Balloon kyphoplasty rapidly reduces pain and improves function, disability, and QOL over the course of two years compared with non-surgical management; the reduction in pain, EQ-5D QOL, patient satisfaction, and kyphotic angulation remain statistically significant at all time points. Peri-operative complications may be reduced with more care in patient positioning.
Keywords: balloon kyphoplasty, vertebral fracture, osteoporosis, kyphosis correction
Abstract
Study Design.
Multicenter randomized controlled trial.
Objective.
To compare the efficacy and safety of balloon kyphoplasty (BKP) with nonsurgical management (NSM) during 24 months in patients with painful vertebral compression fractures (VCFs).
Summary of Background Data.
Recently, several large randomized controlled trials have been conducted and reported how vertebral augmentation compares with NSM for patients with acute VCFs. Few of these trials report on the surgical aspects and radiographical vertebral deformity results.
Methods.
Adults with 1 to 3 VCFs were randomized within 3 months of pain to undergo bilateral BKP (n = 149) or NSM (n = 151). Surgical parameters, subjective quality of life assessments and objective functional (timed up and go) and radiographical assessments were collected.
Results.
Compared with NSM, the BKP group had greater improvements in SF-36 physical component summary (PCS) scores at 1 month (5.35 points; 95% CI, 3.41−7.30; P < 0.0001) and when averaged across the 24 months (overall treatment effect 2.71 points; 95% CI, 1.34–4.09; P = 0.0001). The kyphoplasty group also had greater functionality by assessing timed up and go (overall treatment effect −2.49 s; 95% CI, −0.82 to −4.15; P = 0.0036). At 24 months, the change in index fracture kyphotic angulation was statistically significantly improved in the kyphoplasty group (average 3.13° of correction for kyphoplasty compared with 0.82° in the control, P = 0.003). Number of baseline prevalent fractures (P = 0.0003) and treatment assignment (P = 0.004) are the most predictive variables for PCS improvement; however, in patients who underwent BKP, there may also be a link with kyphotic angulation. In BKP, the highest quart for kyphotic angulation correction had higher PCS improvement (13.4 points) than the quart having lowest correction of angulation (7.40 points, P = 0.0146 for difference). The most common adverse events temporally related to surgery (i.e., within 30 d) were back pain (20 BKP, 11 NSM) new VCF (11 BKP, 7 NSM), nausea/vomiting (12 BKP, 4 NSM), and urinary tract infection (10 BKP, 3 NSM). Several other adverse events were possibly related to patient positioning in the operating room.
Conclusion.
Compared with NSM, BKP improves patient quality of life and pain averaged during 24 months and results in better improvement of index vertebral body kyphotic angulation. Perioperative complications may be reduced with more care in patient positioning.
Level of Evidence: 2
Clinical vertebral fractures affect an estimated 1.4 million individuals worldwide every year.1 Current therapies for vertebral fractures include nonsurgical and surgical treatment. Balloon kyphoplasty (BKP) is a percutaneous surgical option that aims to reduce pain and disability and correct vertebral body deformity using orthopedic balloons. Within the current literature, there are well over 300 unique cohorts of at least 10 patients or more with vertebral compression fracture due to osteoporosis or cancer treated with kyphoplasty or vertebroplasty. Importantly, within the current literature, several studies have demonstrated that kyphoplasty and vertebroplasty provide better clinical outcomes to NSM in randomized controlled studies2–6; however, surgical parameters and radiographical outcomes have either been limited or not collected or reported. We recently reported 1- and 2-year quality of life (QOL) outcomes of the fracture reduction evaluation (FREE) study, a multinational, randomized controlled trial.2,3 The primary objective here is to report the surgical aspects and compare the clinical and radiographical outcomes and 30-day safety of kyphoplasty treatment with standard NSM.
MATERIALS AND METHODS
Participants
The FREE trial was a randomized, nonblinded trial comparing NSM with BKP, for the treatment of acute painful vertebral fractures; details of the participants/protocol have been previously reported.2,3 Participants gave written informed consent before enrollment. The protocol and consent forms were approved by local ethics committees.
Procedures
Patients were randomized to either BKP (n = 149) or NSM (n = 151) as previously described.2,3 Kyphoplasty surgery was to be scheduled no more than 10 days following study entry. BKP was performed using introducer tools, inflatable bone tamps, and polymethylmethacrylate bone cement, and delivery devices (manufactured by Medtronic Spine LLC, Sunnyvale, CA), using a percutaneous, bilateral, transpedicular, or extrapedicular approach that has been described in detail elsewhere.2,3,7,8 Intraoperative assessments included procedural duration, anesthesia and surgical approach, balloon pressures and balloon and cement volumes, intraoperative cement leakage, and adverse events (AEs).
NSM consisted of analgesics, bed rest, bracing, physiotherapy, rehabilitation programs, and walking aids according to standard practices of participating physicians and hospitals; patients who underwent BKP also received these therapies as required. All patients were referred for treatment with calcium and vitamin D supplements and antiresorptive or anabolic agents; please refer to previous reports for results of these treatment modalities.2,3 At 24 months, data were available for 120 patients who underwent BKP and 112 NSM.2
Methods for pain, function, and QOL outcome measures have been described previously.2,3 The timed up and go (TUG) test of mobility is an objective, timed test that requires the subject to get out of a chair, walk 3 meters away from the chair, return, and sit down again, which was assessed at 1, 3, 6, 12, and 24 months.9
Standing lateral spine radiographs were taken at baseline, postoperatively (BKP subjects), 3, 12, and 24 months and read centrally (BioClinica Inc, Newtown, PA); 2 radiologists independently used semiquantitative grading for fracture determination10 and a single reader made quantitative morphometric measurements from endplate to endplate at the posterior, anterior, and midpoint of the vertebral body.11 Kyphosis angle was defined as the angle formed by lines drawn parallel to the caudal and cranial fractured vertebral body endplates. For vertebral body height, a fractured-to-nonfractured ratio was calculated. The prefracture height was estimated by averaging the measurements for the adjacent superior and inferior nonfractured vertebral bodies up to 4 levels away; otherwise the index fracture was considered nonevaluable. All AEs, were reported and evaluated by investigators for device or procedure relationship; AEs were categorized by body system according to Medical Dictionary for Regulatory Activities (MedDRA).12
Statistical Analysis
Analyses were by intent to treat, including all data available from the full analysis set; for physical component summary (PCS), visual analogue scale (VAS), Roland-Morris Disability Questionnaire (RMDQ), EuroQol 5 dimension (EQ-5D) assessment, TUG and satisfaction outcomes, we used repeated-measures analysis of variance using mixed models.2,3,13,14 Patient proportions were compared using the stratified Cochran-Mantel-Haenszel χ2 test. These analyses included randomization stratification factors and baseline as covariates. Angulation and vertebral height data were assessed for normality using Shapiro-Wilk test statistics and P values for nonparametric tests were used as a result. P values for within group change from baseline are based on the signed-rank test and within group change across all time points was assessed using the Friedman test; comparison between groups at each time point is based on the Mann-Whitney test. In the quart analyses, kyphosis correction and PCS, VAS, EQ-5D and RMDQ data were assessed for normality using Shapiro-Wilk normality test statistic; Mann-Whitney nonparametric P values were used if the normality test failed, otherwise, the parametric t test P values were used. No adjustments were made for multiple tests and a P value of 0.05 or less was considered statistically significant.
