Abstract
The aim of this study was to examine the effect of genistein on human neuroblastoma cell proliferation induced by two common environmental endocrine disruptors, bisphenol A (BPA) and Di-2-ethylhexyl phthalate (DEHP), and to investigate its underlying mechanism. SK-N-SH human neuroblastoma cells were treated with E2 (1 ng/ml), BPA (2 μg/ml) or DEHP (100 μM), with or without genistein (12.5 μM) in vitro. The number of viable cells was detected with an absorbance reader after 0, 24, 48 or 72 h treatment. The percentage of cells in different phases, and expression of Akt and its phosphorylation levels were also assessed by flow cytometry and western blot analysis at 72 h, respectively. The BPA and DEHP groups had a 30% higher number of viable cells compared to the non-treated group at 48 h (P<0.001). However, the cell numbers did not increase significantly in the groups with additional treatment with genistein (P>0.05 vs. control) and the same trend was observed at 72 h. The expression of phospho-Akt protein was increased in the groups treated with BPA or DEHP compared to the control group at 72 h (P<0.05), while no significant elevation in the expression of phospho-Akt was observed (P>0.05) in genistein-treated groups. Cells were arrested at the G2/M phase by genistein. Similar effects were observed in the E2 group with or without genistein treatment. Akt protein expression had no significant change among all the groups (P>0.05). In conclusion, estradiol- or environmental endocrine disruptor-induced proliferation of human neuroblastoma cells is effectively abolished by genistein, likely in a cell cycle- and Akt pathway-dependent manner.
Keywords: neuroblastoma, environmental endocrine disruptors, genistein, Akt
Introduction
Neuroblastoma is a common extracranial childhood solid tumor (1,2). The majority of neuroblastomas are highly metastastic with poor clinical outcome despite the application of intensive multimodal therapy (3). However, its oncogenesis is still poorly understood. Our previous studies showed that environmental endocrine disruptors (EEDs) may be one of the most important causes of the carcinogenesis of neuroblastoma (4,5). A recent multicenter case-control survey also showed that paternal exposure to hydrocarbons was associated with an increased incidence of neuroblastoma in children (6). Therefore, specific antagonists blocking EED-dependent adverse effects may potentially protect against neuroblastoma occurrence and become a new option for the treatment of patients with neuroblastoma in the clinical practice.
Isoflavones are natural compounds that commonly exist in soy-based foods. They display a variety of biological activities including suppression of activity of several enzymes that regulate cell proliferation (7), prevention of cell-cycle progression (8) and inhibition of tumor growth (7). Genistein 4′,5,7-trihydroxyisoflavone, also known as genistein, is one of the major soy isoflavones. Genistein is considered to be a promising therapeutic candidate for various cancers (9–11).
However, the effects of genistein on neuroblastoma cell growth, especially those induced by EED and its molecular mechanisms, are rarely reported. In this study, we used SK-N-SH human neuroblastoma cells as a cell model, and investigated the effect of genistein on the bisphenol A (BPA)-and Di-2-ethylhexl phthalate (DEHP)-induced proliferation of human neuroblastoma in vitro.
Materials and methods
Cell line, reagents and antibodies
SK-N-SH, a human neuroblastoma cell line, was purchased from Shanghai Institute for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). Roswell Park Memorial Institute-1640 (RPMI-1640) medium, phenol red-free RPMI-1640 medium and fetal bovine serum (FBS) were obtained from Gibco (Invitrogen, Carlsbad, CA, USA). 17β-estradiol (E2), genistein, sulphatase and dextran-coated charcoal were purchased from Sigma (St. Louis, MO, USA). Cell counting kit-8 (CCK-8) was obtained from Dojindo Laboratory (Kumamoto, Japan). BPA and DEHP were purchased from Shanghai Chemical Reagents Company (Shanghai, China). Akt and phospho-Akt (Ser473; p-Akt) antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA). E2, BPA, DEHP and genistein were dissolved in Dimethyl Sulfoxide (DMSO), and then diluted with phenol red-free RPMI-1640 medium containing charcoal-dextran-stripped FBS (cd-FBS) to final concentration for culturing at 1 ng/ml E2, 2 μg/ml BPA, 100 μM/l DEHP and 12.5 μM/l genistein, respectively. The final solvent concentration in the culture did not exceed 0.1%.
