Abstract
Objectives
to explore relationships among serum adipokines, vitamin D, clinical and microbial parameters of chronic periodontitis before and after treatment.
Methods
weight, height and smoking status were recorded for 56 patients with chronic periodontitis. Plaque, gingivitis, bleeding on probing (BOP), suppuration, pocket depth (PD) and attachment level (AL) were measured at all teeth present. Subgingival biofilm samples from each tooth were analyzed for levels of 40 bacterial species using checkerboard DNA-DNA hybridization. Serum levels of interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), adiponectin, leptin, resistin and vitamin D were measured at baseline. Sample collection was then performed in a subset of the population 6 months post-therapy (n=17). Serum samples were analyzed using ELISA and immunoassays. Differences in clinical, microbial and serum factors among groups were sought using the Mann-Whitney test. Correlations among factors were evaluated using regression analysis. Effects of therapy were sought using the Wilcoxon signed ranks test
Results
There were positive correlations between adiponectin/vitamin D and between IL-6/leptin; negative correlations between IL-6/vitamin D, and leptin/vitamin D, but no associations between serum analytes and clinical or microbial parameters. Gender and BMI were associated with levels of adipokines. Periodontal therapy improved clinical and microbiological parameters, but did not influence the levels of serum analytes.
Conclusions
Adipokines and IL-6 levels were affected by gender and BMI. Serum analytes were not influenced by periodontal therapy.
Keywords: adipokines, cytokines, calcitriol, periodontitis, subgingival scaling, biofilm, microbiota
INTRODUCTION
Periodontal diseases are initiated by a consortia of oral bacteria that elicit local inflammatory responses that lead to bleeding on probing, loss of periodontal attachment, bone and tooth loss.1 They have been linked to systemic conditions, including heart disease 2, diabetes 3, obesity 4 and metabolic syndrome.5 The association between periodontal diseases and these systemic conditions seems to be due to a low grade inflammatory burden that links them through a common pathophysiological mechanism. Conceivably, locally secreted cytokines and periodontal pathogens can enter into the bloodstream and contribute to damage elsewhere in the body and there appears to be some evidence for that burden.6-9
Tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) are key cytokines in the initiation and maintenance of systemic inflammation which have been implicated in progression and severity of periodontitis.10, 11 In addition, higher serum levels of these cytokines have been observed in periodontitis patients than in periodontally healthy individuals.9, 12-15.
Leptin, adiponectin and resistin are adipokines that are secreted primarily by adipose tissues, but also produced by monocytes and macrophages and are able to directly influence inflammation.16 Leptin regulates T lymphocyte proliferation, activation and cytokine production and elevated levels are present in infection and inflammation17, 18. Resistin levels increase upon endotoxin exposure.19 Adiponectin is associated with modulation of inflammatory responses, via attenuation of TNF-α effects 20, inhibition of nuclear factor κB (NF-κB), inhibition of IL-6 production and induction of anti-inflammatory cytokines IL-10 and IL1 receptor antagonist. 21
Altered adipokine levels have been observed in systemic inflammatory conditions, including inflammatory bowel disease (IBD) and rheumatoid arthritis.22 Little is known regarding the potential association between adipokines and chronic periodontitis in systemically healthy individuals. 23-25 In addition, studies that investigated the effects of periodontal treatment on serum levels of adipokines, TNF-α and IL-6 in such patients have shown conflicting results. 9, 15, 26-30
Vitamin D has an important role in bone growth and maintenance, which might be beneficial for maintaining periodontal health. Recently, it has been suggested to have positive effects on periodontal diseases, tooth loss and gingival inflammation not through its effects on bone metabolism, but through anti-inflammatory mechanisms.31 Hence, adequate serum values of Vitamin D could be important in the prevention and treatment of periodontal diseases.
The primary goal of this exploratory study was to examine relationships among serum levels of IL6, TNF-α, adipokines, vitamin D and clinical and microbial parameters of chronic periodontitis. The secondary objective of the present investigation was to assess the effects of therapy on the levels of those serum analytes before and after non-surgical periodontal therapy.
MATERIAL AND METHODS
Subject population
The subject population consisted of 56 subjects with chronic periodontitis. The inclusion and exclusion criteria were as follows:
Inclusion criteria
> 20 years of age, > 15 natural teeth, > 5% sites (approximately 8 sites) with pocket depth > 4 mm and/or 5% sites with attachment level > 4 mm and willingness and ability to sign informed consent and overall systemic health as determined upon completion of a medical questionnaire.
Exclusion criteria
Pregnancy or nursing, periodontal or antibiotic therapy in the previous 3 months, any systemic condition which might influence the course of periodontal disease or treatment (e.g. diabetes, AIDS), any systemic condition which requires antibiotic coverage for routine periodontal procedures (e.g. heart conditions, joint replacements).
Attempts were made to recruit approximately equal numbers of males and females. In addition, subjects of any racial/ethnic group were accepted for the study, which was performed between September 2003 and April 2008. All subjects were recruited at The Forsyth Institute. The study was approved by The Forsyth Institute Institutional Review Board and all subjects signed informed consent prior to entering the study.
