Abstract
Background
The objective of this study was to examine the safety and intraocular pressure (IOP)-lowering efficacy of a fixed combination of brinzolamide 1% + brimonidine 0.2% (BBFC) after six months of treatment in patients with open-angle glaucoma or ocular hypertension.
Methods
This was a randomized, multicenter, double-masked, three-month, three-arm contribution-of-elements study with a three-month safety extension. Patients were randomly assigned 1:1:1 to treatment with BBFC, brinzolamide 1%, or brimonidine 0.2% after a washout period. Patients dosed their study medications three times daily at 8 am, 3 pm, and 10 pm for six months. Patients returned for visits at two weeks, six weeks, three months, and six months. IOP measurements were used to assess efficacy. Safety assessments were adverse events, corrected distance visual acuity, slit-lamp biomicroscopy, pachymetry, perimetry, fundus parameters, and cardiac parameters.
Results
A total of 690 patients were randomized. Six-month mean IOP values were similar to those at three months, when the mean IOP in patients treated with BBFC was significantly lower than that of either monotherapy group. A total of 175 patients experienced at least one treatment-related adverse event (BBFC, 33.0%; brinzolamide, 18.8%; brimonidine, 24.7%), eight of which were severe, and five resulted in discontinuation. Seventy-seven patients discontinued participation due to treatment-related adverse events (BBFC, 17.2%; brinzolamide, 2.1%; brimonidine, 14.5%). There were 21 serious adverse events (n = 7 in each group), none of which was related to treatment. Resting mean pulse and blood pressure with BBFC were similar to those with brimonidine, demonstrating modest, clinically insignificant decreases. No new or increased risks were identified with use of BBFC relative to either monotherapy.
Conclusion
This study showed that, after six months of treatment, the safety profile of BBFC was similar to that of its individual components and its IOP-lowering activity was similar to its efficacy at three months, when it was superior to both brinzolamide 1% alone and brimonidine 0.2% alone.
Keywords: brimonidine, brinzolamide, fixed combination, ocular hypertension, open-angle glaucoma
Introduction
In recent years, the use of fixed-combination antihypertensive medications by patients with glaucoma or ocular hypertension has increased substantially. These therapies are often favored by patients because they offer the convenience of using a single medication bottle and lower copays than a similar two-bottle regimen.1 Physicians may prefer them because of the potential for increased patient compliance compared with a regimen containing two separate medications,2 avoidance of the potential for washout of the first drug by the second,3 and reduced exposure of these patients to topical preservatives, which have been implicated in the development of ocular surface disease in patients with glaucoma.4,5
Although many different fixed-combination therapies are commercially available in various countries, all of them contain timolol, a beta blocker that is contraindicated in patients with certain respiratory or cardiac conditions. A novel fixed combination has been developed that combines a carbonic anhydrase inhibitor with an alpha agonist, ie, brinzolamide 1% + brimonidine 0.2% (BBFC), representing the only fixed-combination antihypertensive therapy not to include timolol.
The current multicenter, randomized Phase III study measured the contribution of the individual active ingredients of BBFC to the combination and compared the safety of these three agents with one another. The primary endpoint was fulfilled by the interim three-month results, which demonstrated that BBFC has significantly greater intraocular pressure (IOP)-lowering activity compared with either brinzolamide 1% alone or brimonidine 0.2% alone in patients with open-angle glaucoma or ocular hypertension.6 The aim of the current analysis was to examine the safety and IOP-lowering efficacy of BBFC after six months of treatment in this patient population.
Materials and methods
Study design
This was a randomized, multicenter, double-masked, parallel-group, three-month, three-arm contribution-of-elements study with a three-month safety extension in patients with open-angle glaucoma or ocular hypertension. The protocol was approved by all relevant institutional review boards and the study was performed in compliance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice. All participating patients provided written informed consent.
At the screening visit, patients were screened against the inclusion and exclusion criteria. Eligible patients were then instructed to discontinue use of all IOP-lowering medications during a washout period, as follows: 5 ± 1 day for miotics and oral/topical carbonic anhydrase inhibitors, 14 ± 1 day for alpha agonists and alpha/beta agonists, and 28 ± 1 day for beta antagonists and prostaglandin analogs. For combination drugs, the longest washout period of the individual components was used.
