Abstract
Skin engineering provides a new strategy for treating a wide variety of skin defects. In particular, electrospun nanofibrous membranes have been used as carriers for epidermis engineering. The aim of this study was to investigate the feasibility of a modified gelatin and polycaprolactone (GT/PCL) electrospun membrane for epidermis engineering. The biocompatibility of the membranes was evaluated by seeding HaCaT cells (human keratinocyte cell line) on the membrane and the mechanical properties of the membranes were determined with and without cells after culture. A cell proliferation assay showing that HaCaT cells attached and proliferated well on the membranes demonstrated that the membranes possess good biocompatibility. Mechanical tests showed that the membranes are strong enough to be handled during transplantation. Further in vivo transplantation studies revealed that epidermises engineered with GT/PCL membranes were able to repair skin defects in the nude mouse. These results demonstrate that GT/PCL electrospun membranes could be suitable scaffolds for skin engineering.
Keywords: epidermis engineering, electrospun nanofibrous membrane, gelatin, polycaprolactone
Introduction
The early and permanent coverage of extensive skin injury caused by trauma, burns, or diabetic diseases is usually hampered by insufficient supplies of donor skin. Skin engineering provides a new strategy to treat a wide variety of skin defects.1 In early studies, cultured keratinocyte (KC) sheets from autologous skin were applied in clinical use;2 however, the cell sheets are too fragile for engraftment. To improve the mechanical properties of the grafts, various membranes that can support the growth of KCs have been developed.3–6 Such scaffolds can be classified into naturally occurring or artificial substrates, or combinations of the two.
An ideal scaffold for epidermis engineering should provide suitable mechanical properties that can support the transfer of engineered graft from a culture dish to the wound. It should also have good biocompatibility that can provide a favorable environment for KC growth.7 In addition, the quality of the scaffolds should be controllable so that stable clinical outcomes can be achieved. Electrospinning technology, which can easily mass-produce thin nanofibrous membranes with good conformability, could offer a solution to the manufacture of scaffolds for epidermis engineering. Electrospun nanofibers resemble the native topographical features of the natural extracellular matrix and may thus promote the cell’s natural functions in a biomimetic fashion.8,9 Several electrospun nanofibrous membranes have been tested in epidermis engineering, including those fabricated from pure natural materials or natural materials combined with synthetic polymers.3,5,10–14 The latter hybrid materials, which combine the merits of the natural and synthetic polymeric components, have shown great advantages. One applicable hybrid scaffold is the gelatin and polycaprolactone (GT/ PCL) electrospun membrane. The presence of GT enhances the biodegradability and biocompatibility of the membranes, whereas PCL improves the mechanical properties of the sheets. This hybrid material has been used in both skin and nerve engineering.12,15
However, electrospinning of GT and PCL could raise a phase separation problem during electrospinning because of the dissimilarity of the two materials, which might be detrimental to the resultant fiber performance. Fiber inhomogeneities at the ultrastructural level could lead to unfavorable performance (eg, weakened mechanical properties). 3,16–18 To address phase separation related issues, we previously used a tiny amount (<0.3%) of acetic acid to improve miscibility, which clarified the originally turbid solution to be single-phase stable for more than 1 week. The resultant nanofibers appeared to be thinner, smooth, and homogeneous, with enhanced performance in wettability and mechanical properties.19 Thus, it was of great interest to determine whether this improved membrane could be used for epidermis engineering.
In this study, the mechanical properties and biocompatibility of the improved GT/PCL nanofibrous membranes were investigated in vitro by seeding with human KCs. The potential of the scaffold for skin engineering was further evaluated in vivo by transplantation of engineered epidermis into a wound-healing model in the nude mouse.
Materials and methods
Preparation of GT/PCL membranes
Composite GT/PCL (50:50) nanofibers were fabricated as described previously.20 The GT/PCL membranes were tailored into round shapes (diameter 15.6 mm), sterilized for 30 minutes under ultraviolet irradiation, and then lyophilized in a vacuum freeze-drier (Virtis Benchtop 6.6; SP Industries, Inc, Gardiner, NY, USA).
HaCaT cell culture on GT/PCL membranes
The human KC cell line, HaCaT, was purchased from Fuxiang Biological Technology Co, Ltd (Shanghai, People’s Republic of China) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) (HyClone, Logan City, UT, USA) with 10% fetal bovine serum (FBS; Hyclone). Cells at 90% confluency were trypsinized, resuspended in medium, and counted using a hemocytometer. Cells were seeded onto GT/PCL membranes at 2.5 × 104 cells/cm2 and maintained with medium changes every 2 days. Cell proliferation and biomechanical properties were measured after 7 days of culture.