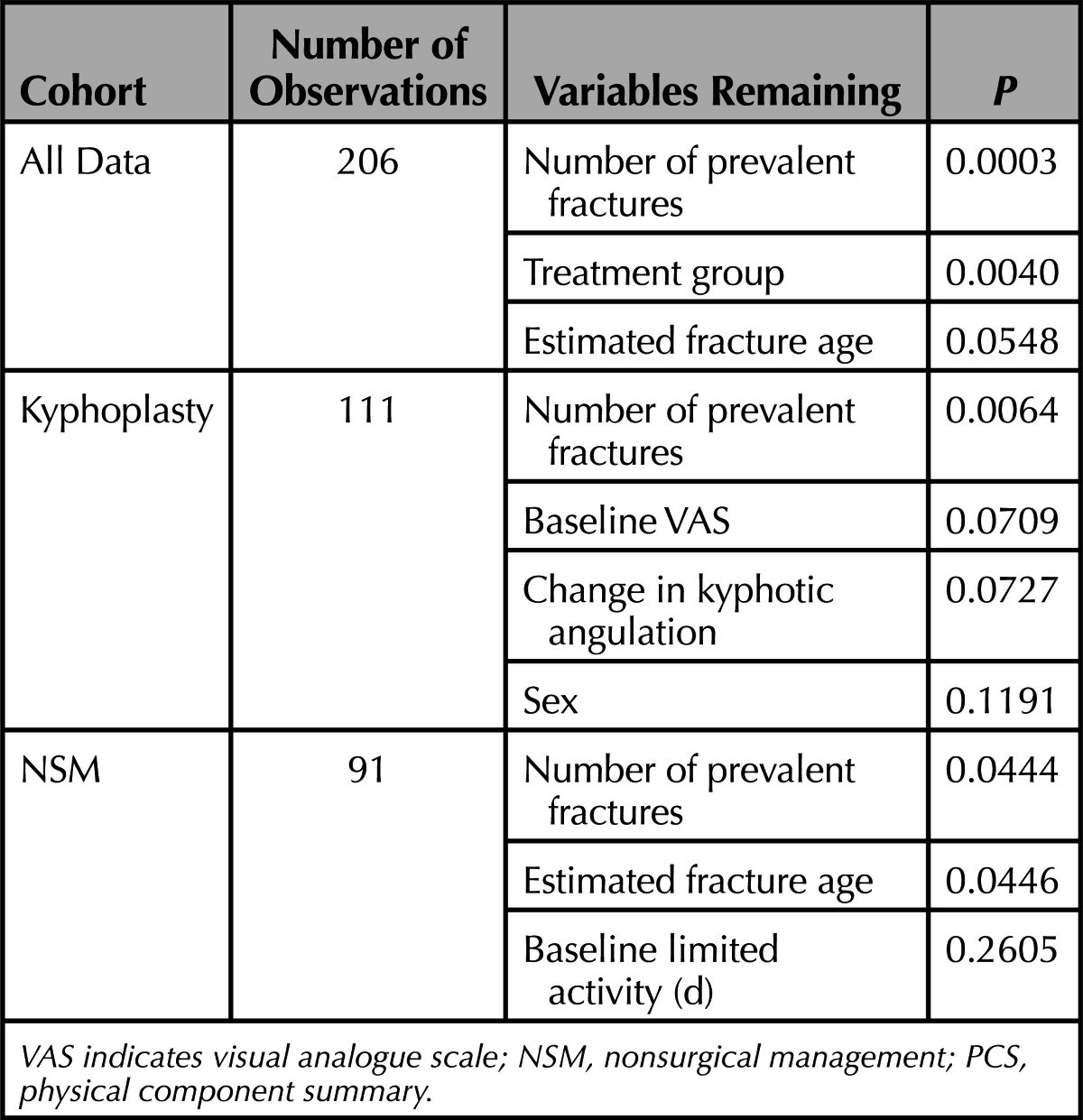
For regression analyses, improvement in PCS (at 3 mo) was used as the dependent variable with the following explanatory variables (treatment, baseline steroid use, baseline RMDQ, VAS and limited activity days, sex, spine T-score, age, baseline bisphosphonate use, estimated fracture age, number of prevalent fractures, and change in kyphotic angulation); baseline EQ-5D, hip T-score and etiology were not included in the model due to high autocorrelation with RMDQ, spine T-score and baseline steroid use, respectively. The regression model was run first for backward selection of variables with P ≤ 0.1 with a second run of the model with remaining variables to obtain adjusted P values.
RESULTS
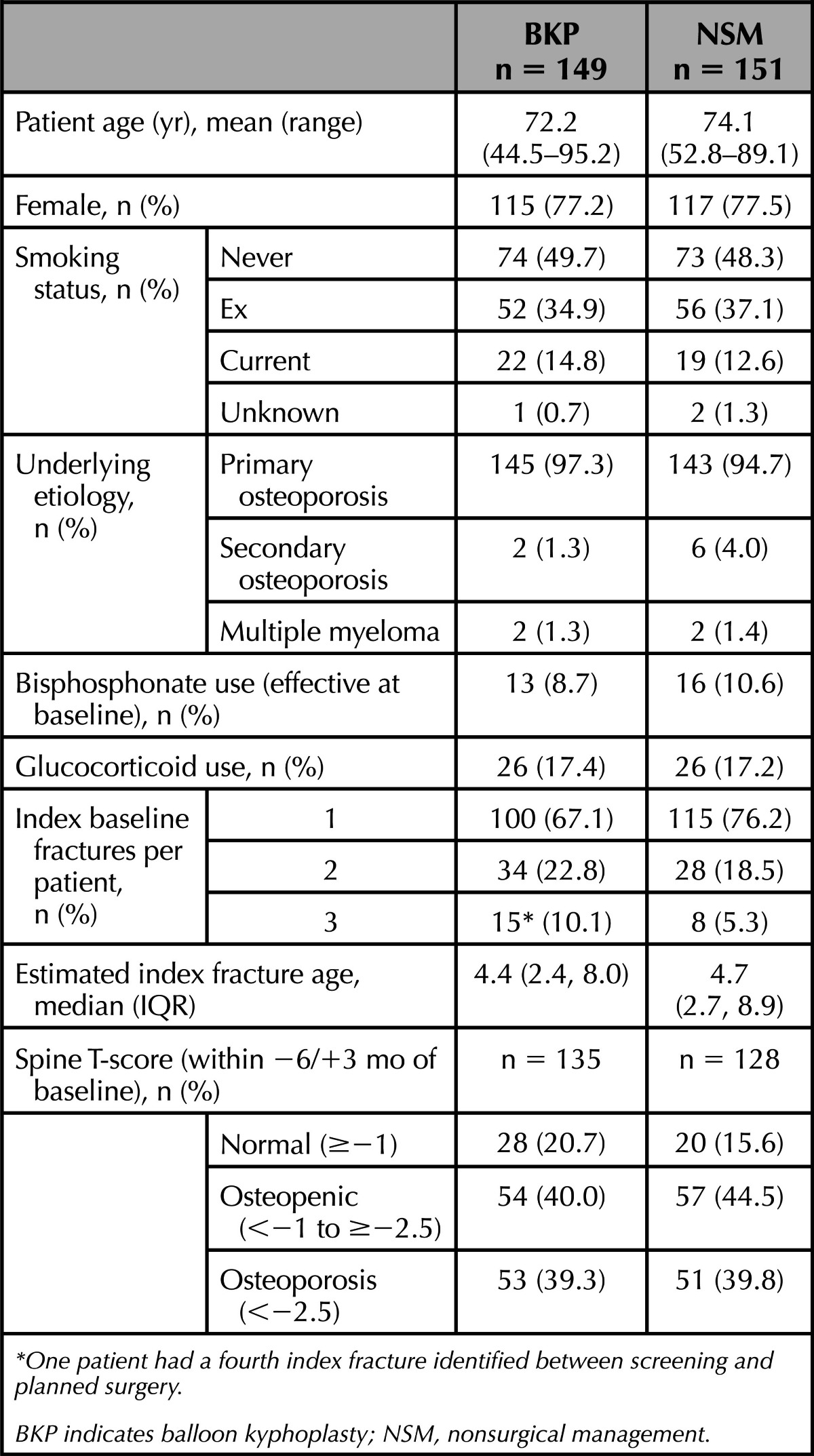
The 2 groups were comparable at baseline for demographic variables (Table 1).
TABLE 1. Demographics.
