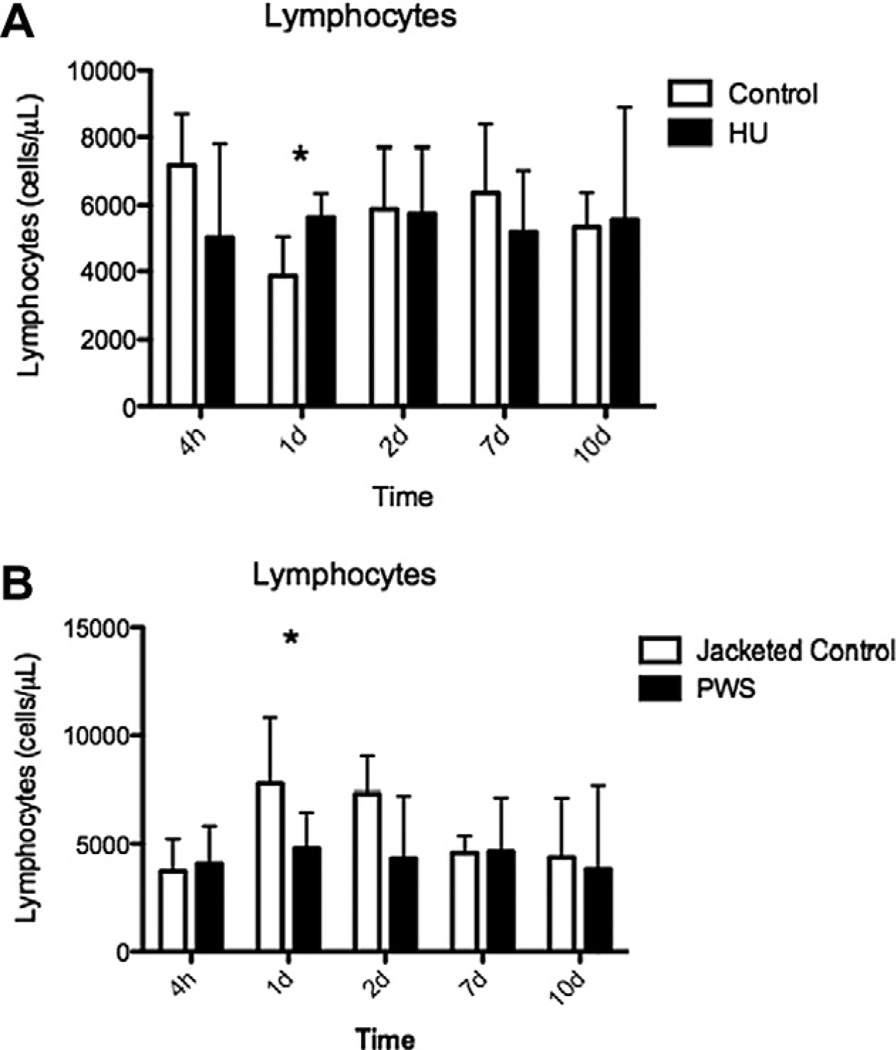
Statistically significant changes in blood cell counts were observed in the WBC, lymphocyte, neutrophil, and monocyte populations only at the 1 day time point, when comparing the hindlimb unloaded animals to their respective controls (Figs. 6–10A). Unlike the partially suspended animals, the cell counts increased in the hindlimb unloaded animals, when compared to the control animals. Eosinophil counts were elevated in the control animals, compared to the hindlimb unloaded animals, at the 4 h time point. There were no statistically significant differences in the eosinophil counts between the suspended and non-suspended animals at the 1 day time point (Fig. 9A). While statistically significant changes in lymphocyte, neutrophil, and monocyte counts were observed at 1 day after HU, the percentage of these cells did not change between HU and control groups (Table 2). Although there was an overall increase in WBC count, the percentage of each cell type was constant between the HU and control groups.