Confocal microscopic analyses
GT/PCL membranes seeded with HaCaT cells were rinsed with phosphate-buffered saline (PBS), fixed with 4% para-formaldehyde for 15 minutes at room temperature, and then counterstained by 4′,6-diamidino-2-phenylindole (DAPI 1:1000; Life Technologies, Carlsbad, CA, USA). Samples were sealed and examined using a confocal microscope (Leica Microsystems, Wetzlar, Germany).
Scanning electron microscopy (SEM) analyses
GT/PCL membranes, with or without cells, were rinsed using PBS, and fixed overnight in 0.05% glutaraldehyde at 4°C. After dehydration through a graded ethanol series, samples were critical-point dried and then examined using a scanning electron microscope (SEM) (JEOL-6380 LV; JEOL, Tokyo, Japan). The pore size of the membrane was measured in SEM by Image J software (National Institutes of Health, Bethesda, MD, USA),21 and the thickness was determined with the aid of a micrometer.12
Cell proliferation assay
The biocompatibility of the scaffold was evaluated by monitoring the cell proliferation of HaCaT cells on the membranes. Briefly, HaCaT cells were seeded on GT/PCL membranes that were commensurate with the size of 24 well plates, at 5 × 104 cells/well in 500 μL of DMEM with 10% FBS. The same amount of culture medium was added into a well with a GT/PCL membrane acting as a control. Cell proliferation was assessed using a Cell Count-8 kit (CCK-8; Dojindo Molecular Technologies, Inc, Kumamoto, Japan) following the manufacturer’s protocol. The plates were then measured at 450 nm wavelength on an enzyme-linked immunosorbent assay instrument (Thermo Fisher Scientific, Waltham, MA, USA). Optical density values were calculated from triplicates of each group, and the experiment was then repeated three times.
Biomechanical test
Mechanical properties of the electrospun fibrous membranes with or without cells were determined using a tabletop uniaxial material testing machine (Instron-3343; Instron, Norwood, MA, USA) equipped with a 50 N load cell. Rectangular shaped specimens (50 mm × 10 mm × 0.10 mm − 0.20 mm) were stretched at a constant cross-head speed of 10 mm/minute. Five samples in each group were tested. The load–elongation behaviors of the scaffolds and failure modes were recorded. The structural properties of the scaffolds were represented by typical stress–strain curves, Young’s modulus (MPa), breaking strength (MPa), and strain at break (%). The original data were transformed into stress–strain values by
| (1) |
and Change in length/original length using Origin 8.5.1 software (OriginLab, Hampton, MA, USA). For each scaffold, the greatest slope in the linear region of the stress–strain curve corresponding to strain between 0%–20% was used to calculate the tensile modulus.
Transplantation of engineered epidermis in a mouse wound-healing model
Two types of cells were used for epidermis engineering: the HaCaT and human KCs. Human KCs were isolated from foreskin specimens, which were obtained from five donors aged from 6 years to 12 years who underwent a routine circumcision procedure at the Shanghai 9th Hospital with informed consent. The study was performed with approval from the local ethics committee (Shanghai Jiao Tong University School of Medicine, People’s Republic of China). KCs were isolated as previously described.22 Primary KCs were suspended in KC-serum free medium (Gibco®; Life Technologies, Carlsbad, CA, USA) and seeded onto tissue culture plates (BD Pharmingen, Franklin Lakes, NJ, USA) at 3.5 × 104 cells/cm2 in 10 mL of complete medium and incubated at 37°C with 5% CO2. Culture medium was changed every other day. Upon reaching 70%–80% confluency, KCs were trypsinized and replated at a 1:3 split ratio. Cells at passage 3 were used for epidermis engineering.
For epidermis engineering, cells (HaCaT or human KCs) were seeded onto GT/PCL membranes (round shape, 1.56 cm in diameter) at 1 × 106 cells/cm2 in DMEM with 10% FBS. Cell cultures were maintained for 7 days with the medium changed every other day. The engineered epidermises were then used for transplantation.