Procedure Characteristics
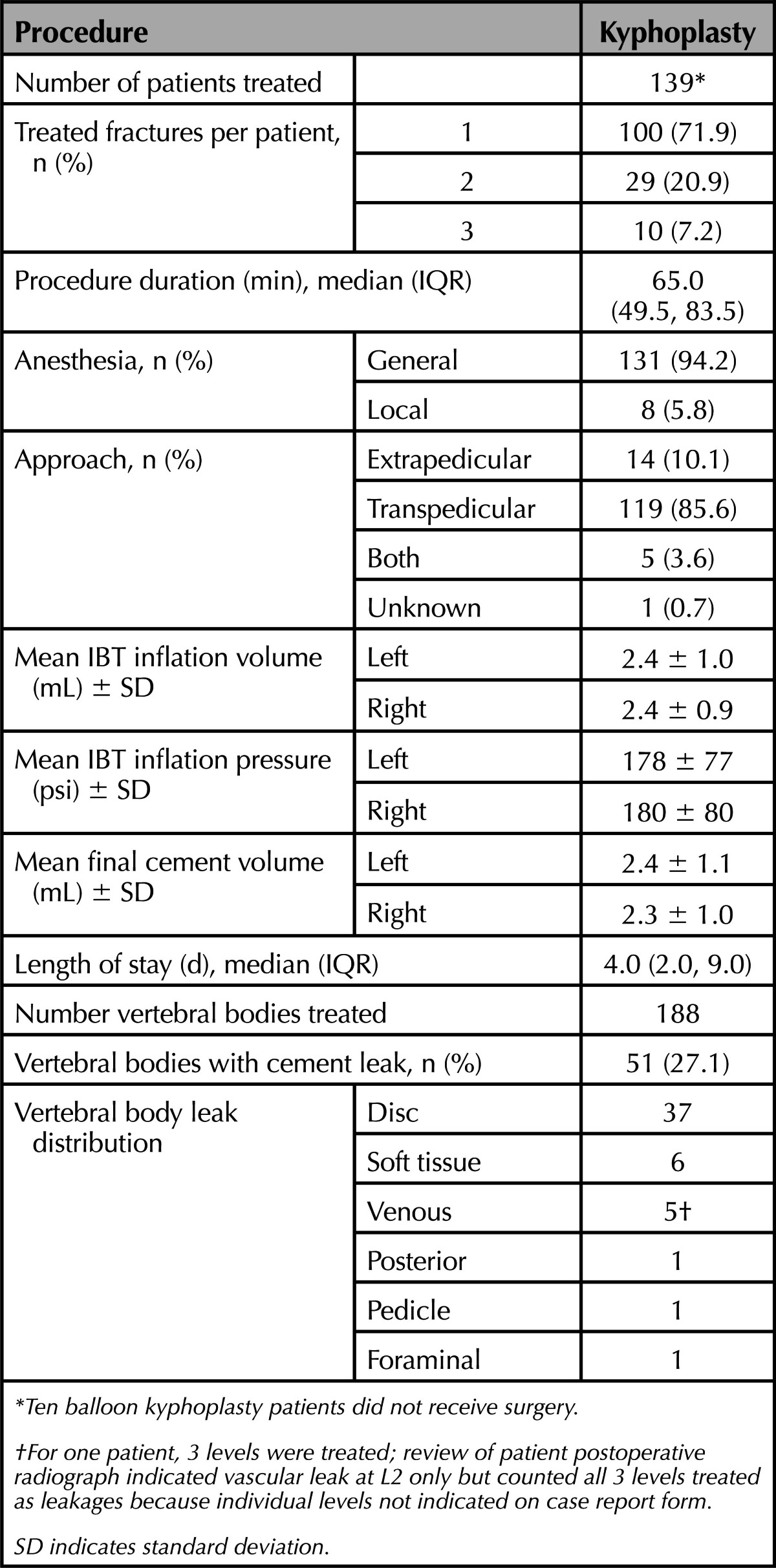
For the 139 patients who received BKP, on average, kyphoplasty was performed 7 days (range, 0–41) following randomization. Most patients were treated using general anesthesia (94.2%) and a bipedicular transpedicular approach (85.6%) with a median procedure duration of 65.0 minutes. On average, final bilateral IBT inflation volumes for BKP were 4.8 (± 1.9) mL that was consistent with cement volume of 4.7 (± 2.1) mL. Cement leakage, assessed by physicians intraoperatively, occurred in 51 of 188 (27.1%) vertebral bodies but no AEs were noted intraoperatively in regard to leakage (Table 2). Hospitalization duration was a median of 4.0 days.
TABLE 2. Procedural Characteristics.
Efficacy Parameters
The kyphoplasty group had 5.35 points (95% CI, 3.41–7.30; P < 0.0001) more on the PCS scale compared with the nonsurgical group at 1 month, (the study primary endpoint) and an average of 2.71 points (95% CI, 1.34−4.09; P = 0.0001) more during the 2-year follow-up with a significant interaction between treatment and follow-up time (P < 0.0001; Table 3). Also, patients assigned to kyphoplasty had statistically significant improvements in EQ-5D, more back pain relief, less Roland-Morris back disability, and were more satisfied on a 20-point Likert scale (Table 3).
TABLE 3. Clinical Outcome Measures*.
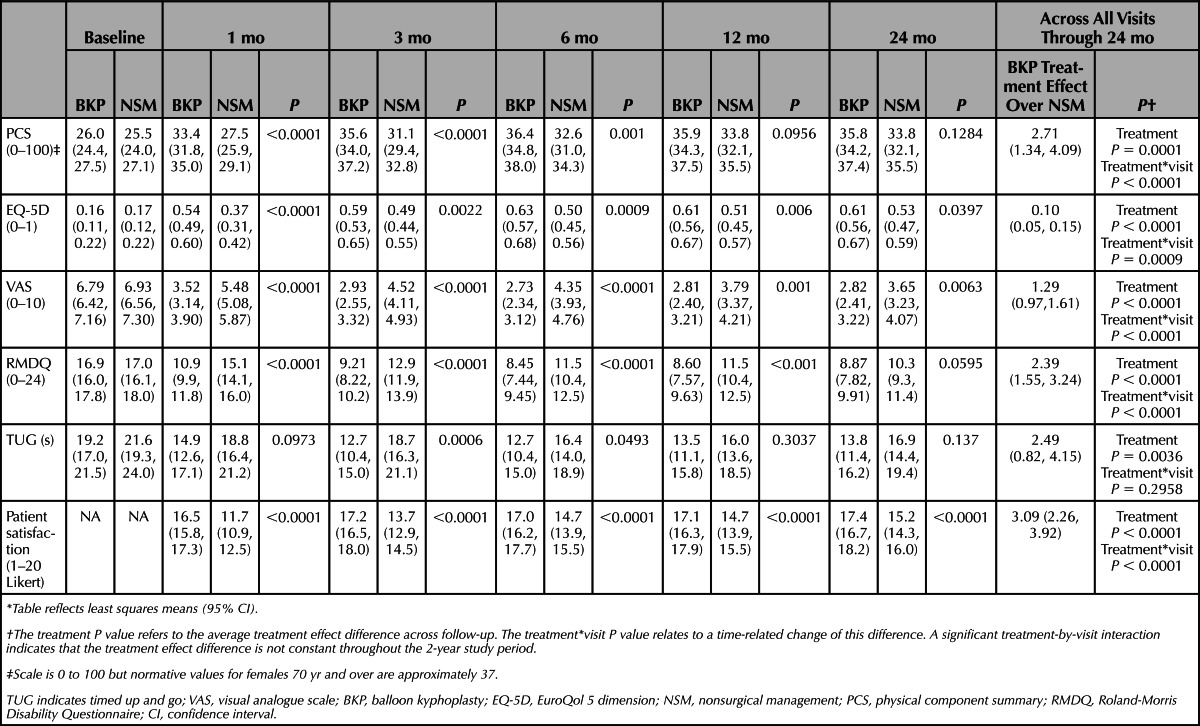
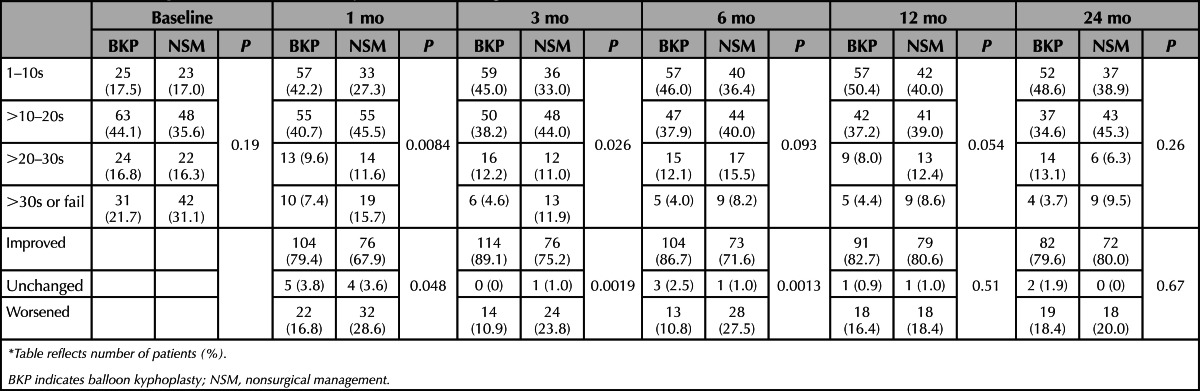
Kyphoplasty resulted in greater functionality by assessing the objective TUG (overall treatment effect −2.49 s, 95% CI, −0.82 to −4.15; P = 0.0036) during the course of 2 years (Table 3). Based on the time required to complete, patients may be categorized into 4 groups that are clinically validated; freely mobile (>0 to ≤10 s), mostly independent (>10 to ≤20 s), variable mobility (>20 to ≤30 s), and impaired mobility (>30 s). The difference in categorical change from baseline between study groups was statistically significant from 1 to 6 months (Table 4).
TABLE 4. Timed Up and Go in Clinically Relevant Categories*.