Cell culture and treatment
SK-N-SH cells were cultured in 75-cm2 flasks with RPMI-1640 medium supplemented with 0.1 M L-glutamine, 10% (v/v) FBS, 100 U/ml of penicillin, 100 μg/ml of streptomycin at 37°C with 5% CO2 in a fully humidified incubator. Prior to treatments, cells were starved in phenol red-free RPMI-1640 medium without FBS for 24 h. The study was approved by the ethics committee of Children’s Hospital, Fudan University.
CCK-8 assay
SK-N-SH cells were seeded into 96-well flat-bottomed microtiter plates at a density of 105/ml and the final volume in each well was 100 μl. After being starved, cells were then treated with 1 ng/ml E2, 2 μg/ml BPA, or 100 μM/l DEHP with or without 12.5 μM/l genistein. The number of viable cells was detected with CCK-8 assay as described previously, at 0, 24, 48 and 72 h (4,5). The treatment wells were in quadruplicates and the whole experiment was repeated independently three times.
Flow cytometry
Cells were cultured into 6-well plates at a density of 3×105/ml, and then treated as described above. After 72 h incubation, 106 cells were fixed with 1 ml 70% ethanol at 4°C for 30 min and then stained with staining solution (2 mg/ml propidium iodide and 10 mg/ml RNase in PBS) at 37°C for 30 min. Stained cells were analyzed for fluorescence intensity with a fluorescence-activated cell sorter (Becton Dickinson, San Jose, CA, USA) equipped with an argon laser emitting at 488 nm, using the CellQuest software (Becton Dickinson). A minimum of 10,000 events were acquired for each determination. The percentages of cells in G2/M phases were calculated by the ModFit program (Becton Dickinson).
Western blot analysis
Neuroblastoma cells were plated in 100-mm dishes and subsequently treated as described above for 72 h. Then cells were collected in RIPA buffer (50 mmol/l Tris-HCl, pH 7.5, 150 mmol/l NaCl, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate) on ice for 15 min. Protein concentrations were determined by the Bradford method and protein extracts (50 μg/lane) were loaded on an 8% SDS-polyacrylamide gels. After gel separation, proteins were transferred to a nitrocellulose membrane. The membrane was blocked with 5% skimmed milk, then separately incubated with anti-Akt antibody (1:1000 dilution), anti-p-Akt antibody (1:200 dilution). Incubation with anti-β-actin antibody (1:5000 dilution) was performed together as an internal control. The membranes were visualized with an enhanced chemiluminescence system according to the manufacturer’s instructions. The bands on the western blot films were scanned by VersaDoc Image Analysis System (Bio-Rad, Hercules, CA, USA), and analyzed with the QualityOne Image Analysis software (Bio-Rad).
Statistical analysis
All results were analyzed using SPSS 11.5 software (SPSS Inc., Chicago, IL, USA). Data were expressed as mean ± standard error of mean (SEM) of separate experiments (n≥3) and compared by one-way analysis of variance (ANOVA). The difference between two treatments was considered significant at P<0.05.
Results
Genistein suppressed SK-N-SH cell proliferation induced by E2, BPA and DEHP
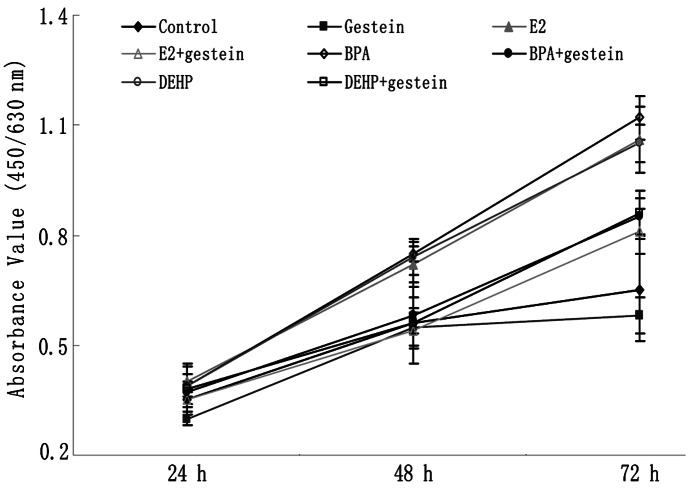
The effect of genistein on the proliferation of neuroblastoma cells was assessed using CCK-8 proliferation assay. Time- and dose-dependent experiments showed that genistein inhibited the SK-N-SH cell growth in a dose-dependent manner (data not shown). After 24 h treatment, absorbance values (AVs) among all the groups showed no significant difference. Then at 48 h, the AVs of the E2, BPA and DEHP groups (0.72±0.06, 0.75±0.02, 0.74±0.05, respectively) were significantly higher than those of the control group (0.56±0.03, P<0.001; Fig. 1). However, there was not a similar increase of AV in groups treated with E2, BPA or DEHP with additional genistein treatment. The AVs in these groups were not significantly higher than those in the control group (P>0.05), but were evidently lower than those in the groups treated with E2, BPA and DEHP alone (P<0.001; Fig. 1). At 72 h, a similar phenomenon was noted (Fig. 1).