The first eligibility visit was scheduled at the end of the washout period for those patients on prior IOP-lowering medications and at least 3 ± 1 day for those patients not on prior IOP-lowering medications. At this visit, investigators assessed IOP in both eyes at 8 am, 10 am, 3 pm, and 5 pm. At the 8 am time point, they also underwent corrected distance visual acuity testing and slit-lamp biomicroscopy in both eyes, and resting pulse and blood pressure was measured at the 8 am and 10 am time points.
At the second eligibility visit, which was scheduled three to eight days after the first eligibility visit, investigators assessed the same parameters as during the first eligibility visit (IOP, corrected distance visual acuity, slit-lamp bio-microscopy, and resting pulse and blood pressure) on the same schedule. All eligible patients were then randomly assigned 1:1:1 to treatment with BBFC, brinzolamide 1%, or brimonidine 0.2%. The randomization was stratified by the mean baseline IOP from both eligibility visits measured at the 8 am time point (24–27 mmHg and 28–36 mmHg) to ensure balanced baseline IOP among the treatment groups. Patients dosed both eyes with the study medications (unless the nonstudy eye had a potential safety issue) three times daily at 8 am (±30 minutes), 3 pm (±30 minutes), and 10 pm (±30 minutes) for six months.
Patients returned for visits two weeks, six weeks, three months, and six months after the second eligibility visit. Patients did not instill the 8 am dose of study medication prior to these visits. At these visits, investigators assessed IOP in both eyes at 8 am (15 minutes after which investigators administered study medication), 10 am, 3 pm, and 5 pm. At the 8 am time point, corrected distance visual acuity measurements and slit-lamp biomicroscopy were performed on both eyes, and resting pulse and blood pressure was measured at both the 8 am and 10 am time points. At the three-month and six-month visits, the investigators also performed automated perimetry on both eyes at 8 am and fundus examination and pachymetry at 5 pm on both eyes.
Participants
Patients from 65 academic and private practice study sites throughout the United States were recruited by the investigators to participate in this study. They had to be at least 18 years of age and have a clinical diagnosis of open-angle glaucoma or ocular hypertension in at least one (study) eye. Intraocular pressure in the study eye had to be between 24 mmHg and 36 mmHg at the 8 am time point and between 21 mmHg and 36 mmHg at the 10 am time point at both eligibility visits. All IOP readings in both eyes at both eligibility visits had to be 36 mmHg or less.
Patients were excluded if they had any history of ocular trauma or intraocular surgery within the past six months or ocular infection, inflammation, or laser surgery within the past three months. They were also excluded if they had: any form of glaucoma other than open-angle glaucoma; chronic, recurrent, or severe inflammatory eye disease; central cornea thickness >620 μm in either eye; Shaffer angle grade <2 in either eye; cup/disc ratio >0.80 (horizontal or vertical measurement) in either eye; severe central visual field loss in either eye, defined as sensitivity ≤10 decibels in at least two of four visual field test points closest to the point of fixation; clinically significant or progressive retinal disease; corrected distance visual acuity worse than 0.6 logMAR; or other ocular pathology that could preclude administration of an alpha-adrenergic agonist and/or a topical carbonic anhydrase inhibitor.
Patients could also not have a recent history of taking medications prohibited during the study, including high-dose salicylate therapy within four weeks of the first eligibility visit and any medications or substances used on a chronic basis that could affect IOP and that had not been on a stable dosing regimen for at least 30 days before the screening visit; current use of any prohibited medications, including monoamine oxidase inhibitors, psychotropic drugs that augment an adrenergic response, and any additional ocular hypotensive medications; history of active, severe, unstable, or uncontrolled systemic disease precluding safe administration of a topical alpha-adrenergic agonist or carbonic anhydrase inhibitor; hypersensitivity to alpha-adrenergic agonist drugs, topical or oral carbonic anhydrase inhibitors, sulfonamide derivatives, or any component of the study medications; or any condition requiring treatment with glucocorticoids, unless the glucocorticoid could be safely discontinued during the study. Women could not be pregnant, lactating, or of childbearing potential (unless they were abstinent or using a highly effective method of birth control).
