Abstract
An examination of the micro-organization of visual cortex using two-photon calcium imaging provides a new level of insight into retinotopic maps, finding that retinotopy is scrambled on fine scales in mouse primary visual cortex.
One fundamental principle of many sensory systems is that they are highly organized. The mammalian retina, for example, has a smooth representation of visual position that is achieved by intricate mosaic tiling of retinal ganglion cells (RGCs). Many types of RGCs independently cover the full visual field. For example, distinct RGC types respond to bright spots versus dark spots. Given such orderly input, one might expect that cells in the primary visual cortex (V1) would follow suit and organize into a smooth continuous retinotopic map that corresponds one-to-one with retinal location. Indeed, at the macroscopic level, there is evidence for such a retinotopic organization. On the other hand, V1 combines inputs from these different RGC classes to represent new features that may supersede retinotopy. Micro-organization of V1 has previously been examined laboriously using electrodes. But with few cells recorded simultaneously and no way of gauging the distance between cells, unequivocal evidence regarding the micro-organization of retinotopic maps in V1 has been unavailable until now.
In this issue, Smith and Häusser1 examined this question using two-photon calcium imaging in vivo2. This relatively new and powerful technique allowed them to simultaneously monitor the firing rates of 20–68 cells, that is, roughly half of all the neurons present in each imaged region (230-μm square) in superficial layers of mouse V1. On the basis of the high spatial and temporal resolution of this data, they were able to determine that fine retinotopy is discarded in mouse V1, possibly in favor of extracting new visual features.
As neuronal receptive fields in the superficial layers of mouse V1 consist of segregated ON and OFF subfields that summate their visual responses linearly3, Smith and Häusser1 used random flashes of bright and dark spots on a gray background to map the receptive fields of the imaged neurons. In this manner, spike-triggered averages of white dot positions revealed the part of the receptive field that received ON-RGC input (on subregion) and the same operation for black dots revealed OFF-RGC input (off subregion). If fine retinotopy were preserved in the mouse V1, subregions of adjacent cells should be closer together in visual space than those of distant cells.
Far from being orderly, the subregions of cells in a local neighborhood in mouse V1 were scattered (average scatter = 5 degrees, subregion diameter = 20 degrees). Smith and Häusser1 found little relationship between the cortical distance separating two neurons and the visual distance between their receptive fields, confirming that local retinotopic structure was violated. This is consistent with prior work using electro-physiology that found that receptive fields of simultaneously recorded neuronal pairs were not perfectly aligned4 (but see refs. 5,6).
Notably, Smith and Häusser1 found that pairs of V1 cells separated by as many as 300 μm (>10% of the full size of mouse V1) appeared to have a significant tendency to share common subregions (~40% of the sampled subregion pairs were fully overlapping), indicating that they used input from the same small population of RGCs to construct their receptive fields. Notably, however, stimulus-driven responses of neurons that shared subregions were hardly more correlated than average, despite a previous study finding enhanced connectivity between neurons sharing common input from layer 4 (ref. 7).
Moreover, Smith and Häusser1 found that the average retinotopic positions of on and off subregions were shifted relative to each other, implying that the retinotopic maps formed by inputs from ON and OFF RGCs are not in register in mouse V1. Such segregation has been observed in several species8,9 and may underlie the development of orientation columns10–13. However, orientation-tuned cells in mice are not organized into columns, so the segregation between ON and OFF pathways may be independent of developing orientation maps. On the other hand, it is also possible that the non-overlapping ON and OFF inputs observed by Smith and Häusser1 may simply reflect the salt and pepper arrangement of RGCs in the mouse retina13 (Fig. 1a).
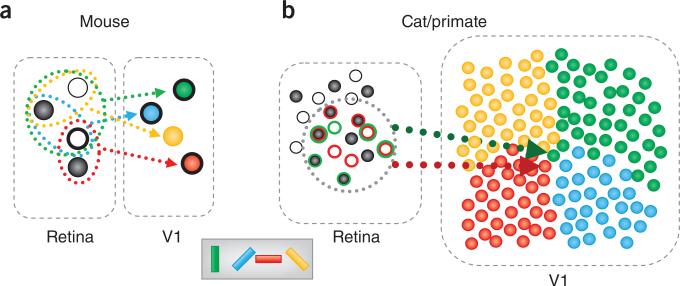
Figure 1.
The mouse visual system is substantially different from those of cats and macaques. (a) In the mouse visual system, the retina contains about one RGC per input-layer neuron in V1. Some of the retinal ganglion cells respond to bright spots (ON center, white circles), whereas others respond to dark spots (OFF center, black circles). Neurons in mouse V1 are tuned for orientation (pictured as cell color) and receive input from a few RGCs. A shared subfield may be a result of common RGC input (heavy outline). The result of this arrangement is breakdown of retinotopy on a fine scale. Vertical (green) and horizontal (red) tuned cells share a subfield, despite being far from each other along cortex. (b) The situation in cats and macaques is quite different. In these systems, there are about 100 cortical recipient neurons for every RGC and these neurons are precisely arranged (for example, by their orientation preference, as illustrated for an orientation pinwheel on the right). Given that many RGCs contribute to a single receptive field in V1, whereas each RGC diverges to contribute to the receptive fields of multiple V1 neurons, many orientations may be adequately represented without the apparent need to sacrifice retinotopy. Compare the inputs into a cell tuned for horizontal (red) versus vertical (green). The net receptive field position of these cells would be quite similar (dotted circle). Other retinotopic positions may be smoothly mapped by sliding the dotted outline. Outline colors of RGCs indicate which RGCs connect to which neuron (those outlined in green connect to the green neuron and similarly for red).