Figure 1.
Growth curves of SK-N-SH cells from 0 to 72 h after drug treatment. SK-N-SH cells were treated with 1 ng/ml 17β-estradiol (E2), 2 μg/ml bisphenol A (BPA) or 100 μM/l di-2-ethylhexl phthalate (DEHP), with or without 12.5 μM/l genistein. BPA, DEHP and E2 significantly promoted the proliferation of SK-N-SH cells in vitro at 48 and 72 h, while genistein inhibited the proliferation effects.
Genistein arrested SK-N-SH cells at G2/M phase of cell cycle
After 72 h treatment, flow cytometric analysis was also applied to investigate whether the effect of genistein inhibition against neuroblastoma cell proliferation occurred in a cell cycle-dependent manner. When cells were treated with additional genistein, the percentage of cells in the G2/M phase significantly increased. Cells were arrested at the G2/M phase of the cell cycle (P>0.05 vs. the control group; P<0.01 vs. E2, BPA or DEHP groups; Table I).
Table I.
G2/M phase analysis of SK-N-SH cells at 72 h.
| Groups | G2/M (%) | Groups | G2/M (%) |
|---|---|---|---|
| Control | 9.15±1.08 | Genistein | 16.58±2.71c |
| E2 | 11.70±1.14a | E2+genistein | 15.56±0.58b |
| BPA | 11.94±0.06a | BPA+genistein | 17.76±1.45c |
| DEHP | 11.64±0.39a | DEHP+genistein | 15.99±0.98c |
Compared with control group,
P<0.05; Compared with groups without genistein,
P<0.01;
P<0.001. BPA, bisphenol A; DEHP, Di-2-ethylhexyl phthalate.
Genistein modulated p-Akt (Ser473) protein expression
Akt and p-Akt expression levels were further detected by western blot analysis to determine the principal signaling mechanism of genistein inhibiting SK-N-SH cell growth at 72 h after drug treatments. The expression of p-Akt was abundant when treated with E2, BPA or DEHP only (Fig. 2). By contrast, there was no increase in the expression of p-Akt protein in the genistein-treated groups (Fig. 2). Akt protein expression had no significant change in any of the groups.
Figure 2.
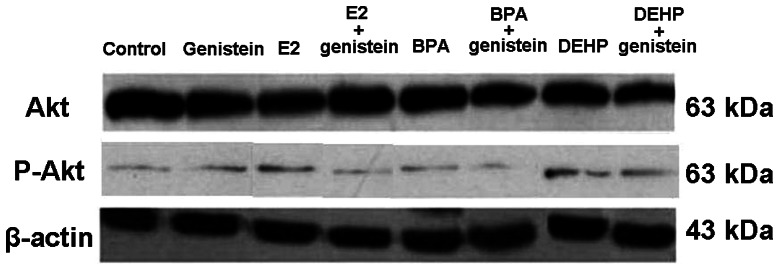
The expression of Akt and p-Akt in SK-N-SH cells detected by western blot analysis. At 72 h, the expression of Akt was abundant in every group. p-Akt was abundant in the E2, BPA and DEHP group, while weak in the control and genistein group. BPA, bisphenol A; DEHP, Di-2-ethylhexyl phthalate.