Outcomes
Safety assessments included solicited and unsolicited adverse events, corrected distance visual acuity, slit-lamp biomicroscopy, pachymetry, automated perimetry, fundus parameters, and resting pulse and blood pressure, all collected through to the six-month visit. Adverse events were collected, monitored, and evaluated throughout the study and were recorded at each visit. Slit-lamp biomicroscopy included examination of the cornea, lens, eyelids, conjunctiva, iris, and anterior chamber of both eyes. Dilated fundus examination included examination of the vitreous, retina, macula, choroid, and optic nerve (including cup/disc ratio) of both eyes. Blood pressure was monitored because of the safety profile of brimonidine, which includes a decrease in blood pressure.7
The efficacy assessment comprised IOP measurement, which were performed using a Goldmann applanation tonometer. The objective of this study was to examine the safety and IOP-lowering efficacy of BBFC when dosed three times daily at 8 am, 3 pm, and 10 pm, after six months of treatment in patients with open-angle glaucoma or ocular hypertension.
Statistical methods
Pairwise comparisons of mean IOP (BBFC versus brinzolamide and BBFC versus brimonidine) at each scheduled on-therapy study visit at all four time points (8 am, 10 am, 3 pm, and 5 pm) were based on the least squares means and made using a two-sample t-test. Assuming a standard deviation between 3.5 mmHg and 3.9 mmHg, this study provided a 90% power to detect a difference of at least 1.5 mmHg between any two treatment groups if at least 143 patients were analyzed for each treatment group. Target enrollment was 250 patients for each treatment group, with the intention of retaining no less than 100 patients per treatment group at the six-month visit.
An α level of 0.05 was used to declare statistical significance. All data analyses, which were intent-to-treat, were two-sided. The data were analyzed for the baseline visit and the three-month visit (primary endpoint). Descriptive statistics were calculated for all safety parameters (adverse events, corrected distance visual acuity, slit-lamp biomicroscopy observations, pachymetry, automated perimetry, fundus parameters, and resting pulse and blood pressure) and for IOP, IOP change from baseline, and IOP percent change from baseline.
If both eyes were eligible, both were treated with the study medication, but only the eye with the higher IOP at 8 am averaged across the two eligibility visits was selected for analysis. If both eyes had equal IOP at 8 am, the eye with the higher IOP at 10 am averaged across the two eligibility visits was selected for analysis. If both eyes had equal IOP at both time points, the right eye was selected for analysis. Last observation carried forward was used for any missing IOP data points at six weeks and three months. Statistical analysis was performed using SAS software (SAS Institute, Cary, NC, USA).
Results
Participant flow
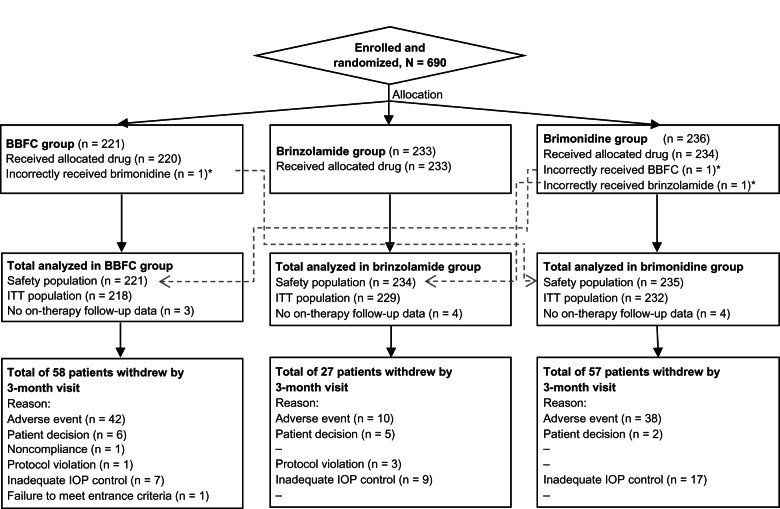
A total of 690 patients were enrolled in the study, with 548 patients completing the six-month visit. Patient disposition is described in Figure 1.
Figure 1.
Participant flow chart.
Note: *Patients receiving incorrect study drug were included in the safety population of the actual drug received and in the ITT population of the intended drug.
Abbreviations: BBFC, brinzolamide/brimonidine fixed combination; IOP, intraocular pressure; ITT, intent-to-treat.
Demographic and baseline characteristics
Demographic and baseline characteristics were well balanced between the three arms (Table 1). The mean age of the total intent-to-treat population was 64.9 ± 10.4 years, 77.9% of the population was white, and 56.1% were women.
Table 1.