Why isn't the activity of neurons with shared subregions more correlated? One possibility is that these neurons encode different features of visual stimuli, such as orientation3. As orientation tuning in simple cells results from the relative positions of the ON and OFF subregions, the relative position between the two subregions can determine orientation selectivity of the cell. Smith and Häusser1 found that the average overlap of the second region was quite high (0.7) for neurons sharing one subregion. It will be important to determine whether such small shifts in relative subregion position can imbue these cells with substantially different orientation preferences, given the sharp orientation tuning observed in most cells3. Alternately, the neuron pairs may have other receptive field differences that were not assessed by Smith and Häusser1, such as direction selectivity3.
Why is fine retinotopy not preserved in mouse V1? A key to interpreting the need for scatter may lie in the limitations imposed by the small size of the mouse visual cortex, where there is roughly one neuron for every RGC (Fig. 1a). For cortical cells to represent additional features of the visual stimulus, RGC inputs must be combined. However, because there are too few cortical cells in mouse V1, some information carried by the RGCs, such as fine retinotopy, may be lost (Fig. 1a). The observations of shared and spanned receptive fields suggest that the mouse visual system may sample portions of the visual field and pass them in parallel through several filters, extracting unique information, but introducing scatter into local retinotopy. Indeed, local scatter appears to be important in mouse auditory cortex (A1), where tonotopy fed forward from the thalamus is markedly disrupted, possibly in favor of more complex stimulus representations such as natural sounds14. It may be that functional scatter is a general principle in mouse sensory cortex, where discrete cell assemblies processing input from the same population of thalamic afferents act in a manner similar to a hypercolumn4 (Fig. 1a).
The situation is likely very different in visual systems of other model species, such as cats and macaques, which are more similar to humans. Because these species have about 100 cells in V1 for every RGC with each receptive field composed of inputs from many RGCs (Fig. 1b), such marked tradeoffs between retinotopy and diversity may not be necessary. Indeed, the organization of orientation tuning in cat V1 is smooth even at single-cell resolution15. It will be important to determine whether retinotopy is equally smooth. Notably, some evidence suggests that maps of orientation and retinotopy may be interrelated5. The microscopic relationship between the two maps may have important implications for the principles governing the organization and development of the cortical microcircuit13. Thus, it will be important to examine how maps of retinotopy and orientation are related in these species on microscopic scales.
By zooming into the mouse visual system with a two-photon microscope, Smith and Häusser1 provide important insights into how cortex produces diverse functional tuning under the constraint of low divergence. Their work will serve as a valuable reference for imaging the visual system at finer resolution in other species. In these systems, their powerful technique could be used to systematically study how cortical representations of visual features, such as orientation and direction of motion, interact with retinotopy. At the same time, developing our understanding of rodent V1 (refs. 3,7) sets up a system for studying visual processing that is amenable to the full spectrum of genetic and imaging techniques. Because of its accessibility, future work in this system will likely produce breakthroughs in our understanding of the general principles underlying cortical processing. Imagine what lies ahead.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Yevgeniy B Sirotin, Shelby White and Leon Levy Center for Mind, Brain and Behavior, The Rockefeller University, New York, New York, USA. ysirotin@rockefeller.edu.
Aniruddha Das, Department of Neuroscience, Columbia University, New York, New York, USA..
References
- 1.Smith SL, Häusser M. Nat. Neurosci. 2010;13:1144–1149. doi: 10.1038/nn.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohki K, Chung S, Ch'ng YH, Kara P, Reid RC. Nature. 2005;433:597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- 3.Niell CM, Stryker MP. J. Neurosci. 2008;28:7520–7536. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hubel DH, Wiesel TN. Proc. R. Soc. Lond. B Biol. Sci. 1977;198:1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- 5.Das A, Gilbert CD. Nature. 1997;387:594–598. doi: 10.1038/42461. [DOI] [PubMed] [Google Scholar]
- 6.DeAngelis GC, Ghose GM, Ohzawa I, Freeman RD. J. Neurosci. 1999;19:4046–4064. doi: 10.1523/JNEUROSCI.19-10-04046.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshimura Y, Dantzker JL, Callaway EM. Nature. 2005;433:868–873. doi: 10.1038/nature03252. [DOI] [PubMed] [Google Scholar]
- 8.Jin JZ, et al. Nat. Neurosci. 2008;11:88–94. doi: 10.1038/nn2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zahs KR, Stryker MP. J. Neurophysiol. 1988;59:1410–1429. doi: 10.1152/jn.1988.59.5.1410. [DOI] [PubMed] [Google Scholar]
- 10.Miller KD. Neuroreport. 1992;3:73–76. doi: 10.1097/00001756-199201000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Miller KD. J. Neurosci. 1994;14:409–441. doi: 10.1523/JNEUROSCI.14-01-00409.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ringach DL. J. Neurophysiol. 2004;92:468–476. doi: 10.1152/jn.01202.2003. [DOI] [PubMed] [Google Scholar]
- 13.Ringach DL. PLoS One. 2007;2:e251. doi: 10.1371/journal.pone.0000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro JB, Kandler K. Nat. Neurosci. 2010;13:271–273. doi: 10.1038/nn0310-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohki K, et al. Nature. 2006;442:925–928. doi: 10.1038/nature05019. [DOI] [PubMed] [Google Scholar]