Radiographical Results
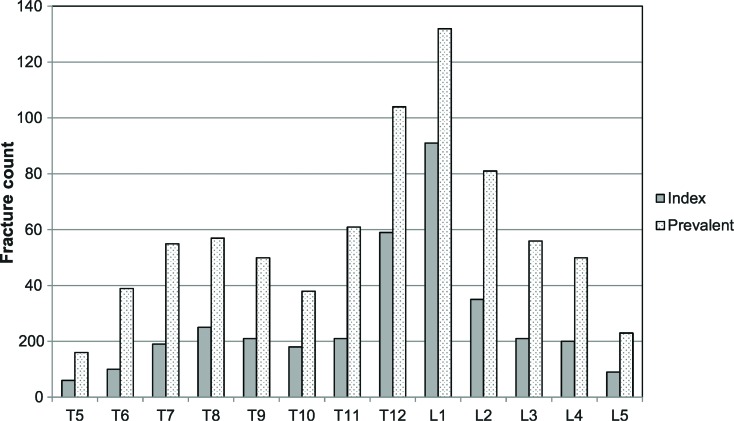
For patients with available plain films at baseline (n = 140 for BKP, n = 133 for NSM), Figure 1 shows the distribution of index levels (those identified as treatment levels) or prevalent fractures (all fractures assessed by the core laboratory). Prevalent fracture determination identified by 2 independent readers were highly concordant (k = 0.795). For both index and prevalent fractures, the majority of fractures occur in the transition zone at T12 and L1. Study patients had many more prevalent fractures compared with those identified clinically (Figure 1).
Figure 1.
Histogram of index and prevalent fractures for BKP and NSM groups, combined. BKP indicates balloon kyphoplasty; NSM, nonsurgical management.
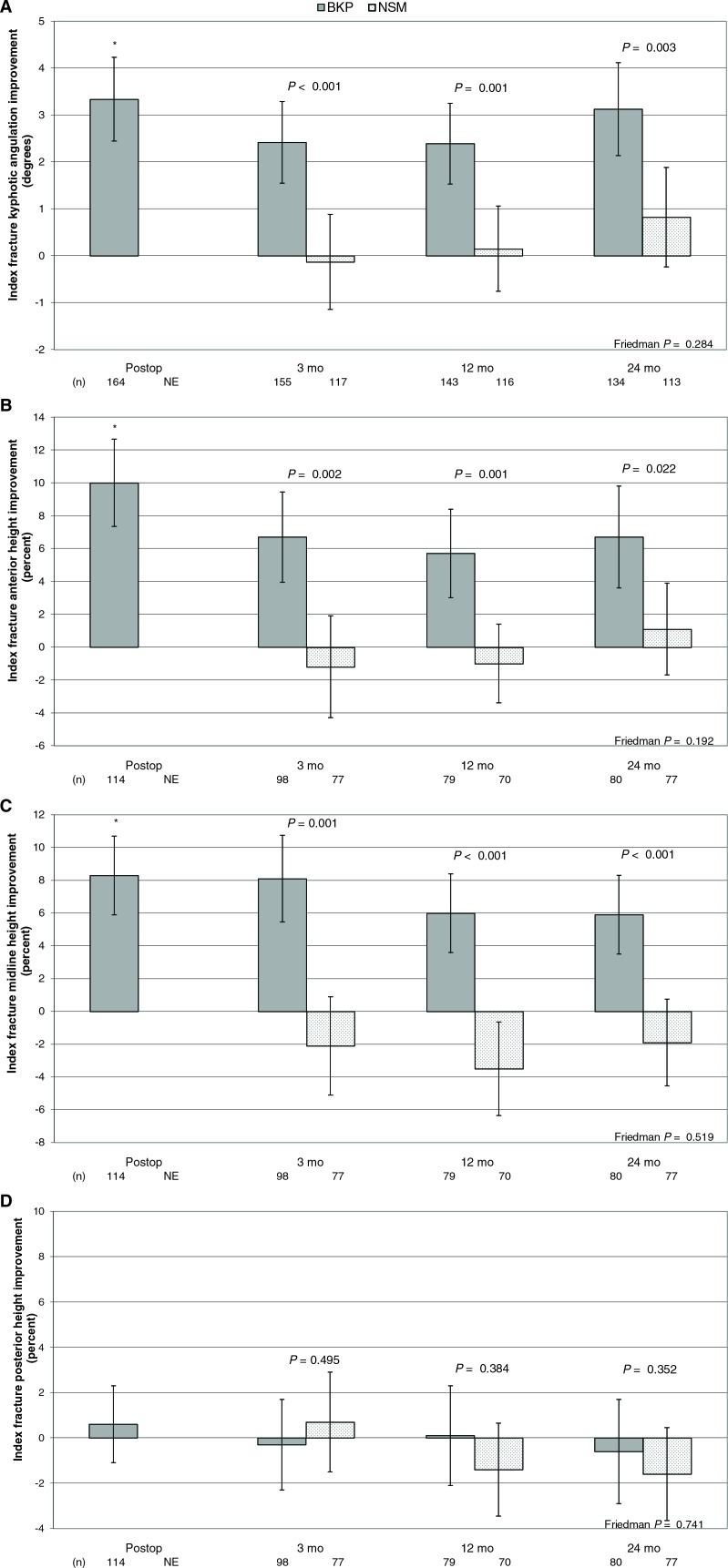
For kyphotic angulation of index fractures (see Materials and Methods section and Figure 2), of 188 BKP-treated fractures, 164 (87%) fractures were evaluable at baseline and the postoperative time point. The postoperative mean change from baseline showed an average improvement of 3.33° that was statistically significant (P < 0.001). At the 3-, 12-, and 24-month visit, the angulation changed −0.89, −0.65, and 0.03°, compared with postoperative; across these time points, differences in angulation were not statistically significantly different (P = 0.284). At 24 months, the change from baseline in index fracture kyphotic angulation was statistically significantly improved in the kyphoplasty group with a correction of 3.13° compared with 0.82° in the control group (P = 0.003 for comparison).
Figure 2.
Index vertebral body kyphotic angulation correction and height restoration. Means and 95% confidence intervals are shown for balloon kyphoplasty and nonsurgical management for (A) kyphotic angulation of index fractures; (B) index fracture anterior height as a percent; (C) index fracture midvertebral height as a percent; (D) index fracture posterior height as a percent. Group comparison P values are shown for each time point. The asterisk (*) indicates a statistically significant improvement from baseline postoperatively (P < 0.001). The Friedman P value represents change across all time point for the BKP group. BKP indicates balloon kyphoplasty; NSM, nonsurgical management; postop indicates postoperative; NE, not evaluated.
Compared with angulation data, there were less evaluable data for assessing vertebral body height (Figure 2); only 114 of the 188 treated vertebrae were evaluable. In this study the mean baseline anterior vertebral height was 62.6% (± 23.0%) and 61.1% (±21.4%) of the predicted height, in the BKP and NSM groups, respectively; similarly, the baseline midvertebral measurement was 65.8% (±19.5%) and 64.5% (±19.2%). In the BKP group, with a mean postoperative anterior gain of 10.0% (± 14.1%) and a midvertebral change in height of 8.3% (±12.6%), this represents an estimated 27% and 25% of lost height restored in the BKP group, respectively. At 24 months, anterior and medial measurements in kyphoplasty were statistically significantly improved 6.7% and 5.9% in the kyphoplasty group compared with the 1.1% improvement and 1.9% worsening in the control group (P = 0.022 and P < 0.001, respectively).
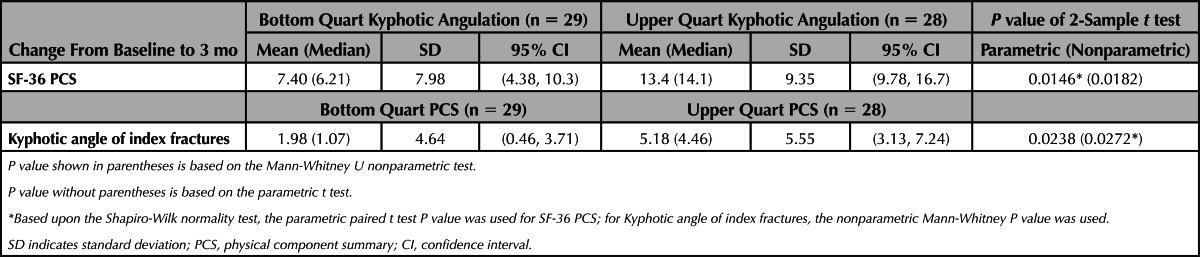
To assess the possible link between BKP kyphotic angulation correction and clinical outcome, we performed a correlation of the change-from-baseline in PCS and kyphotic angulation at 3 months, the first time point in which both of the clinical and radiographical parameters were assessed, seem to be at steady state and maximize evaluable data. Because patients could have multiple fractures, kyphotic angulation correction for multiple fractures were summed for individual patients. In the BKP group, the correlation was relatively weak but statistically significant (Spearman correlation coefficient = 0.201; P = 0.0341). To further evaluate this, patients in the bottom and top quarts of kyphotic angulation improvement were compared with regard to PCS improvement (Table 5). Patients who underwent BKP with highest kyphotic angulation correction had higher PCS improvement (13.4 points) than the subgroup having the lowest correction of angulation (7.40 points, P = 0.0146 for difference). Similarly, patients with highest PCS improvement had better kyphosis correction (5.18°) compared with the subgroup having the lowest amount of PCS improvement (1.98°; P = 0.0272 for difference). In similar analyses with the control NSM group, no correlation was found and there were no statistically significant differences in the upper and lower quarts for PCS or kyphotic angulation (data not shown).