Demographics and baseline characteristics
| Demographic/baseline characteristic | Total population n = 679 | BBFC n = 218 | Brinzolamide n = 229 | Brimonidine n = 232 |
|---|---|---|---|---|
| Age, years | ||||
| Mean ± standard deviation | 64.9 ± 10.4 | 65.7 ± 10.3 | 64.2 ± 10.3 | 64.9 ± 10.5 |
| <65, n (%) | 323 (47.6%) | 98 (45.0%) | 110 (48.0%) | 115 (49.6%) |
| ≥65, n (%) | 356 (52.4%) | 120 (55.0%) | 119 (52.0%) | 117 (50.4%) |
| Race, n (%) | ||||
| White | 529 (77.9%) | 174 (79.8%) | 179 (78.2%) | 176 (75.9%) |
| Black | 130 (19.1%) | 36 (16.5%) | 42 (18.3%) | 52 (22.4%) |
| Asian | 9 (1.3%) | 3 (1.4%) | 5 (2.2%) | 1 (0.4%) |
| Multiracial | 3 (0.4%) | 0 (0%) | 1 (0.4%) | 2 (0.9%) |
| Other | 8 (1.2%) | 5 (2.3%) | 2 (0.9%) | 1 (0.4%) |
| Gender, n (%) | ||||
| Male | 298 (43.9%) | 100 (45.9%) | 97 (42.4%) | 101 (43.5%) |
| Female | 381 (56.1%) | 118 (54.1%) | 132 (57.6%) | 131 (56.5%) |
| Diagnosis, n (%) | ||||
| Ocular hypertension | 168 (24.7%) | 51 (23.4%) | 59 (25.8%) | 58 (25.0%) |
| Open-angle glaucoma | 511 (75.3%) | 167 (76.6%) | 170 (74.2%) | 172 (75.0%) |
Note: Reprinted with permission from Nguyen QH, McMenemy MG, Realini T, et al. Phase 3 randomized 3-month trial with an ongoing 3-month safety extension of fixed-combination brinzolamide 1%/brimonidine 0.2%. J Ocul Pharmacol Ther. 2013;29:290–297.6
Abbreviation: BBFC, brinzolamide-brimonidine fixed combination.
Intraocular pressure
Baseline mean IOP levels were similar between the three treatment groups at each of the four time points. As fully described in a previous publication, three-month mean IOP of the BBFC group was significantly lower than that of either the brinzolamide group or the brimonidine group.6 Mean IOP values at six months were similar to those at three months for each treatment group at each time point, with the percent IOP reduction from baseline to six months ranging from 20.0% to 30.7% for BBFC, 16.4% to 22.0% for brinzolamide, and 12.4% to 24.8% for brimonidine across all four time points. Absolute IOP reductions from baseline to six months ranged from 4.9 to 8.0 mmHg for BBFC, 4.1 to 5.8 mmHg for brinzolamide, and 3.0 to 6.3 mmHg for brimonidine across all four time points.
Adverse events
A total of 175 patients experienced at least one treatment-related adverse event (BBFC group, n = 73, 33.0%; brinzolamide group, n = 44, 18.8%; brimonidine group, n = 58, 24.7%). The majority of these were local ocular adverse events (Table 2). The brinzolamide-containing groups included all cases of blurred vision (BBFC group, 4.5%; brinzolamide group, 6.8%) and nearly all cases of dysgeusia (BBFC group, 4.1%; brinzolamide group, 10.3%; brimonidine group, 0.4%), whereas the brimonidine-containing groups included all cases of conjunctivitis (BBFC group, 5.0%; brimonidine group, 6.0%), dry mouth (BBFC group, 3.2%; brimonidine group, 2.1%), and nearly all cases of eye allergy (BBFC group, 6.3%; brimonidine group, 2.1%, brinzolamide group, 0.4%).
Table 2.