Fig. 6.
Statistically significant changes in WBC counts were observed. An unpaired t-test is used to determine significance between suspended and non-suspended animals. Data are shown as the average blood cell counts ± SD, n ≥ 3. (A) WBC counts increased for HU when compared to the control group 1 day after exposure in a statistically significant manner. No changes in WBC counts are observed at the latter timepoints. (B) WBC counts decreased for PWS when compared to the jacketed control group 1 and 2 days after exposure in a statistically significant manner. No changes in WBC counts are observed at 10 days after exposure.
Fig. 10.
Statistically significant changes in monocyte counts were observed. An unpaired t-test is used to determine significance between suspended and non-suspended animals. Data are shown as the average blood cell counts ± SD, n ≥ 3. (A) Monocyte counts increased for HU when compared to the control group 1 day after exposure in a statistically significant manner. No changes in monocyte counts are observed at the latter timepoints. (B) Monocyte counts decreased for PWS when compared to the jacketed control group 1 day after exposure in a statistically significant manner. No changes in lymphocyte counts are observed at the latter timepoints.
Fig. 9.
Statistically significant changes in eosinophil counts were observed. An unpaired t-test is used to determine significance between suspended and non-suspended animals. Data are shown as the average blood cell counts ± SD, n ≥ 3. (A) Eosinophil counts decreased for HU when compared to the control group 4 h after exposure in a statistically significant manner. No changes in eosinophil counts are observed at the latter timepoints. (B) No changes in eosinophil counts are reported for PWS when compared to the jacketed control group.
Table 2.
A lack of statistical significance was observed with white blood cell count differentials for hindlimb unloading (HU) and control groups over time. Lymphocytes, neutrophils, eosinophils and monocytes are reported as the percentage of total white blood cells. p-Values are reported for an unpaired t-test performed on HU and control groups.
| Lymphocytes |
Neutrophils |
Eosinophils |
Monocytes |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | HU | p-Value | Control | HU | p-Value | Control | HU | p-Value | Control | HU | p-Value | |
| 4 h | 75.2 ± 4.15 | 78.7 ± 7.97 | p > 0.05 | 14.8 ± 1.64 | 15.3 ± 7.94 | p > 0.05 | 5.00 ± 2.00 | 3.13 ± 2.03 | p > 0.05 | 3.60 ± 2.07 | 2.44 ± 1.59 | p > 0.05 |
| 1 day | 78.6 ± 5.32 | 74.5 ± 8.46 | p > 0.05 | 15.2 ± 4.71 | 20.2 ± 8.23 | p > 0.05 | 2.86 ± 2.07 | 2.60 ± 0.843 | p > 0.05 | 2.00 ± 0.707 | 2.30 ± 0.823 | p > 0.05 |
| 2 days | 78.6 ± 3.78 | 79.7 ± 6.24 | p > 0.05 | 17.0 ± 5.48 | 14.3 ± 4.97 | p > 0.05 | 2.40 ± 0.894 | 4.01 ± 3.75 | p > 0.05 | 2.18 ± 1.04 | 2.10 ± 0.994 | p > 0.05 |
| 7 days | 79.0 ± 4.24 | 66.5 ± 10.5 | p > 0.05 | 15.3 ± 7.09 | 31.0 ± 10.4 | p > 0.05 | 2.00 ± 1.00 | 1.67 ± 0.577 | p > 0.05 | 4.25 ± 5.25 | 1.25 ± 0.500 | p > 0.05 |
| 10 days | 72.7 ± 6.35 | 59.8 ± 21.1 | p > 0.05 | 21.0 ± 7.81 | 34.8 ± 17.0 | p > 0.05 | 3.83 ± 1.66 | 3.00 ± 1.73 | p > 0.05 | 2.00 ± 3.29E−10 | 3.00 ± 2.83 | p > 0.05 |
3.3. Blood cell count changes induced by the partial weight suspension system
Statistical analyses were performed between the jacketed control groups and the partial weight suspended groups at each time point. Statistically significant differences between the results observed for WBC counts in the two systems were observed at the 1 day and 2 day time points (Fig. 6B). Interestingly, the non-suspended animals wearing the jacket had statistically significant elevations in WBC counts at these time points when compared to the suspended animals (also wearing the jacket). At 4 h, and by days 7 and 10, the changes in WBC counts were not statistically significant for the jacketed controls and PWS animals.