Twelve male BALB/c nude mice were purchased from the Shanghai Laboratory Animal Center National Rodent Laboratory Animal Resources (Shanghai, People’s Republic of China). Mice were anesthetized with intraperitoneal injections of pentobarbital sodium (20 mg/kg body weight). One full-thickness circular wound, 1 cm in diameter, was created on the back of each mouse. The wound was covered by a GT/PCL membrane or engineered epidermis (with HaCaT or human KCs; n = 4 for each group), fixed by 50 nylon sutures at the corners, followed by covering with a Vaseline (Unilever House, London, UK) gauze and adhesive bandages. Animal behavior and bandage integrity were monitored throughout the experiment. Wound healings were evaluated at days 0, 4, 7, 11, and 14 postoperation. Images were recorded with a digital camera (Panasonic Corporation, Osaka, Japan). The wound area was measured by tracing the wound margin using Image-Pro Plus Software (version 5.0; Media Cybernetics LP). The person taking measurements was blinded to the groups and to treatment. Wound-healing rates were calculated as:
| (2) |
Histological analyses and immunofluorescence staining
For histological analyses and immunofluorescence staining, skin grafts were harvested at day 14, embedded in Tissue- Tek® OCT™ compound (Sakura Finetek, Torrance, CA, USA), followed by snap-freezing and sectioning into 5 μm sections. Cells of donor origin were detected by antihuman human leukocyte antigen (HLA)-ABC staining. Briefly, sections were labeled with a mouse antihuman HLA-ABC monoclonal antibody (1:200; Abcam plc, Cambridge, MA, USA), followed by a secondary Alexa–Fluor 488-labeled goat antimouse immunoglobulin G (1:1000; Life Technologies). The slides were then counterstained by DAPI (1:1000; Life Technologies) and observed under a confocal microscope. After immunofluorescence observation, the slides were further stained with hematoxylin and eosin for histological structure analyses.
Statistical analysis
Data were expressed as the mean ± standard deviation. A two-way analysis of variance was used to determine the statistical significance between groups, and a value of P < 0.05 was considered statistically significant.
Results
Preparation of the electrospun GT/PCL membranes
The electrospun GT/PCL membranes were first tailored into a round shape, 1.56 cm in diameter, which was commensurate with the size of 24-well plates (Figure 1A). SEM analysis was performed to evaluate the microscopic structure. As shown in Figures 1B and C, the GT/PCL (50:50) fibers were smooth, uniform, and fine, with an average fiber diameter of 409 nm ± 88 nm (Figure 1D). The average pore size of the membrane was about 7.2 μm ± 1.5 μm and the thickness was 25 μm ± 4 μm (Table 1).
Figure 1.

Characterization of electrospun GT/PCL membranes. (A) Gross view of a round membrane; (B and C) Microscopic structure of the membrane by SEM analysis.
Note: Scale bars: 5 μm.
Abbreviations: GT/PCL, gelatin and polycaprolactone; SEM, scanning electron microscopy; D, diameter of fibers.
Table 1.
Diameter, thickness, and pore size of GT/PCL nanofibrous membrane
| Diameter (nm) | Thickness (μm) | Pore size (μm) |
|---|---|---|
| 409 ± 88 | 25 ± 4 | 7.2 ± 1.5 |
Notes: Data are representative of three independent experiments, and all data are recorded as the mean values ± SD.
Abbreviations: GT/PCL, gelatin and polycaprolactone; SD, standard deviation.
Biocompatibility of GT/PCL membranes in vitro
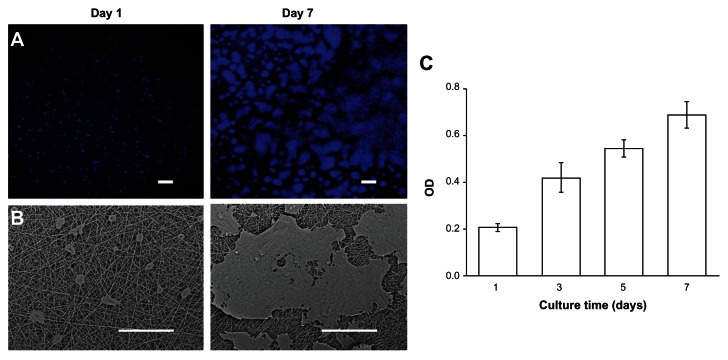
The biocompatibility of the GT/PCL membranes was tested by seeding HaCaT cells on top of the membrane. HaCaT cells adhered to and spread on the membrane 1 day after seeding. Confocal microscope and SEM analyses showed that cells proliferated continuously on the membrane and reached approximately 85% confluency after 7 days of culture (Figures 2A and B). The results were confirmed by CCK-8 analysis. An increase in the optical density value was observed with increasing culture time (Figure 2C). These data proved that GT/PCL membranes possess good biocompatibility.