Treatment-related adverse events (incidence ≥1%)
| Treatment-related adverse event | BBFC n = 221 n (%) | Brinzolamide n = 234 n (%) | Brimonidine n = 235 n (%) |
|---|---|---|---|
| Ocular | |||
| Eye irritation | 14 (6.3%) | 3 (1.3%) | 8 (3.4%) |
| Eye allergy | 14 (6.3%) | 1 (0.4%) | 5 (2.1%) |
| Conjunctivitis | 11 (5.0%) | 0 (0%) | 14 (6.0%) |
| Vision blurred | 10 (4.5%) | 16 (6.8%) | 0 (0%) |
| Conjunctivitis allergic | 8 (3.6%) | 1 (0.4%) | 10 (4.3%) |
| Eye pruritus | 7 (3.2%) | 2 (0.9%) | 3 (1.3%) |
| Eye pain | 6 (2.7%) | 4 (1.7%) | 3 (1.3%) |
| Ocular hyperemia | 6 (2.7%) | 1 (0.4%) | 9 (3.8%) |
| Conjunctival hyperemia | 5 (2.3%) | 1 (0.4%) | 3 (1.3%) |
| Dry eye | 4 (1.8%) | 2 (0.9%) | 2 (0.9%) |
| Lacrimation increased | 3 (1.4%) | 1 (0.4%) | 2 (0.9%) |
| Conjunctival follicles | 1 (0.5%) | 0 (0%) | 4 (1.7%) |
| Nonocular | |||
| Dysgeusia | 9 (4.1%) | 24 (10.3%) | 1 (0.4%) |
| Dry mouth | 7 (3.2%) | 0 (0%) | 5 (2.1%) |
| Fatigue | 1 (0.5%) | 0 (0%) | 4 (1.7%) |
Abbreviation: BBFC, brinzolamide-brimonidine fixed combination.
Seventy-seven patients discontinued participation due to treatment-related adverse events (BBFC, n = 38, 17.2%; brinzolamide, n = 5, 2.1%; brimonidine, n = 34, 14.5%). Eight of the treatment-related adverse events were severe (allergic conjunctivitis, conjunctivitis, eye irritation, eye discharge, and ocular hyperemia in the BBFC group; blurred vision in the brinzolamide group; and atopic dermatitis and eye allergy in the brimonidine group). Five of the eight severe adverse events resulted in discontinuation. The allergic conjunctivitis, conjunctivitis, and atopic dermatitis resolved with treatment, and the blurred vision, ocular hyperemia, eye discharge, eye irritation, and eye allergy resolved without treatment. Patients experienced a total of 21 serious adverse events (n = 7 in each treatment group), none of which was judged to be related to treatment.
Other safety measures
From the baseline visit to the six-month visit, the change in mean number of letters read was less than one letter in all groups (BBFC group, −0.5 letters; brinzolamide group, -0.3 letters; brimonidine group, 0.0 letters). Of two patients observed to have a clinically relevant decrease in corrected distance visual acuity, neither was judged to be related to treatment.
Using slit-lamp biomicroscopy, evidence of inflammation or significant structural changes in or discharge from the eyelids and/or conjunctiva was the most commonly observed change from the baseline visit to the six-month visit (BBFC group, 26.7%; brimonidine group, 5.6%; brinzolamide group, 22.1%, Table 3). Pachymetry revealed minimal mean changes from baseline in corneal thickness for all three treatment groups. From the baseline visit to the exit visit, all three groups showed mean changes in corneal thickness ≤0.006 mm. One patient from the brinzolamide group experienced an increase in corneal thickness of 0.2 mm.
Table 3.
Changes in ocular signs from baseline to any visit through month 6
| Ocular sign | Changes from baseline through month 6
|
||
|---|---|---|---|
| BBFC n = 221 n (%) | Brinzolamide n = 232 n (%) | Brimonidine n = 235 n (%) | |
| Cornea | 15 (6.8%) | 9 (3.9%) | 6 (2.6%) |
| Iris/anterior chamber | 2 (0.9%) | 2 (0.9%) | 1 (0.4%) |
| Lens | 8 (3.6%) | 2 (0.9%) | 2 (0.9%) |
| Eyelids/conjunctiva | 59 (26.7%) | 13 (5.6%) | 52 (22.1%) |
Abbreviation: BBFC, brinzolamide-brimonidine fixed combination.
All three groups showed changes in ≤3% of patients for all fundus parameters (Table 4). No clinically meaningful differences were noted for changes from baseline to the exit visit in cup/disc ratio, with each group showing mean horizontal and vertical ratio changes ≥−0.002 and ≤0.004.
Table 4.