TABLE 5. Upper and Lower Quarts of Kyphotic Angulation and PCS.
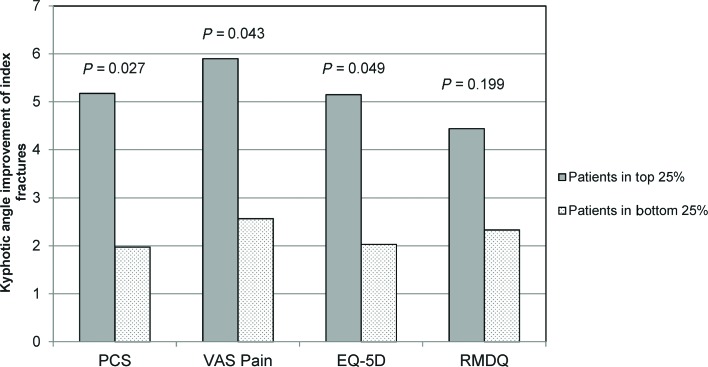
We also analyzed the upper and lower quarts for VAS back pain, EQ-5D QOL and Roland-Morris disability in the BKP group (Figure 3); angulation correction was statistically significantly higher in the upper quarts for each of these parameters except for RMDQ.
Figure 3.
Kyphotic angulation for upper and lower quarts of PCS, VAS, EQ-5D, and RMDQ. The figure shows the kyphotic angulation improvement for the upper and lower quarts for each clinical outcome measure. For each assessment, Shapiro-Wilk normality test was conducted. Based upon this test result, Mann-Whitney U nonparametric test P values are shown for SF-36 PCS and parametric t test P values are shown for VAS, EQ-5D, and RMDQ. EQ-5D indicates EuroQol 5 dimension; VAS, visual analogue scale; RMDQ, Roland-Morris Disability Questionnaire; PCS, physical component summary.
To evaluate what variables are the most predictive of PCS QOL, we performed a multivariate backward elimination regression model. Table 6 shows the results of the final model with remaining variables and adjusted P values. It is clear that the number of baseline prevalent fractures and treatment group are the most predictive variables overall (better outcomes in the BKP group and better outcomes in patients with fewer prevalent fractures at baseline) and fracture age to a lesser degree (younger fractures correlating to better outcomes). Similarly, within each treatment group, the number of prevalent fractures was most predictive. Additionally, in the BKP group, the baseline pain score and change in kyphotic angulation remained with adjusted P values of P = 0.0709 and P = 0.0727, respectively. In the NSM group, the estimated fracture age remained with P = 0.0446.
TABLE 6. Regression Analysis for Explanatory Variables for Improvement in PCS.
Intraoperative Safety
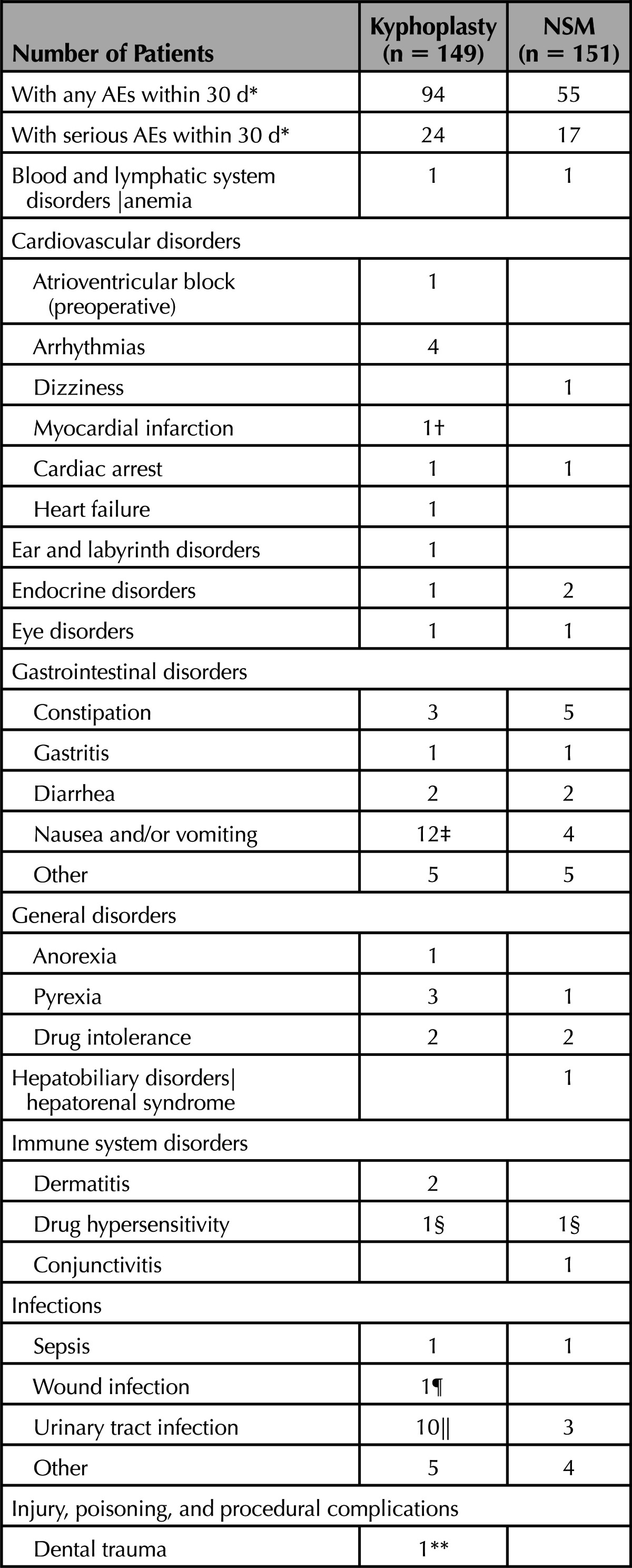
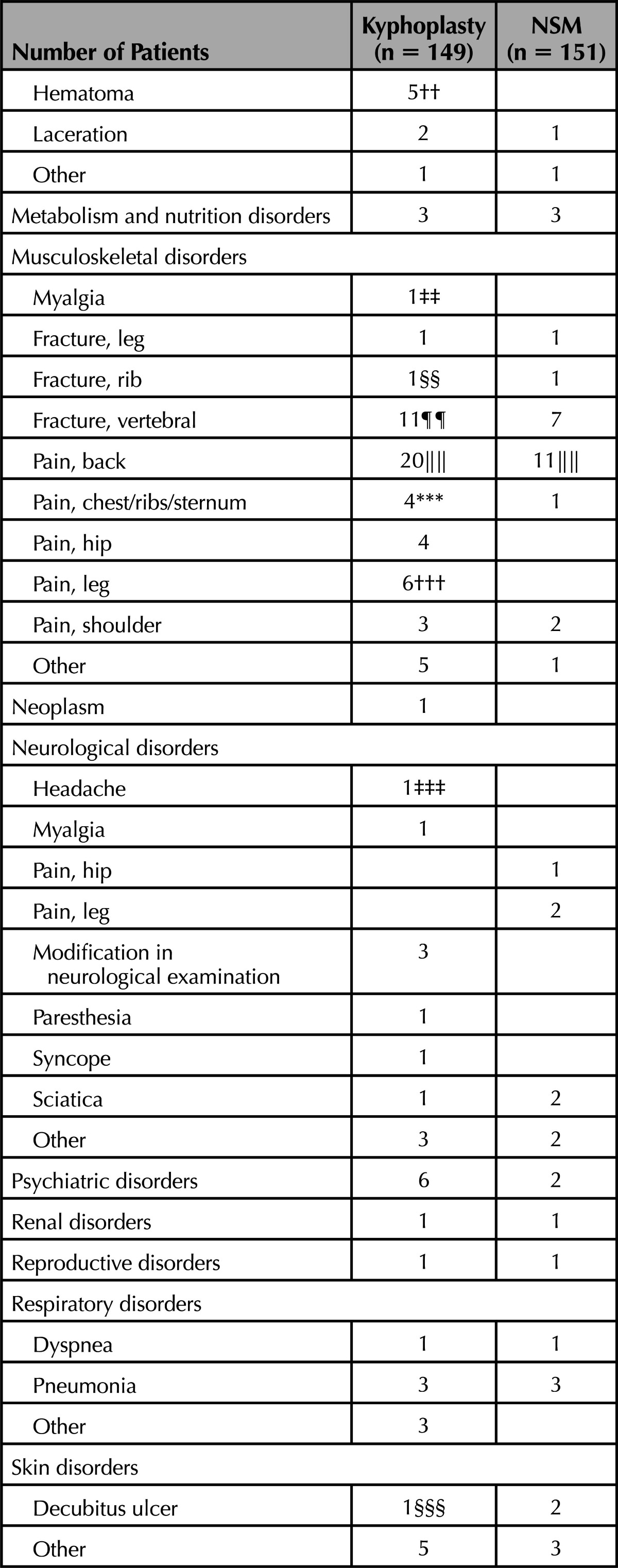
To assess safety events temporally related to surgery, we report all AEs occurring within the first 30 days (Table 7). The most common AEs in this timeframe were back pain (20 BKP, 11 NSM) new vertebral compression fracture (11 BKP, 7 NSM), nausea/vomiting (12 BKP, 4 NSM), and urinary tract infection (UTI; 10 BKP, 3 NSM). A few of the back pain AEs (3 BKP and 2 NSM) were considered related to treatment received. For 6 patients who underwent BKP, nausea/vomiting postoperative was likely due to anesthesia, whereas in another it was attributed to back brace therapy pressing on the stomach; none of these events were serious. For 2 BKP patients with UTI, the events were possibly due to catheterization during the procedure; 1 was a serious AE. Four hematomas occurred in the BKP group; 2 were considered procedure-related and 2 device-related (1 of these was a serious AE). Two nonserious AEs were related to intubation, hypersensitivity to atracurium besilate and damage to dental bridge (Table 7); the latter was the only AE noted to have occurred on the operative case report form. Nine nonserious AEs were possibly related to prone positioning on the operating room table (rib fracture, chest/rib/sternum pain, leg pain, hematoma, headache, facial decubitus ulcer). Five patients who underwent BKP had new vertebral fractures (4 were serious) considered possibly related to cement by the treating physician; however, there was no difference in the number of radiographical fractures observed at 3 months (27/123 for BKP vs. 27/100 for NSM; P = 0.43 for difference).