Figure 2.
Biocompatibility of GT/PCL membranes. (A) SEM images of HaCaT cells on a membrane at day 1 and day 7. Scale bars: 200 μm; (B) Confocal microscope images of HaCaT cells on a membrane at day 1 and day 7. Scale bars: 100 μm; (C) Proliferation of HaCaT cells on a membrane measured by a CCK-8 kit.
Abbreviations: GT/PCL, gelatin and polycaprolactone; SEM, scanning electron microscopy; CCK-8, Cell Counting Kit-8; OD, optical density.
Mechanical properties of GT/PCL membranes and engineered epidermis
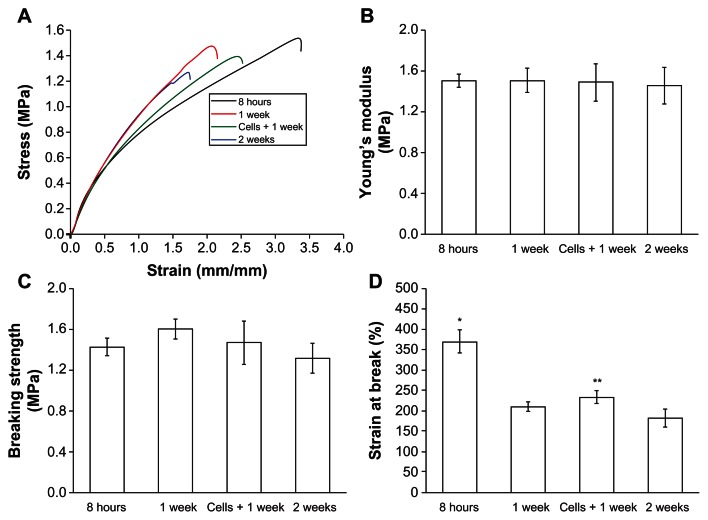
The engineered epidermis should ideally be mechanically strong for handling during transplantation. Thus, the mechanical properties of GT/PCL membranes with or without cells were evaluated after culture. The representative stress–strain behaviors of electrospun GT/PCL 50:50 nanofibers soaked in culture medium for 8 hours, 1 week, 2 weeks, or seeded with HaCaT cells for 1 week are shown in Figure 3A. Young’s modulus and breaking strengths were comparable, and no significant difference was observed between groups (Figures 3B and C). The elasticity (elongation) of the nanofibrous membranes decreased with extended immersion time (Figure 3D), which is likely because of the rapid degradation of GT. Interestingly, seeding of the HaCaT cells could improve the elasticity of the membranes (P < 0.05, compared with membranes at 1 week without cells). The membranes were strong enough to be handled after 1 week of culture.
Figure 3.
Mechanical properties of the GT/PCL membranes with or without cells. (A) Representative stress–strain curves of membranes with or without cells. (B) Young’s modulus; no significant difference was observed between groups. (C) Tensile strength; no significant difference was observed between groups. (D) Strain at break; the elasticity of the membranes decreased with extended immersion time.
Abbreviation: GT/PCL, gelatin and polycaprolactone.