Discussion
During the last decade, soy isoflavones mainly derived from soybean received much attention as dietary components having inhibitory effects on cancers. The lower risk of breast and prostate cancers in Asians, who consume 20–50 times more soy than Americans, has raised the question whether compounds in the soy diet act as a natural chemopreventive agent. Indeed, a cross-national study involving 59 countries identified that soy products have a highly significant effect against the development of prostate cancer (12). Elevated levels of soy isoflavones in the micromolar range have been detected in the serum, urine, prostatic fluid and prostate tissue in vegetarians and Asian men who consume a soy-rich diet and have low risk of prostate cancer (13). In contrast, serum concentration of genistein in Americans and Europeans is in the nanomolar range (13).
Soy isoflavones include genistein, daidzein and glycitein. However, genistein is the principal isoflavone in soy that has been demonstrated to be responsible for reducing the incidence of hormone-related cancers. In laboratory in vitro experiments, genistein has been found to inhibit the growth of various cancer cell lines including prostate and breast cancer cells (14). Moreover, the evidence from in vitro studies has demonstrated that genistein exerts its inhibitory effects on the development of cancers, cancer cell growth, cancer progression, cancer cell invasion, metastasis and angiogenesis (14).
The direct anti-tumor activity, action mechanisms and therapeutic potential of genistein on neuroblastoma cells have been investigated (8,15). We have previously reported that environmental endocrine disruptor BPA promoted the proliferation of SK-N-SH cells in vitro and in vivo(4,5). However, the effects of genistein on the growth-promoted action of BPA and DEHP on human neuroblastoma cells and its action mechanisms remain poorly understood. In the current study, we hypothesized that genistein may also inhibit the human neuroblastoma cell proliferation induced by EED and estrodiol in vitro.
Our results showed that the proliferation of neuroblastoma cells is enhanced by estrogen and certain environmental estrogen-like contaminants. When genistein was used in combination with E2, BPA or DEHP, cell growth was inhibited effectively. This is in agreement with other studies involving several types of cells (16–18). The present results demonstrated that genistein suppressed SK-N-SH cell proliferation induced by BPA and DEHP.
In addition, genistein prevented the SK-N-SH cells from entering the G2/M phase during the cell cycle, suggesting that the observed growth-inhibitory effect of genistein was mediated through modulation of cell cycle progression in SK-N-SH cells. Our results are in line with others which showed that genistein exerts its anti-tumor effects by arresting SK-N-SH cells in G2/M phase and inhibiting cancer cell death (19).
The phosphatidylinositol-3 kinase/Akt (PI3K/Akt) signaling pathway plays a critical role in cell survival and apoptosis. Inhibition of the PI3K/Akt pathway has been considered as a therapeutic target for cancer where PI3K/Akt activation is a causative factor. It has also been reported that genistein inhibits the activation of the Akt signaling pathway in prostate and breast cancer cell lines (20,21). In our study, the expression of p-Akt was abundant in the E2, BPA and DEHP groups, but genistein caused a decrease in active p-Akt levels.
In summary, BPA and DEHP have growth-promoting effects on human neuroblastoma SK-N-SH cells in vitro, which can be inhibited by genistein. Genistein significantly inhibited the growth of neuroblastoma cells through modulation of cell cycle progression and the PI3K/Akt pathway. The results of our study provide the experimental basis for the application of genistein in additional preventive or therapeutic strategies in neuroblastoma.
Acknowledgments
These results were presented at the 42nd Annual Meeting of the Pacific Association of Pediatric Surgeons, May 9 to May 14, 2009, Hongkong, China. We thank the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, and the Chinese Academy of Sciences for their help with the flow cytometric analysis. This study was supported by the National Natural Science Foundation Commission (30801198/C1611) and the Doctoral Fund of Ministry of Education of China.