TABLE 7. Adverse Events Within 30 Days of Surgery/Enrollment.

DISCUSSION
To provide a context of this research, in September 2012, a thorough evaluation of the kyphoplasty literature was conducted using PubMed. For clinical studies consisting of at least 10 patients with vertebral compression fracture due to osteoporosis or cancer treated with kyphoplasty, we found what seemed to be 140 unique cohorts and a total of 8250 patients treated with kyphoplasty, most reporting similar improvements in pain, function, and QOL. The literature also contains several meta-analyses on kyphoplasty and/or vertebroplasty15–24 and a few comparing these surgical therapies to NSM.5,25,26 Several very large retrospective studies using Medicare data have also been conducted investigating kyphoplasty, vertebroplasty, and nonsurgical therapies.27–30
Within the current literature we have found several randomized clinical trials comparing either kyphoplasty or vertebroplasty to nonsurgical management (NSM),2–4,6,31–34 and several reports that compare the cost-effectiveness of these procedures compared with NSM.4,29,35–37 Thus, mounting evidence document the benefits of kyphoplasty and vertebroplasty over standard NSM.
Although 2 randomized trials concluded that vertebroplasty was no different than a “sham” intervention in back pain and disability,38,39 it is important to note that several limitations make it hard to draw definitive conclusions.40,41 Further trials comparing sham with kyphoplasty and/or vertebroplasty, designed to address these limitations, are needed. However, it is important to note in this study, for most criteria, maximal benefit, and stabilization for each group occurs between 3 and 12 months, and we have demonstrated better pain and EQ-5D QOL outcomes compared with NSM throughout 2 years,2 an outcome not consistent with placebo effects.
To date none of the major RCTs for kyphoplasty or vertebroplasty have described detailed radiographical outcomes. Therefore, we have extended our previous results to describe the surgical parameters, vertebral body kyphosis correction and height restoration, the objective TUG test results, and intraoperative safety and analyses exploring the link between vertebral body anatomy correction and QOL.
The postoperative mean change from baseline showed an average improvement of 3.33° in the BKP that was statistically significantly different from the baseline measurement (P < 0.001); across all follow-up time points, differences in index fracture kyphotic angulation correction were not statistically significantly different (P = 0.284), indicating that postoperative deformity correction is maintained during the 24 months of follow-up. The correction was, not surprisingly, better in the BKP group compared with the NSM group at all time points through 24 months. Overall, the final kyphosis correction/vertebral body height restoration in our study is lesser to some extent when compared with other results such as that by Voggenreiter et al42; this may be due to the multicenter nature of our study, larger sample size, and the independent radiographical core lab assessments, which minimizes reader bias. In this study the mean preoperative anterior vertebral height in the BKP group was approximately 63% of the predicted height, with a mean postoperative gain of 10%, representing an estimated 27% of lost height restored; this result is similar to other published studies.43,44 It will be of interest in future studies to understand deformity correction outcomes with new inflatable bone tamp technologies that are made of less compliant materials.
The difference between the treatment groups met the 0.08-point minimally clinically important difference (MCID) for EQ-5D at all time points (Table 3).45 Similarly for pain, the 0.83 point difference at 24 months is a 29% difference; a 25% to 30% difference is also considered clinically relevant.46 The objective TUG test matched fairly well with the subjective RMDQ; improvement in the clinically relevant TUG categories9 was significant through 6 months and the categories themselves were marginally significant at 12 months (Table 4). For RMDQ, using 2 to 3 points as the MCID,47 results were statistically and clinically significant through 12 months and marginally statistically significant at 24 months. For PCS, using the MCID of 3.5 points, the results were both statistically and clinically relevant through 6 months.48 In our study, in multivariable backward elimination regression, it was clear that treatment assignment (P = 0.004) and the number of baseline prevalent fractures (P = 0.0003) were the best predictors of PCS outcome. A few studies have demonstrated a possible connection between angulation and pulmonary function,49,50 but few studies have demonstrated a clinical benefit of height restoration. To our knowledge, no MCID has been established for kyphosis correction and the clinical relevance of the average 2.3°-treatment effect of BKP over NSM at 24 months is dubious. However, we observed a relatively weak correlation between PCS and kyphosis correction in the BKP group (Spearman correlation coefficient = 0.201; P = 0.0341) and kyphosis correction remained in the multivariable backward elimination regression (P = 0.0727). Moreover, patients with the highest amount of kyphosis correction had approximately 6 points more PCS improvement at 3 months; putting this in a clinical perspective, 3.5 points is the MCID for PCS.48 Furthermore, when comparing the upper and lower quarts for PCS, EQ-5D and back pain, those with greater clinical improvement had more kyphosis correction at the treated vertebrae (Figure 3). Fracture mobility, fracture age, patient positioning (bolstering), IBT use and possibly anesthesia type are important parameters that may influence fracture reduction.51 Techniques of vertebroplasty, primarily attributed to postural reduction, may also yield height restoration52,53 but nonetheless, 1 small vertebroplasty versus kyphoplasty RCT showed similar pain outcomes for the 2 procedures but better height in BKP.54 Taken all together, our results suggest that BKP treatment and fewer prevalent fractures at baseline are the most important predictors for providing better PCS QOL outcomes. However, our data also suggest, to a lesser extent, that treating physicians should also consider all parameters in achieving maximal vertebral anatomy correction when performing vertebral augmentation which is in line with orthopedic principles.