Repair of skin defects with engineered epidermis in vivo
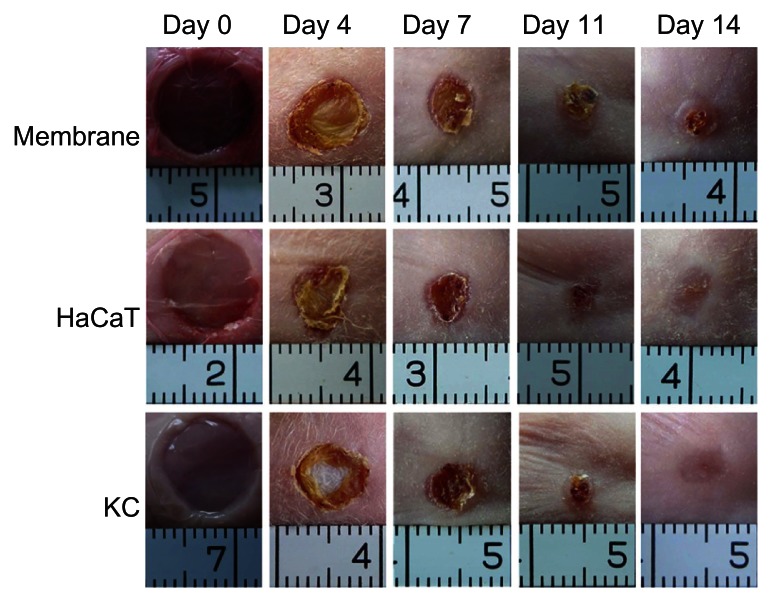
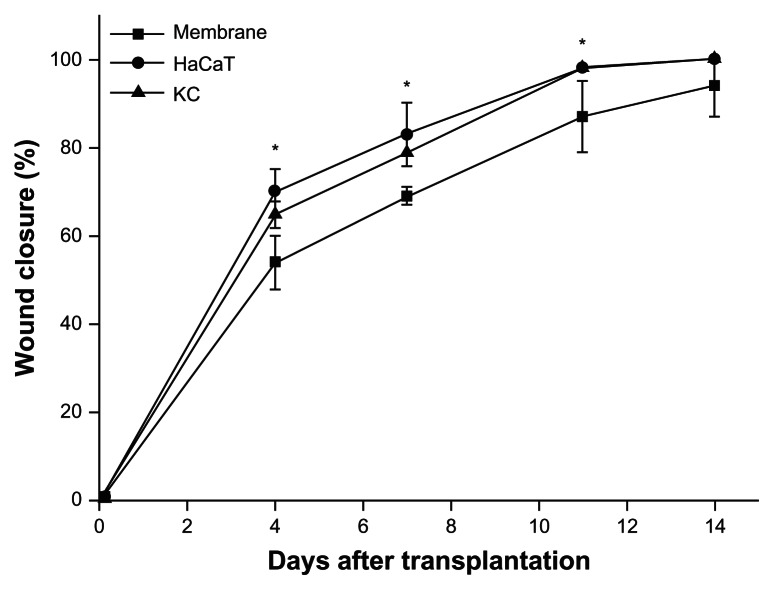
The appearances of animals treated with HaCaT-membranes, KC membranes, or membranes alone were digitally recorded on days 0, 4, 7, 11, and 14 postsurgery. Representative views in each group are shown in Figure 4. Wound closure with shrinking of wound size was observed in all groups at day 14. Statistical analyses from four animals in each group showed that wound closure rates in the groups treated with engineered epidermises, made of either HaCaT cells or human KCs, were significantly (P < 0.05) higher than the group treated with membranes alone at days 4, 7, and 11. However, no significant difference was observed between the three groups at day 14, and the majority of the defects were closed (94.4% ± 6.8% in the membrane-alone group; 99.7% ± 0.6% in the HaCaT-membrane group; and 99.8% ± 0.3% in the KC membrane group) (Figure 5). These results indicate that the engineered epidermis could accelerate the wound closure progress.
Figure 4.
Repair of skin defects with engineered epidermis in the nude mouse.
Notes: Animals were treated with engineered epidermis (HaCaT or KC) or membrane alone, and observed for 14 days.
Abbreviation: KC, keratinocyte.
Figure 5.
Wound closure rates in each group (n = 4 in each group).
Notes: The groups treated with engineered epidermises were significantly (*P < 0.05) higher than the group treated with membranes alone at days 4, 7, and 11, but not at day 14.
Abbreviation: KC, keratinocyte.