The absolute neutrophil count was significantly higher in the jacketed control animals at the 1 and 2 day time points, when compared to the 4 h, 7 day and 10 day time points. At the 10 day time point, the jacketed control animals had a significantly decreased neutrophil count when compared to the PWS animals at each time point (Fig. 8B). The only statistically significant differences observed in the lymphocyte and monocyte counts were at the 1 day time point, when the jacketed controls had a significant increase in cell count when compared to the PWS groups (Figs. 7B and 10B). While there were decreases observed for the various cell types, the percentage of lymphocytes, neutrophils, eosinophils, and monocytes remained constant (Table 3).
Fig. 8.
Statistically significant changes in neutrophil counts were observed. An unpaired t-test is used to determine significance between suspended and non-suspended animals. Data are shown as the average blood cell counts ± SD, n ≥ 3. (A) Neutrophil counts increased for HU when compared to the control group 1 day after exposure in a statistically significant manner. No changes in neutrophil counts are observed at the latter timepoints. (B) Neutrophil counts decreased for PWS when compared to the jacketed control group 1 and 2 days after exposure in a statistically significant manner. An increase in neutrophil counts was observed between the PWS and the jacketed control 10 days after exposure.
Fig. 7.
Statistically significant changes in lymphocyte counts were observed. An unpaired t-test is used to determine significance between suspended and non-suspended animals. Data are shown as the average blood cell counts ± SD, n ≥ 3. (A) Lymphocyte counts increased for HU when compared to the control group 1 day after exposure in a statistically significant manner. No changes in lymphocyte counts are observed at the latter timepoints. (B) Lymphocyte counts decreased for PWS when compared to the jacketed control group 1 day after exposure in a statistically significant manner. No changes in lymphocyte counts are observed at the latter timepoints.
Table 3.
A lack of statistical significance was observed with white blood cell count differentials for partial weight suspension (PWS) and jacketed control groups over time. Lymphocytes, neutrophils, eosinophils, and monocytes are reported as the percentage of total white blood cells. p-Values are reported for an unpaired t-test performed on PWS and jacketed control groups.
| Lymphocytes |
Neutrophils |
Eosinophils |
Monocytes |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jacketed control |
PWS | p-Value | Jacketed control |
PWS | p-Value | Jacketed control |
PWS | p-Value | Jacketed control |
PWS | p-Value | |
| 4 h | 78.7 ± 6.81 | 70.4 ± 9.96 | p > 0.05 | 16.0 ± 4.36 | 24.1 ± 11.2 | p > 0.05 | 3.37 ± 2.57 | 3.00 ± 1.73 | p > 0.05 | 1.00 ± 0.00 | 2.57 ± 1.40 | p > 0.05 |
| 1 day | 65.0 ± 15.0 | 70.4 ± 8.81 | p > 0.05 | 25.3 ± 10.0 | 21.2 ± 8.38 | p > 0.05 | 4.50 ± 1.29 | 4.92 ± 2.29 | p > 0.05 | 5.00 ± 4.00 | 3.61 ± 5.11 | p < 0.05 |
| 2 days | 64.4 ± 11.9 | 64.9 ± 15.4 | p > 0.05 | 29.8 ± 11.8 | 22.3 ± 6.02 | p > 0.05 | 3.60 ± 0.548 | 6.21 ± 6.05 | p > 0.05 | 1.20 ± 0.447 | 4.80 ± 4.73 | p > 0.05 |
| 7 days | 71.7 ± 4.51 | 66.6 ± 6.58 | p > 0.05 | 24.7 ± 5.51 | 28.2 ± 8.14 | p > 0.05 | 1.67 ± 0.577 | 3.20 ± 0.837 | p > 0.05 | 1.33 ± 0.577 | 2.00 ± 1.22 | p > 0.05 |
| 10 days | 76.3 ± 7.77 | 53.7 ± 22.9 | p > 0.05 | 19.0 ± 7.21 | 39.0 ± 22.9 | p > 0.05 | 2.33 ± 2.52 | 5.33 ± 2.08 | p > 0.05 | 2.33 ± 1.53 | 1.67 ± 0.577 | p > 0.05 |
4. Discussion
The main objective of this study was to investigate hematologic responses in a reduced weight bearing environment comparing two rodent systems, the hindlimb unloading system and the partial weight suspension system. The results show that there are differences in leukocyte parameters within the first 48 h of exposure to each system. For the most part, the changes in blood cell counts between the PWS or HU animals and their respective non-suspended controls are not statistically significant after 2 days in the suspension apparatus.