References
- 1.van Noesel MM, Versteeg R. Pediatric neuroblastomas: genetic and epigenetic ‘danse macabre’. Gene. 2004;325:1–15. doi: 10.1016/j.gene.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 2.Izbicka E, Izbicki T. Therapeutic strategies for the treatment of neuroblastoma. Curr Opin Investig Drugs. 2005;6:1200–1214. [PubMed] [Google Scholar]
- 3.DuBois SG, Kalika Y, Lukens JN, et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. Journal of pediatric hematology/oncology. 1999;21:181–189. doi: 10.1097/00043426-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Zheng J, Xiao X, Liu J, Zheng S, Yin Q, Yu Y. Growth-promoting effect of environmental endocrine disruptors on human neuroblastoma SK-N-SH cells. Environ Toxicol Pharmacol. 2007;24:189–193. doi: 10.1016/j.etap.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Zhu H, Xiao X, Zheng J, Zheng S, Dong K, Yu Y. Growth-promoting effect of bisphenol A on neuroblastoma in vitro and in vivo. J Pediatr Surg. 2009;44:672–680. doi: 10.1016/j.jpedsurg.2008.10.067. [DOI] [PubMed] [Google Scholar]
- 6.De Roos AJ, Olshan AF, Teschke K, et al. Parental occupational exposures to chemicals and incidence of neuroblastoma in offspring. Am J Epidemiol. 2001;154:106–114. doi: 10.1093/aje/154.2.106. [DOI] [PubMed] [Google Scholar]
- 7.Formica JV, Regelson W. Review of the biology of Quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33:1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- 8.Ismail IA, Kang KS, Lee HA, Kim JW, Sohn YK. Genistein-induced neuronal apoptosis and G2/M cell cycle arrest is associated with MDC1 up-regulation and PLK1 down-regulation. Eur J Pharmacol. 2007;575:12–20. doi: 10.1016/j.ejphar.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 9.Park OJ, Surh YJ. Chemopreventive potential of epigallocatechin gallate and genistein: evidence from epidemiological and laboratory studies. Toxicol Lett. 2004;150:43–56. doi: 10.1016/j.toxlet.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Magee PJ, Rowland IR. Phyto-oestrogens, their mechanism of action: current evidence for a role in breast and prostate cancer. Br J Nutr. 2004;91:513–531. doi: 10.1079/BJN20031075. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer investigation. 2003;21:744–757. doi: 10.1081/cnv-120023773. [DOI] [PubMed] [Google Scholar]
- 12.Hebert JR, Hurley TG, Olendzki BC, Teas J, Ma Y, Hampl JS. Nutritional and socioeconomic factors in relation to prostate cancer mortality: a cross-national study. J Natl Cancer Inst. 1998;90:1637–1647. doi: 10.1093/jnci/90.21.1637. [DOI] [PubMed] [Google Scholar]
- 13.Rannikko A, Petas A, Rannikko S, Adlercreutz H. Plasma and prostate phytoestrogen concentrations in prostate cancer patients after oral phytoestogen supplementation. The Prostate. 2006;66:82–87. doi: 10.1002/pros.20315. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Sarkar FH. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res. 2002;8:2369–2377. [PubMed] [Google Scholar]
- 15.Lo FH, Mak NK, Leung KN. Studies on the anti-tumor activities of the soy isoflavone daidzein on murine neuroblastoma cells. Biomed Pharmacother. 2007;61:591–595. doi: 10.1016/j.biopha.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Linford NJ, Yang Y, Cook DG, Dorsa DM. Neuronal apoptosis resulting from high doses of the isoflavone genistein: role for calcium and p42/44 mitogen-activated protein kinase. J Pharmacol Exp Ther. 2001;299:67–75. [PubMed] [Google Scholar]
- 17.Hewitt AL, Singletary KW. Soy extract inhibits mammary adenocarcinoma growth in a syngeneic mouse model. Cancer Lett. 2003;192:133–143. doi: 10.1016/s0304-3835(02)00712-7. [DOI] [PubMed] [Google Scholar]
- 18.Chang KL, Kung ML, Chow NH, Su SJ. Genistein arrests hepatoma cells at G2/M phase: involvement of ATM activation and upregulation of p21waf1/cip1 and Wee1. Biochem Pharmacol. 2004;67:717–726. doi: 10.1016/j.bcp.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Sarkar FH, Adsule S, Padhye S, Kulkarni S, Li Y. The role of genistein and synthetic derivatives of isoflavone in cancer prevention and therapy. Mini reviews in medicinal chemistry. 2006;6:401–407. doi: 10.2174/138955706776361439. [DOI] [PubMed] [Google Scholar]
- 20.Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH. Inactivation of NF-kappaB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene. 2003;22:4702–4709. doi: 10.1038/sj.onc.1206583. [DOI] [PubMed] [Google Scholar]
- 21.Park SS, Kim YN, Jeon YK, et al. Genistein-induced apoptosis via Akt signaling pathway in anaplastic large-cell lymphoma. Cancer chemother Pharmacol. 2005;56:271–278. doi: 10.1007/s00280-004-0974-z. [DOI] [PubMed] [Google Scholar]