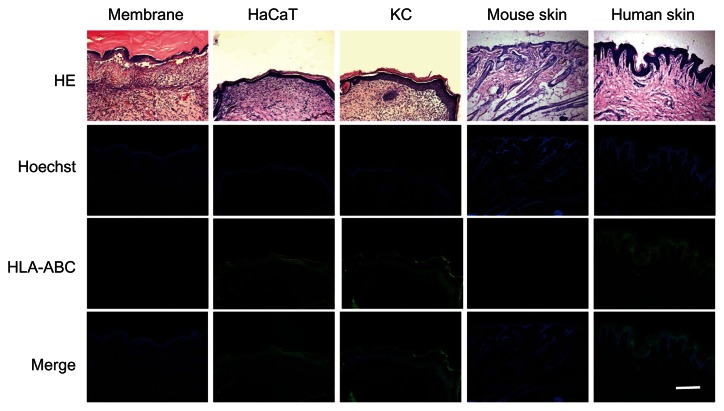
Histological analyses of repaired skin at day 14 showed that in the epidermis-treated groups, multiple layers of epithelial cells covered the wound area. The repaired area could be distinguished by the absence of skin appendages, which only existed in the native mouse skin (Figure 6). To further determine whether HaCaT cells and human KCs contributed to the wound healing, immunofluorescence staining of antihuman HLA-ABC was performed using cell nuclei count stained by Hoechst. As shown in Figure 6, positive staining of HLA-ABC on the epithelial layers was observed in the groups treated with engineered epidermises, but not in the group treated with the membrane alone, suggesting that donor cells survived and contributed to the wound healing.
Figure 6.
Histology of repaired skin.
Notes: HLA-ABC staining indicates the origin of cells from engineered epidermis. Cell nuclei were count-stained by Hoechst. Scale bar: 200 μm.
Abbreviation: HE, hematoxylin and eosin staining; HLA, human leukocyte antigens; KC, keratinocyte.
Discussion
Good biocompatibility and sufficient mechanical strength are basic requirements for membranes in epidermis engineering. The electrospun nanofibrous membranes of GT/PCL meet the requirements by taking advantage of both material properties. GT is a natural component of the extracellular matrix that can provide a suitable ground for cell adhesion, proliferation, and differentiation, whereas PCL provides good mechanical properties because of its slow degradation rate.20 Several ratios of GT/PCL combinations were tested in the preliminary studies (unpublished data). An increase in GT content aided in cell adhesion, but decreased the mechanical strength of the membrane. On the contrary, an increase in PCL content improved the mechanical strength but reduced cell adhesion. The current GT/PCL ratio (50:50) takes the requirements of both epidermis engineering and further surgical operation into account. The in vitro and in vivo experiments demonstrated that the GT/PCL (50:50) membranes are suitable for epidermis engineering.
Degradation of the GT/PCL membranes is slow. Undegraded membranes could still be observed even after 9 months when used for cartilage engineering (unpublished data). The membrane scaffold in epidermis engineering acted simply as a carrier that supports KC growth. After the KCs covered the wounds and wound healing had progressed, the membranes eventually detached from the skin; therefore, the slow degradation of the membrane is not problematic in practice. Our previous work demonstrated that chitosan–gelatin membranes, which are also slowly degrading materials, could be used for epidermis engineering in animal models as well as in patients.6,22 Compared with chitosan–gelatin membranes, the current GT/PCL nanofibrous membranes could provide a biomimetic substrate for cell adhesion and proliferation. In addition, the fiber networks create better permeability, which allows for the easy passage of liquid. With this latter advantage, an epidermis engineered with GT/PCL membranes would give a better performance in the repair of wounds with inflammation and exudates. A comparative study of the two membranes in a skin burn model is under investigation.
It is well known that wound repair processes are initiated immediately after injury by various growth factors and cytokine secretions, cellular proliferation, and neovascularization.23 The improvement of wound healing in the groups treated with engineered epidermis was demonstrated by accelerating the wound closure progress at early time points (Figure 5). The wounds treated with membrane alone were also healed at day 14. This is due to the contraction of the wound in the mouse model. Although a shrinking of wound size was also observed in the experiment groups, HLA-ABC staining confirmed that the wounds were covered by grafted cells, indicating that donor cells played a role in this wound healing process. Studies have shown that engineered epidermis can not only cover the wound, but also secrete growth factors to stimulate skin regeneration.24–26 Therefore, instead of using epithelial cells, many studies have focused on the modification of membranes with growth factors. Choi et al27 immobilized recombinant human epidermal growth factor on an electrospun scaffold for diabetic ulcers, whereas Yang et al28 imbedded human basic fibroblast growth factor into nanofibers to improve the wound recovery rate. Other factors, including granulocyte colony-stimulating factor and platelet-derived growth factor-BB, have also been used for skin tissue engineering.29,30 Blending of growth factors into the GT/PCL electrospun nanofibers is worthy of investigation in the future.