We previously reported and discussed the long-term serious AEs and those related to device or procedure (1 patient with UTI and subsequent spondylitis, 1 patient with a hematoma and 1 with anterior cement migration).2,3 With regard to AEs that occurred within 30 days, the most common were nausea, back pain, and UTIs (Table 7). A few of these were considered related to treatment received; nausea, mostly attributed to anesthesia, is not surprising because 94% of patients who underwent BKP were treated using general anesthesia. Recently, there is a trend toward more local anesthesia use, likely due to more interventional radiologists performing BKP, but this is a challenge in elderly patients with scoliosis and facet arthritis. Similarly, a few of the UTIs, common in the elderly, were exacerbated by catheterization. There were 9 nonserious AEs possibly related to prone positioning in the operating room; this safety data suggest the need for extreme care in preparing these elderly patients for surgery. A few new fractures were considered possibly related to bone cement; however, there was no statistically significant difference in the number of patients with new fractures or adjacent fractures.2 Finally, regarding cement leakages, those into the venous system or within posterior elements are typically of most concern. A few such patients in our study were regarded as asymptomatic because no intraoperative AEs were noted by investigators and in reviewing all AEs throughout the study for these patients, only a new fracture AE was considered cement related. Thus, we have found, similar to those of several meta-analyses,16,18,55 that such leakages occur least often (e.g., compared with intradiscal leakages) and that clinically symptomatic leakages are relatively rare. It is thought that creation of a void with the IBT with a known volume for cement fill and the compaction of cancellous bone along with use of radiopaque, viscous, cement and fluoroscopy use during cementation can help reduce such leakages.8,56
This is the largest randomized trial of surgical treatment for vertebral fractures, with relatively high rates of follow-up for 2 years and assessment of multiple clinical and radiographical endpoints compared with standard practice; however, our study has several limitations. Knowledge of treatment assignment may have influenced patient responses or radiologist assessments. The study, powered to detect differences between groups in SF-36 PCS at 1 month, may not be adequately powered to detect differences in other radiographical, clinical, or safety outcomes. The radiographical data collection and therefore, the analysis, had several limitations. Due to the multicenter nature of the trial, the quality of the films and use of standard markers to control magnification error was variable despite the use of a detailed radiographical protocol and training. Therefore, for height restoration, we had to rely on normal adjacent vertebrae to estimate height. We used standard thoracic and lumbar films to capture all vertebral bodies from T5–L5; because most fractures occur in the transition zone, these areas are often on the extreme ends of the films where parallax is maximized and the next normal vertebrae may not be captured on the same film. Adjacent prevalent fractures (Figure 1) also diminished these evaluations. As a result, compared with vertebral body height restoration data, there was more evaluable radiographical data for kyphotic angulation of index fractures; this is because this measurement does not necessitate controlling for magnification error and does not depend on including normal adjacent vertebrae. Finally, the analyses of clinical outcomes and radiographical results (kyphotic angulation correction) suggest a link but were exploratory in nature and less predictive than other variables such as baseline prevalent fracture; more studies are required where the study design is appropriately focused to confirm this hypothesis.
CONCLUSION
We conclude that, compared with NSM, BKP rapidly reduces pain and improves function, disability, and QOL during the course of 2 years and the reduction in pain, EQ-5D QOL, patient satisfaction, and kyphotic angulation remain statistically significant at all time points. Perioperative complications could be minimized with more care in patient positioning.
KEY POINTS
Compared with the control group, BKP rapidly improves pain, function, disability, and QOL during the course of 2 years.
Number of baseline prevalent fractures and treatment assignment are the most predictive variables for SF-36 PCS QOL improvement.
At 24 months, the change from baseline in index fracture kyphotic angulation was statistically significantly improved in the kyphoplasty group; on average 3.1° of correction for kyphoplasty compared with 0.8° in the control.
Patients who underwent BKP in the top 25% for kyphotic angulation correction had higher PCS improvement (13.4 points) than those in the bottom 25% (7.40 points).
Assessment of 30 day AEs suggests that perioperative complications could be minimized with more care in patient positioning.
Acknowledgments
The authors and study investigators are indebted to the patients, who consented to participate in the FREE trial, and to all participating staff at the investigational centers. The authors thank Li Ni, PhD (Medtronic), for contributions in statistical analysis.
Clinicaltrials.gov number: NCT00211211; Registration date: September 13, 2005.
Country of Origin (number of patients enrolled for each country), city and investigator name: Austria (4): Vienna—S. Becker; Belgium (63): Brugge—J. Van Meirhaeghe; Liège—V. Bex, L. Collignon, F. Sacré; France (25): Caen—H. Huet, C. Marcelli; Lille—P. Chastanet, B. Cortet; Paris—M. Cohen-Solal, J. Laredo; Toulouse—B. Mazières, J. Railhac; Germany (54): Berlin—D. Felsenberg; Hannover—L. Bastian, C. Krettek; Heidelberg—C. Kasperk, P. Meeder; Rosenheim—G. Regel; Italy (7): Catania—T. Russo; Verona—P. Bartolozzi; The Netherlands (6): Tilburg—P. N. M. Lohle; Sweden (70): Falun—P. Fritzell; Malmö—R. Hasserius, O. Johnell, M. Karlsson, A. Ohlin; Stockholm—G. Ordeberg; Uppsala—M. Cornefjord, P Försth; United Kingdom (71): Aberdeen—F. Smith, D. Wardlaw; London—K. Lam, T. Sabharwal; Oxford—J. Wass, D. Wilson.
Footnotes
Address correspondence and reprint requests to Jan Van Meirhaeghe, MD, Dienst Orthopedie en Traumatologie, Algemeen Ziekenhuis Sint-Jan Brugge-Oostende AV, Brugge, Belgium; E-mail: Jan.VanMeirhaeghe@azsintjan.be
The device(s)/drug(s) is/are FDA-approved or approved by corresponding national agency for this indication.
Medtronic Spine LLC was the sponsor, contributed to the study design, data monitoring, and reporting of results, and paid for statistical analysis (Advanced Research Associates, Mountain View, CA), core laboratory services and open access of article.
Relevant financial activities outside the submitted work: consulting fee or honorarium, support for travel to meetings for the study or other purposes, fees for participation in review activities, payment for writing or reviewing the manuscript, provision of writing assistance, medicines, equipment, or administrative support, payment for lectures, payment for manuscript preparation, travel/accommodations/meeting expenses unrelated to activities, and stock/stock options.
FREE Investigators
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivitives 3.0 License, where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially.
References
- 1.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17:1726–33 [DOI] [PubMed] [Google Scholar]
- 2.Boonen S, Van Meirhaeghe J, Bastian L, et al. Balloon kyphoplasty for the treatment of acute vertebral compression fractures: 2-year results from a randomized trial. J Bone Miner Res 2011;26:1627–37 [DOI] [PubMed] [Google Scholar]
- 3.Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet 2009;373:1016–24 [DOI] [PubMed] [Google Scholar]
- 4.Klazen CA, Lohle PN, de Vries J, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet 2010;376:1085–92 [DOI] [PubMed] [Google Scholar]
- 5.Papanastassiou ID, Phillips FM, Van Meirhaeghe J, et al. Comparing effects of kyphoplasty, vertebroplasty, and non-surgical management in a systematic review of randomized and non-randomized controlled studies. Eur Spine J 2012;21:1826–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrokhi MR, Alibai E, Maghami Z. Randomized controlled trial of percutaneous vertebroplasty versus optimal medical management for the relief of pain and disability in acute osteoporotic vertebral compression fractures. J Neurosurg Spine 2011;14:561–9 [DOI] [PubMed] [Google Scholar]
- 7.Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine 2001;26:1511–5 [DOI] [PubMed] [Google Scholar]
- 8.Wardlaw D, Van Meirhaeghe J, Ranstam J, et al. Balloon kyphoplasty in patients with osteoporotic vertebral compression fractures. Expert Review of Medical Devices 2012;9:423–36 [DOI] [PubMed] [Google Scholar]
- 9.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8 [DOI] [PubMed] [Google Scholar]
- 10.Genant HK, Wu CY, van Kuijk C, et al. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993;8:1137–48 [DOI] [PubMed] [Google Scholar]
- 11.Eastell R, Cedel SL, Wahner HW, et al. Classification of vertebral fractures. J Bone Miner Res 1991;6:207–15 [DOI] [PubMed] [Google Scholar]
- 12.MedDRA Data Retrieval and Presentation: Points to Consider ICH-Endorsed Guide for MedDRA Users on Data Output. Available at: http://www.ich.org/products/meddra/meddraptc.html Accessed March 18, 2012.