Linear regression was used to compare the two systems to take into account the differences in the control or non-suspended animals. Interestingly enough, there were substantial changes in blood cell counts between the control animals from each system. Within the first 48 h, the PWS controls (the jacketed, non-suspended controls) showed an increase in blood cell counts, while the HU controls (non-jacketed, non-suspended controls) had an initial decrease in blood cell counts; although all animals were allotted time to acclimate to the jackets and to the special caging, in both systems, significant differences were observed between the two suspension methods within that time frame. Both suspension systems had contrasting effects within the first 48 h; however, these changes subsided after 48 h. The differences between the blood cell counts from the 7 day time point forward in the experimental and control animals were not statistically significant for the WBC, lymphocytes, eosinophils, and monocyte counts. Although the trend in blood cell count changes appear to fluctuate in opposite directions (increased in the PWS mice or decreased in the HU mice), compared to the respective non-suspended controls, the suspended animals, either PWS or HU, resulted in similar cell counts at the end of the designated suspension period, as shown in Figs 2–5. Further experiments are warranted to investigate the contrasting direction of blood cell count changes between the two systems at the earlier time points.
As shown in Tables 2 and 3, the percentage of lymphocytes, neutrophils, eosinophils, and monocytes remained unchanged over the course of the 10 day study, indicating that changes in overall WBC counts will be reflected in the total number of individual cell types.
Normal mice have 3 times more lymphocytes in circulation than neutrophils. Although leukocyte parameters can vary based on sex, strain, age, time of day of collection and other variables, a typical white blood cell count of mice is 2000–10,000/µL (Fox et al., 2007). All averaged values in this study were within that range. Humans and rats flown in space have experienced neutrophilia, lymphopenia and eosinopenia immediately following spaceflight, while mice returning from space have shown no differences in leukocyte, neutrophil, or lymphocyte counts (Taylor et al., 1986; Taylor and Dardano, 1983; Allebban et al., 1994; Ichiki et al., 1996; Pecaut et al., 2003). In studies of rats, no changes have been observed between animals anesthetized with isoflurane and those unanesthetized (Deckardt et al., 2007; Chassagne et al., 2000). Therefore, it is not believed that there are any effects of the anesthesia on the PWS and jacketed controls in these experiments. It is predicted that the changes in leukocyte parameters observed in humans and rats are likely secondary to stress, with minimal direct association with a reduced gravity state (Taylor and Dardano, 1983; Lange et al., 1987; Ichiki et al., 1996; Chapes et al., 1993). Elevated cortisol levels are reported in astronauts whose lymphocyte numbers are also decreased upon landing (Stowe et al., 1999). Stress hormones were not measured in this study, but may be a potential mechanism for space-flight induced alterations in blood cell counts, since stress hormones are reported to affect the distribution of circulating blood cell numbers (Crary et al., 1983; Dhabhar et al., 1996).