Electrospun nanofibers have been proven to be a promising scaffold for tissue engineering with limited host response.31 One of the components, GT, is the nature products of the extracellular matrix that would barely induce an immune response for repairing murine skin trauma,32 while PCL would not induce inflammatory reactions when used in vivo as a wound dressing.33 In this study, no significant immune reactivity was observed in the animal study (Figure 6). Whether the GT/PCL membranes possess antiinflammation activity is worthy of investigation in wounds with infection. In addition, nanofibrous membranes embedded with antibiotic drugs are also worthy of being developed.34
Conclusion
The current study demonstrated that GT/PCL nanofibrous membranes, improved by the inclusion of acetic acid during the spinning process, possess good biocompatibility and mechanical properties. The membranes may thus be a suitable scaffold for epidermis engineering.
Acknowledgments
This work was supported by the Major State Basic Research Development Program of China (2007CB948004, 2011CB964704); National Basic Research Program of China (30800231, 31170944); Basic Research Program of Shanghai (10ZR1424400, to Li Zhao); Shanghai Shu Guang Foundation (to Zhang WJ); and the Hi-Tech Research and Development Program of China (2006AA02A126). The authors also appreciate technical support from Demin Ying, Lijuan Zong, and Juanjuan Wu.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mansbridge JN. Tissue-engineered skin substitutes in regenerative medicine. Curr Opin Biotechnol. 2009;20(5):563–567. doi: 10.1016/j.copbio.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981;1(8211):75–78. [No authors listed] [PubMed] [Google Scholar]
- 3.Powell HM, Boyce ST. Engineered human skin fabricated using electrospun collagen-PCL blends: morphogenesis and mechanical properties. Tissue Eng Part A. 2009;15(8):2177–2187. doi: 10.1089/ten.tea.2008.0473. [DOI] [PubMed] [Google Scholar]
- 4.Ji SZ, Xiao SC, Luo PF, et al. An epidermal stem cells niche microenvironment created by engineered human amniotic membrane. Biomaterials. 2011;32(31):7801–7811. doi: 10.1016/j.biomaterials.2011.06.076. [DOI] [PubMed] [Google Scholar]
- 5.Kempf M, Miyamura Y, Liu PY, et al. A denatured collagen microfiber scaffold seeded with human fibroblasts and keratinocytes for skin grafting. Biomaterials. 2011;32(21):4782–4792. doi: 10.1016/j.biomaterials.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Woo SL, Yang G, et al. Construction and clinical application of a human tissue-engineered epidermal membrane. Plast Reconstr Surg. 2010;125(3):901–909. doi: 10.1097/PRS.0b013e3181cc9665. [DOI] [PubMed] [Google Scholar]
- 7.Mohamed A, Xing MM. Nanomaterials and nanotechnology for skin tissue engineering. Int J Burns Trauma. 2012;2(1):29–41. [PMC free article] [PubMed] [Google Scholar]
- 8.Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29(13):1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Yoo HS, Kim TG, Park TG. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv Drug Deliv Rev. 2009;61(12):1033–1042. doi: 10.1016/j.addr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Franco RA, Nguyen TH, Lee BT. Preparation and characterization of electrospun PCL/PLGA membranes and chitosan/gelatin hydrogels for skin bioengineering applications. J Mater Sci Mater Med. 2011;22(10):2207–2218. doi: 10.1007/s10856-011-4402-8. [DOI] [PubMed] [Google Scholar]
- 11.Hajiali H, Shahgasempour S, Naimi-Jamal MR, Peirovi H. Electrospun PGA/gelatin nanofibrous scaffolds and their potential application in vascular tissue engineering. Int J Nanomedicine. 2011;6:2133–2141. doi: 10.2147/IJN.S24312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong EJ, Phan TT, Lim IJ, et al. Evaluation of electrospun PCL/ gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007;3(3):321–330. doi: 10.1016/j.actbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Franco RA, Min YK, Yang HM, Lee BT. Fabrication and biocompatibility of novel bilayer scaffold for skin tissue engineering applications. J Biomater Appl. 2013;27(5):605–615. doi: 10.1177/0885328211416527. [DOI] [PubMed] [Google Scholar]
- 14.Long JH, Tan WY, Jiang RW, Zhang YZ. Experimental study on gelatin/polycaprolactam composite nanofiber scaffold in wound healing. Zhonghua Shao Shang Za Zhi. 2008;24(1:):42–44. Chinese. [PubMed] [Google Scholar]