Changes in fundus parameters from baseline to any visit through month 6
| Fundus parameter | Changes from baseline through month 6 visit
|
||
|---|---|---|---|
| BBFC n = 211 n (%) | Brinzolamide n = 229 n (%) | Brimonidine n = 221 n (%) | |
| Vitreous, n (%) | 2 (0.9%) | 3 (1.3%) | 2 (0.9%) |
| Retina/macula/choroid, n (%) | 6 (2.8%) | 1 (0.4%) | 2 (0.9%) |
| Optic nerve, n (%) | 4 (1.9%) | 7 (3.1%) | 3 (1.4%) |
| Cup/disc ratio, mean ± SD* | |||
| Horizontal | −0.002 ± 0.02 | 0.004 ± 0.03 | 0.002 ± 0.03 |
| Vertical | −0.002 ± 0.03 | 0.004 ± 0.05 | 0.002 ± 0.03 |
Note:
Changes in cup/disc ratio were from baseline to exit visit.
Abbreviations: BBFC, brinzolamide-brimonidine fixed combination; SD, standard deviation.
Automated perimetry showed no decrease in retinal ganglion sensitivity or loss. From the baseline visit to the exit visit, the BBFC group, brinzolamide group, and brimonidine group showed changes in mean visual field deviation of 0.08, 0.06, and −0.08, respectively.
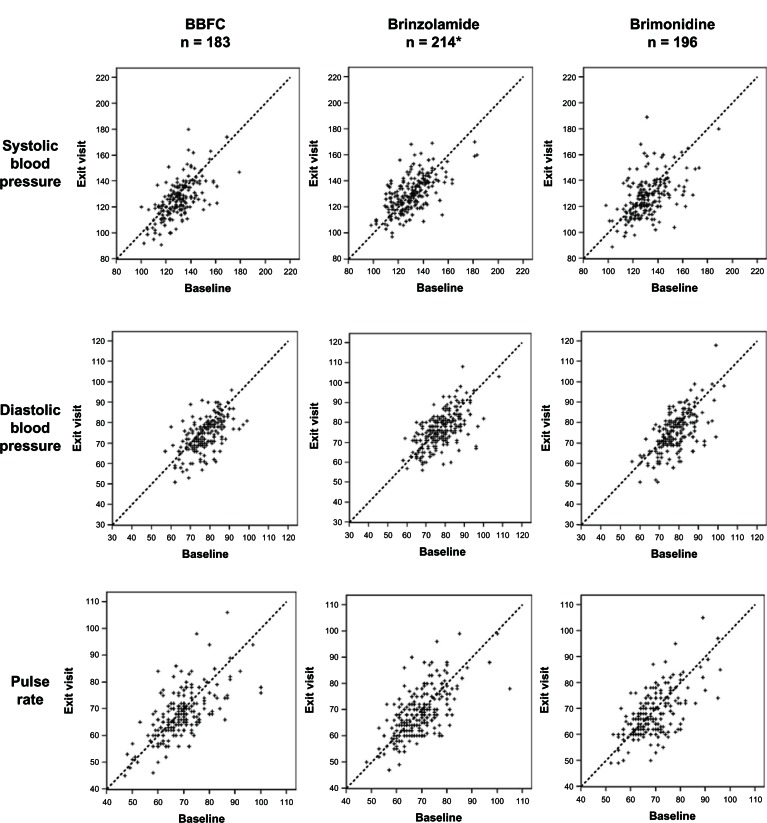
A slight trend towards a decrease in blood pressure was observed from the baseline visit to the six-month visit for patients in the BBFC group (3.3–4.2 mmHg decrease) and the brimonidine group (2.9–5.0 mmHg decrease), but the scatter plots in Figure 2 show that blood pressure in individual patients remained relatively stable from baseline to six months, regardless of the study medication used. No more than a 1.2 beat per minute decrease was noted in mean pulse rate from the baseline visit to the six-month visit for any of the three treatment groups.
Figure 2.
Distribution of systolic and diastolic blood pressure and pulse rate at 10 am: baseline visit versus six-month visit.
Notes: Blood pressure was analyzed using the safety population. *For pulse rate, n = 212.
Abbreviation: BBFC, brinzolamide/brimonidine fixed combination.
Discussion
Examining efficacy after six months did not change the conclusions drawn at three months regarding the IOP activity of BBFC. Mean IOP values at six months were similar to those at three months for each treatment group at each time point, when the BBFC group had significantly lower mean IOP than either monotherapy group.