- 13.Mallinckrodt CH, Clark WS, David SR. Accounting for dropout bias using mixed-effects models. J Biopharm Stat 2001;11:9–21 [DOI] [PubMed] [Google Scholar]
- 14.Ranstam J, Turkiewicz A, Boonen S, et al. Alternative analyses for handling incomplete follow-up in the intention-to-treat analysis: the randomized controlled trial of balloon kyphoplasty versus non-surgical care for vertebral compression fracture (FREE). BMC Med Res Methodol 2012;12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulme PA, Krebs J, Ferguson SJ, et al. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine 2006;31:1983–2001 [DOI] [PubMed] [Google Scholar]
- 16.Eck JC, Nachtigall D, Humphreys SC, et al. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta-analysis of the literature. Spine J 2008;8:488–97 [DOI] [PubMed] [Google Scholar]
- 17.Lee MJ, Dumonski M, Cahill P, et al. Percutaneous treatment of vertebral compression fractures: a meta-analysis of complications. Spine 2009;34:1228–32 [DOI] [PubMed] [Google Scholar]
- 18.Taylor RS, Fritzell P, Taylor RJ. Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J 2007;16:1085–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor RS, Taylor RJ, Fritzell P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety. Spine 2006;31:2747–55 [DOI] [PubMed] [Google Scholar]
- 20.Zou J, Mei X, Zhu X, et al. The long-term incidence of subsequent vertebral body fracture after vertebral augmentation therapy: a systemic review and meta-analysis. Pain Physician 2012;15:E515–22 [PubMed] [Google Scholar]
- 21.Han S, Wan S, Ning L, et al. Percutaneous vertebroplasty versus balloon kyphoplasty for treatment of osteoporotic vertebral compression fracture: a meta-analysis of randomised and non-randomised controlled trials. Int Orthop 2011;35:1349–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouza C, Lopez T, Magro A, et al. Efficacy and safety of balloon kyphoplasty in the treatment of vertebral compression fractures: a systematic review. Eur Spine J 2006;15:1050–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouza C, Lopez-Cuadrado T, Cediel P, et al. Balloon kyphoplasty in malignant spinal fractures: a systematic review and meta-analysis. BMC Palliat Care 2009;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ploeg WT, Veldhuizen AG, The B, et al. Percutaneous vertebroplasty as a treatment for osteoporotic vertebral compression fractures: a systematic review. Eur Spine J 2006;15:1749–58 [DOI] [PubMed] [Google Scholar]
- 25.Shi MM, Cai XZ, Lin T, et al. Is There Really No Benefit of Vertebroplasty for Osteoporotic Vertebral Fractures? A Meta-analysis. Clin Orthop Relat Res 2012;470:2785–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson PA, Froyshteter AB, Tontz WL., Jr Meta-analysis of vertebral augmentation compared to conservative treatment for osteoporotic spinal fractures. J Bone Miner Res 2013;28:372–82 [DOI] [PubMed] [Google Scholar]
- 27.Edidin AA, Ong KL, Lau E, et al. Mortality risk for operated and nonoperated vertebral fracture patients in the medicare population. J Bone Miner Res 2011;26:1617–26 [DOI] [PubMed] [Google Scholar]
- 28.Edidin AA, Ong KL, Lau E, et al. Life expectancy following diagnosis of a vertebral compression fracture. Osteoporos Int 2013;24:451–8 [DOI] [PubMed] [Google Scholar]
- 29.Edidin AA, Ong KL, Lau E, et al. Cost-Effectiveness Analysis of Treatments for Vertebral Compression Fractures. Appl Health Econ Health Policy 2012;10:273–84 [DOI] [PubMed] [Google Scholar]
- 30.Zampini JM, White AP, McGuire KJ. Comparison of 5766 vertebral compression fractures treated with or without kyphoplasty. Clin Orthop Relat Res 2010;468:1773–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berenson J, Pflugmacher R, Jarzem P, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol 2011;12:225–35 [DOI] [PubMed] [Google Scholar]
- 32.Rousing R, Hansen KL, Andersen MO, et al. Twelve-months follow-up in forty-nine patients with acute/semiacute osteoporotic vertebral fractures treated conservatively or with percutaneous vertebroplasty: a clinical randomized study. Spine 2010;35:478–82 [DOI] [PubMed] [Google Scholar]
- 33.Wang HK, Lu K, Liang CL, et al. Comparing clinical outcomes following percutaneous vertebroplasty with conservative therapy for acute osteoporotic vertebral compression fractures. Pain Med 2010;11:1659–65 [DOI] [PubMed] [Google Scholar]
- 34.Blasco Andaluz J, Martinez-Ferrer A, Macho Fernandez J, et al. Effect of vertebroplasty on pain relief, quality of life and the incidence of new vertebral fractures. A 12-month randomised follow-up, controlled trial. J Bone Miner Res 2012 [DOI] [PubMed] [Google Scholar]
- 35.Strom O, Leonard C, Marsh D, et al. Cost-effectiveness of balloon kyphoplasty in patients with symptomatic vertebral compression fractures in a UK setting. Osteoporos Int 2009;21:1599–608 [DOI] [PubMed] [Google Scholar]
- 36.Svedbom A, Alvares L, Cooper C, et al. Balloon kyphoplasty compared to vertebroplasty and nonsurgical management in patients hospitalised with acute osteoporotic vertebral compression fracture: a UK cost-effectiveness analysis. Osteoporos Int 2013;24:355–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fritzell P, Ohlin A, Borgstrom F. Cost-effectiveness of balloon kyphoplasty (BKP) vs. standard medical treatment in patients with osteoporotic vertebral compression fracture - a Swedish multicenter RCT with two year FU. Spine 2011;36:2243–51 [DOI] [PubMed] [Google Scholar]
- 38.Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009;361:569–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009;361:557–68 [DOI] [PubMed] [Google Scholar]
- 40.Bono CM, Heggeness M, Mick C, et al. North American Spine Society: newly released vertebroplasty randomized controlled trials: a tale of two trials. Spine J 2010;10:238–40 [DOI] [PubMed] [Google Scholar]
- 41.Clark W, Lyon S, Burnes J, et al. Trials of vertebroplasty for vertebral fractures. N Engl J Med 2009;361:2097–100 [DOI] [PubMed] [Google Scholar]
- 42.Voggenreiter G. Balloon kyphoplasty is effective in deformity correction of osteoporotic vertebral compression fractures. Spine 2005;30:2806–12 [DOI] [PubMed] [Google Scholar]
- 43.Garfin SR, Buckley RA, Ledlie J. Balloon kyphoplasty for symptomatic vertebral body compression fractures results in rapid, significant, and sustained improvements in back pain, function, and quality of life for elderly patients. Spine 2006;31:2213–20 [DOI] [PubMed] [Google Scholar]
- 44.Ledlie JT, Renfro MB. Kyphoplasty treatment of vertebral fractures: 2-year outcomes show sustained benefits. Spine 2006;31:57–64 [DOI] [PubMed] [Google Scholar]
- 45.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005;14:1523–32 [DOI] [PubMed] [Google Scholar]
- 46.Farrar JT, Young JP, Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58 [DOI] [PubMed] [Google Scholar]
- 47.Roland M, Fairbank J. The roland-morris disability questionnaire and the oswestry disability questionnaire. Spine 2000;25:3115–24 [DOI] [PubMed] [Google Scholar]
- 48.Copay AG, Glassman SD, Subach BR, et al. The minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and Pain Scales. Spine J 2008 [DOI] [PubMed] [Google Scholar]
- 49.Yang HL, Zhao L, Liu J, et al. Changes of pulmonary function for patients with osteoporotic vertebral compression fractures after kyphoplasty. J Spinal Disord Tech 2007;20:221–5 [DOI] [PubMed] [Google Scholar]
- 50.Dong R, Chen L, Gu Y, et al. Improvement in respiratory function after vertebroplasty and kyphoplasty. Int Orthop 2009;33:1689–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bastian L, Schils F, Tillman JB, et al. A randomized trial comparing 2 techniques of balloon kyphoplasty and curette use for obtaining vertebral body height restoration and angular-deformity correction in vertebral compression fractures due to osteoporosis. AJNR Am J Neuroradiol 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chin DK, Kim YS, Cho YE, et al. Efficacy of postural reduction in osteoporotic vertebral compression fractures followed by percutaneous vertebroplasty. Neurosurgery 2006;58:695–700; discussion 695–700. [DOI] [PubMed] [Google Scholar]
- 53.McKiernan F, Jensen R, Faciszewski T. The dynamic mobility of vertebral compression fractures. J Bone Miner Res 2003;18:24–9 [DOI] [PubMed] [Google Scholar]
- 54.Liu JT, Liao WJ, Tan WC, et al. Balloon kyphoplasty versus vertebroplasty for treatment of osteoporotic vertebral compression fracture: a prospective, comparative, and randomized clinical study. Osteoporos Int 2010;21:359–64 [DOI] [PubMed] [Google Scholar]
- 55.Hadjipavlou AG, Tzermiadianos MN, Katonis PG, et al. Percutaneous vertebroplasty and balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures and osteolytic tumours. J Bone Joint Surg Br 2005;87:1595–604 [DOI] [PubMed] [Google Scholar]
- 56.Phillips FM, Todd Wetzel F, Lieberman I, et al. An in vivo comparison of the potential for extravertebral cement leak after vertebroplasty and kyphoplasty. Spine 2002;27:2173–8; discussion 78–9. [DOI] [PubMed] [Google Scholar]