- 15.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Electrospun poly(epsilon-caprolactone)/ gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials. 2008;29(34):4532–4539. doi: 10.1016/j.biomaterials.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Kwon IK, Matsuda T. Co-electrospun nanofiber fabrics of poly(L-lactide-co-epsilon-caprolactone) with type I collagen or heparin. Biomacromolecules. 2005;6(4):2096–2105. doi: 10.1021/bm050086u. [DOI] [PubMed] [Google Scholar]
- 17.Zhang YZ, Feng Y, Huang ZM, Ramakrishna S, Lim CT. Fabrication of porous electrospun nanofibres. Nanotechnology. 2006;17(3):901–908. [Google Scholar]
- 18.Bhattarai N, Li ZS, Gunn J, et al. Natural-synthetic polyblend nanofibers for biomedical applications. Adv Mater. 2009;21(27):2792–2797. [Google Scholar]
- 19.Feng B, Tu H, Yuan H, Peng H, Zhang Y. Acetic-acid-mediated miscibility toward electrospinning homogeneous composite nanofibers of GT/ PCL. Biomacromolecules. 2012;13(12):3917–3925. doi: 10.1021/bm3009389. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Ouyang H, Lim CT, Ramakrishna S, Huang ZM. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J Biomed Mater Res B Appl Biomater. 2005;72(1):156–165. doi: 10.1002/jbm.b.30128. [DOI] [PubMed] [Google Scholar]
- 21.Tutak W, Sarkar S, Lin-Gibson S, et al. The support of bone marrow stromal cell differentiation by airbrushed nanofiber scaffolds. Biomaterials. 2013;34(10):2389–2398. doi: 10.1016/j.biomaterials.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Chen F, Zhang W, Wu W, et al. Cryopreservation of tissue-engineered epithelial sheets in trehalose. Biomaterials. 2011;32(33):8426–8435. doi: 10.1016/j.biomaterials.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Broughton G, 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117(Suppl 7):12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 24.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127(5):998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 25.Buschke S, Stark HJ, Cerezo A, et al. A decisive function of transforming growth factor-β/Smad signaling in tissue morphogenesis and differentiation of human HaCaT keratinocytes. Mol Biol Cell. 2011;22(6):782–794. doi: 10.1091/mbc.E10-11-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansbridge J. Skin substitutes to enhance wound healing. Expert Opin Investig Drugs. 1998;7(5):803–809. doi: 10.1517/13543784.7.5.803. [DOI] [PubMed] [Google Scholar]
- 27.Choi JS, Leong KW, Yoo HS. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF) Biomaterials. 2008;29(5):587–596. doi: 10.1016/j.biomaterials.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Xia T, Zhi W, et al. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials. 2011;32(18):4243–4254. doi: 10.1016/j.biomaterials.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 29.Spadaccio C, Rainer A, De Porcellinis S, et al. A G-CSF functionalized PLLA scaffold for wound repair: An in vitro preliminary study. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:843–846. doi: 10.1109/IEMBS.2010.5626796. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, Yoo JJ, Atala A, Lee SJ. The effect of controlled release of PDGF-BB from heparin-conjugated electrospun PCL/gelatin scaffolds on cellular bioactivity and infiltration. Biomaterials. 2012;33(28):6709–6720. doi: 10.1016/j.biomaterials.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao H, McHugh K, Chew SY, Anderson JM. The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J Biomed Mater Res A. 2010;93(3):1151–1159. doi: 10.1002/jbm.a.32609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang WH, Chang Y, Lai PH, Sung HW. A genipin-crosslinked gelatin membrane as wound-dressing material: in vitro and in vivo studies. J Biomater Sci Polym Ed. 2003;14(5):481–495. doi: 10.1163/156856203766652084. [DOI] [PubMed] [Google Scholar]
- 33.Ng KW, Achuth HN, Moochhala S, Lim TC, Hutmacher DW. In vivo evaluation of an ultra-thin polycaprolactone film as a wound dressing. J Biomater Sci Polym Ed. 2007;18(7):925–938. doi: 10.1163/156856207781367693. [DOI] [PubMed] [Google Scholar]
- 34.Chen DW, Hsu YH, Liao JY, Liu SJ, Chen JK, Ueng SW. Sustainable release of vancomycin, gentamicin and lidocaine from novel electrospun sandwich-structured PLGA/collagen nanofibrous membranes. Int J Pharm. 2012;430(1–2):335–341. doi: 10.1016/j.ijpharm.2012.04.010. [DOI] [PubMed] [Google Scholar]