Hindlimb unloading at a 30° angle has been shown to result in a cephalic fluid shift similar to that experienced in humans during spaceflight (Tipton et al., 1987). However, hindlimb unloading results in complete unloading in the hindlimbs with full weightbearing in the forelimbs, which is not directly comparable to the experience in space where astronauts minimally change their upper body loading. Although the partial weight suspension system does not result in a fluid shift, it may provide a more accurate model for the actual response of quadrupeds to spaceflight. Previous studies of antiorthostatic suspension have used harnesses on rats that allowed movement in an arc of 140°. A transient rise in corticosterone for 1–3 days was observed in the harness model, which appears to be consistent with changes in white blood cell counts in this study that may be attributed to stress (Chapes et al., 1993). However, the level of stress observed appears to be comparable to stress produced by spaceflight (Chapes et al., 1993). To best determine the effectiveness of the PWS system as a model for hematologic changes in mice exposed to partial weightbearing conditions, more studies examining the stress experienced by the animals in the system and adding other factors present in space, such as radiation, are necessary.
In conclusion, mouse exposure to PWS and HU results in equivalent hematologic responses only after the first 2 days of exposure to each system. Both of these model systems are useful for evaluating the effects of simulated microgravity on blood cell parameters.
Acknowledgments
This work was supported by the Center of Acute Radiation Research (CARR) grant from the National Space Biomedical Research Institute (NSBRI) through NASA NCC 9-58 and NIH Training Grant 2T32CA00967.
Keywords
- HU
hindlimb unloading
- PWS
partial weight suspension
- WBC
white blood cells
Contributor Information
Jolaine M Wilson, Email: wilsonj2@email.chop.edu.
Gabriel S. Krigsfeld, Email: stuartg@mail.med.upenn.edu.
Jenine K. Sanzari, Email: sanzari@mail.med.upenn.edu.
Erika B. Wagner, Email: erika@mit.edu.
Rosemarie Mick, Email: rmick@mail.med.upenn.edu.
Ann R. Kennedy, Email: akennedy@mail.med.upenn.edu.
References
- Allebban Z, Ichiki AT, Gibson LA, Jones JB, Congdon CC, Lange RD. Effects of spaceflight on the number of rat peripheral blood leukocytes and lymphocyte subsets. J. Leukoc. Biol. 1994;55:209–213. doi: 10.1002/jlb.55.2.209. [DOI] [PubMed] [Google Scholar]
- Aviles H, Belay T, Vance M, Sonnenfeld G. Effects of space flight conditions on the function of the immune system and catecholamine production simulated in a rodent model of hindlimb unloading. NeuroImmunoModulation. 2005;12:173–181. doi: 10.1159/000084850. [DOI] [PubMed] [Google Scholar]
- Chapes SK, Mastro AM, Sonnenfeld G, Berry WD. Antiorthostatic suspension as a model for the effects of spaceflight on the immune system. J. Leukoc. Biol. 1993;54:227–235. doi: 10.1002/jlb.54.3.227. [DOI] [PubMed] [Google Scholar]
- Chassagne P, Jego A, Gloc P, Capet C, Trivalle C, Doucet J, Denis P, Bercoff E. Does treatment of constipation improve faecal incontinence in institutionalized elderly patients? Age Ageing. 2000;29:159–164. doi: 10.1093/ageing/29.2.159. [DOI] [PubMed] [Google Scholar]
- Crary B, Hauser SL, Borysenko M, Kutz I, Hoban C, Ault KA, Weiner HL, Benson H. Epinephrine-induced changes in the distribution of lymphocyte subsets in peripheral blood of humans. J. Immunol. 1983;131:1178–1181. [PubMed] [Google Scholar]