No serious treatment-related adverse events were observed in the BBFC group, nor did nonserious adverse events reveal any unexpected findings, with treatment-related adverse events occurring in 33% of the BBFC group, 19% of the brinzolamide group, and 25% of the brimonidine group. Overall, 11.2% of patients discontinued participation due to treatment-related adverse events, and most of these early withdrawals were from the brimonidine-containing groups (BBFC, n = 38 of 77; brimonidine, n = 34 of 77). Resting mean pulse rate and systolic and diastolic blood pressure in the BBFC group were similar to those in the brimonidine group, demonstrating modest and clinically insignificant decreases. No other safety outcomes, corrected distance visual acuity, slit-lamp examination, pachymetry, or perimetry, revealed any unexpected results.
Because all currently available fixed-combination products contain the beta blocker, timolol, these agents are not suitable for use by patients in whom beta blockers are contraindicated, including patients with asthma, severe chronic obstructive pulmonary disease, sinus bradycardia, second-degree or third-degree atrioventricular block, cardiogenic shock, or overt cardiac failure.8 A study of nearly 26,000 veterans with glaucoma revealed how frequently patients with glaucoma have such contraindications. In that study, 23% of patients had reactive airways disease (an umbrella term encompassing diagnoses of bronchitis, emphysema, asthma, and chronic obstructive pulmonary disease) and 12% had congestive heart failure.9 This represents a substantial portion of an elderly population (median age 80 years) in which timolol is contraindicated. Further, the investigators from this study reported that 88% of patients with reactive airways disease were dispensed glaucoma medications (primarily beta blockers) that had the potential to aggravate bronchoconstriction. These results highlight the unmet need for a fixed-combination glaucoma medication that does not contain timolol.
The limitations of the current study included its six-month time frame, which prevented examination of the long-term effects of BBFC in patients with open-angle glaucoma or ocular hypertension. In addition, the contribution-of-elements study design did not examine how BBFC might compare with other fixed-combination products or with the concomitant unfixed use of brinzolamide 1% and brimonidine 0.2%.
In conclusion, this study demonstrated that, after six months of treatment, the safety profile of a fixed combination of brinzolamide 1% + brimonidine 0.2% was similar to that of its individual components and that its IOP-lowering activity was similar to its activity at three months, when it was significantly superior to both brinzolamide 1% alone and brimonidine 0.2% alone.
Footnotes
Disclosure
This study was sponsored by Alcon Research Ltd. Jennifer Klem provided medical writing assistance, which was also funded by Alcon Research Ltd.
References
- 1.Higginbotham EJ. Considerations in glaucoma therapy: fixed combinations versus their component medications. Clin Ophthalmol. 2010;4:1–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Syed MF, Loucks EK. Update and optimal use of a brinzolamide-timolol fixed combination in open-angle glaucoma and ocular hypertension. Clin Ophthalmol. 2011;5:1291–1296. doi: 10.2147/OPTH.S13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chrai SS, Makoid MC, Eriksen SP, Robinson JR. Drop size and initial dosing frequency problems of topically applied ophthalmic drugs. J Pharm Sci. 1974;63:333–338. doi: 10.1002/jps.2600630304. [DOI] [PubMed] [Google Scholar]
- 4.Jaenen N, Baudouin C, Pouliquen P, et al. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007;17:341–349. doi: 10.1177/112067210701700311. [DOI] [PubMed] [Google Scholar]
- 5.Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86:418–423. doi: 10.1136/bjo.86.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen QH, McMenemy MG, Realini T, et al. Phase 3 randomized 3-month trial with an ongoing 3-month safety extension of fixed-combination brinzolamide 1%/brimonidine 0.2% J Ocul Pharmacol Ther. 2013;29:290–297. doi: 10.1089/jop.2012.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordlund JR, Pasquale LR, Robin AL, et al. The cardiovascular, pulmonary, and ocular hypotensive effects of 0.2% brimonidine. Arch Ophthalmol. 1995;113:77–83. doi: 10.1001/archopht.1995.01100010079024. [DOI] [PubMed] [Google Scholar]
- 8.Timolol maleate ophthalmic solution [package insert] Fort Worth, TX: Falcon Pharmaceuticals Ltd; 2004. [Google Scholar]
- 9.Roughead EE, Kalisch LM, Pratt NL, et al. Managing glaucoma in those with co-morbidity: not as easy as it seems. Ophthalmic Epidemiol. 2012;19:74–82. doi: 10.3109/09286586.2011.638743. [DOI] [PubMed] [Google Scholar]