- Deckardt K, Weber I, Kaspers U, Hellwig J, Tennekes H, Van ravenzwaay B. The effects of inhalation anaesthetics on common clinical pathology parameters in laboratory rats. Food Chem. Toxicol. 2007;45:1709–1718. doi: 10.1016/j.fct.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, Mcewen BS, Spencer RL. Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J. Immunol. 1996;157:1638–1644. [PubMed] [Google Scholar]
- Fox JG, Barthold S, Davisson M, Newcomer CE, Quimby FW, Smith AL, editors. The Mouse in Biomedical Research: Normative Biology, Husbandry, and Models. Burlington, MA: Academic Press; 2007. [Google Scholar]
- Ichiki AT, Gibson LA, Jago TL, Strickland KM, Johnson DL, Lange RD, Allebban Z. Effects of spaceflight on rat peripheral blood leukocytes and bone marrow progenitor cells. J. Leukoc. Biol. 1996;60:37–43. doi: 10.1002/jlb.60.1.37. [DOI] [PubMed] [Google Scholar]
- Lange RD, Andrews RB, Gibson LA, Congdon CC, Wright P, Dunn CD, Jones JB. Hematological measurements in rats flown on Spacelab shuttle, SL-3. Am. J. Physiol. 1987;252:R216–R221. doi: 10.1152/ajpregu.1987.252.2.R216. [DOI] [PubMed] [Google Scholar]
- Lee EH, Ding W, Kulkarni AD, Granstein RD. Tumor growth and immune function in mice during hind-limb unloading. Aviat. Space Environ. Med. 2005;76:536–540. [PubMed] [Google Scholar]
- Milstead JR, Simske SJ, Bateman TA. Spaceflight and hindlimb suspension disuse models in mice. Biomed. Sci. Instrum. 2004;40:105–110. [PubMed] [Google Scholar]
- Morey-holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J. Appl. Physiol. 2002;92:1367–1377. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- Pecaut MJ, Nelson GA, Peters LL, Kostenuik PJ, Bateman TA, Morony S, Stodieck LS, Lacey DL, Simske SJ, Gridley DS. Genetic models in applied physiology: selected contribution: effects of spaceflight on immunity in the C57BL/6 mouse. I. Immune population distributions. J. Appl. Physiol. 2003;94:2085–2094. doi: 10.1152/japplphysiol.01052.2002. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld G. Animal models for the study of the effects of spaceflight on the immune system. Adv. Space Res. 2003;32:1473–1476. doi: 10.1016/S0273-1177(03)90383-8. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld G. Use of animal models for space flight physiology studies, with special focus on the immune system. Gravit. Space Biol. Bull. 2005;18:31–35. [PubMed] [Google Scholar]
- Stowe RP, Sams CF, Mehta SK, Kaur I, Jones ML, Feeback DL, Pierson DL. Leukocyte subsets and neutrophil function after short-term spaceflight. J. Leukoc. Biol. 1999;65:179–186. doi: 10.1002/jlb.65.2.179. [DOI] [PubMed] [Google Scholar]
- Taylor GR, Dardano JR. Human cellular immune responsiveness following space flight. Aviat. Space Environ. Med. 1983;54:S55–S59. [PubMed] [Google Scholar]
- Taylor GR, Neale LS, Dardano JR. Immunological analyses of U.S. Space Shuttle crewmembers. Aviat. Space Environ. Med. 1986;57:213–217. [PubMed] [Google Scholar]
- Tipton CM, Overton JM, Joyner MJ, Hargens AR. Local fluid shifts in humans and rats: comparison of simulation models with actual weightlessness. Physiologist. 1987;30:S117–S120. [PubMed] [Google Scholar]
- Wagner EB, Granzella NP, Saito H, Newman DJ, Young LR, Bouxsein ML. Partial weight suspension: a novel murine model for investigating adaptation to reduced musculoskeletal loading. J. Appl. Physiol. 2010;109:350–357. doi: 10.1152/japplphysiol.00014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]