Clinical monitoring and Periodontal Therapy
After determination of suitability and obtaining informed consent, subjects entered the study. Prior to clinical monitoring visit, height and weight were recorded for the calculation of the body mass index (BMI). Subjects were clinically monitored at baseline and 6 months after periodontal therapy. Clinical measurements were taken at 6 sites per tooth (mesiobuccal, buccal, distobuccal, distolingual, lingual, and mesiolingual) for all teeth excluding third molars (a maximum of 168 sites per subject) as previously described.32 The clinical parameters were measured in the following order: 1) gingival redness (0 or 1); 2) plaque accumulation (0 or 1) 3) pocket depth (mm); 4) attachment level (mm); 5) bleeding on probing (0 or 1); 6) suppuration (0 or 1).
Pocket depth and attachment level measurements were made to the nearest mm using a North Carolina periodontal probe. Pocket depth and attachment level measurements were measured twice and the average of the pair of measurements was used for analysis. All clinical data were recorded on data sheets and entered into a computer using a prompted data entry program. Subgingival plaque samples were taken prior to the clinical measurements. The same calibrated examiner took clinical measurements and samples at both monitoring visits for a given subject.
After the initial monitoring and sampling, all subjects received scaling and root planing (SRP) at four visits (one quadrant per visit), using manual curettes and ultrasonic devices and also received oral hygiene instructions. All subjects received maintenance subgingival scaling at 3 months. Clinical parameters and subgingival biofilm samples were collected again 6 months after treatment.
Microbiological sample taking and enumeration of organisms
After removal of supragingival plaque, individual subgingival plaque samples were taken separately from the mesio-buccal aspect of up to 28 teeth in each subject at entry and at the 6 months post-treatment visit. Samples were taken using individual sterile Gracey curettes and were evaluated for their content of 40 subgingival species using checkerboard DNA-DNA hybridization.33, 34 Each sample was placed in individual tubes containing 0.15 ml TE (10 mM Tris-HCL, 0.1 mM EDTA, pH 7.6). 0.15 ml of freshly-prepared 0.5 M NaOH was added. The samples were boiled for 5 min and neutralized using 0.8 ml of 5 M ammonium acetate and placed into the extended slots of a Minislot‡ and then concentrated onto a positively charged nylon membrane§ by vacuum and fixed to the membrane by exposure to ultraviolet light followed by baking at 120°C for 20 min. The counts of the 40 species in each sample were determined using checkerboard DNA-DNA hybridization.
Serum samples and analytes
Serum samples were collected at baseline and 6 months after periodontal therapy. Samples were analyzed at the Harvard Catalyst Central Laboratory (Harvard Medical School, Boston, MA) for their levels of adiponectin, leptin, resistin, IL-6, TNF-α and vitamin D. ELISA was used to measure levels of adiponectinII , resistin¶ and TNF-α¶. Radioimmunoassays were employed to assess levels of leptin# and vitamin D**. IL-6 was measured using a chemiluminescent immunoassay††. The dynamic ranges of the assays were as follows: 0.10 to 24 ug/mL for adiponectin, 0.1-10 ng/mL for leptin, 0.21 -50 ng/mL for resistin, 1.9 6.5 pg/mL for IL-6, 0.106-16 pg/mL for TNF-α and 1.5-100 ng/mL for vitamin D.
Data Evaluation
Clinical parameters including plaque index, gingival index, % of sites with bleeding on probing and suppuration as well as mean pocket depth and attachment level were computed for each subject averaged within a subject and then averaged across subjects. In the same fashion, counts of each bacterial species were determined at each sampled site, averaged within a subject and then averaged across subjects.
Relationships among serum analytes were sought using the Spearman correlation coefficient rho (r). Significant correlations were sought using a t statistic. Measures of periodontal disease severity (PD, CAL, %BOP) were used to stratify the population into tertiles and search for associations between periodontal parameters and the serum biomarkers tested. Differences in clinical, microbial and serological parameters among groups were sought using the Kruskal-Wallis and the Chi-square test. The same approach was used to search for those associations based on tertiles of the distribution for each of the serum analytes. Multiple linear regression was used to assess the value of explanatory variables (gender, BMI, age as well as serum and clinical variables) in predicting the response of selected outcomes (adiponectin, IL-6, leptin, vitamin. D, resistin and TNF-α). The effects of periodontal treatment on clinical and microbial parameters as well as on serum analytes were sought using the Wilcoxon signed ranks test. Because of the exploratory nature of the present study no attempts were made to correct for multiple comparisons.
RESULTS
Table 1 presents the baseline clinical parameters, as well as levels of serum analytes of the 56 subjects enrolled in the study. The overall mean PD and CAL were 3.2 mm and 3.6 mm, respectively. Males represented 53% of the subject population and 49% of enrolled subjects were current smokers. BMI values ranged from 15 to 63 kg/m2.
Table 1.
Clinical and serum analytes characteristics of the study population at baseline (n=56).
| Clinical Features | Median | IQR |
|---|---|---|
| % sites with | ||
| Plaque | 64 | 32 |
| Gingival Redness | 67 | 32 |
| BOP | 29 | 25 |
| Suppuration | 0 | 1 |
| PD (mm) | 3.1 | 0.9 |
| AL (mm) | 3.4 | 1.5 |
| N missing teeth | 2 | 4 |
| BMI (kg/m^2) | 26 | 6 |
| Mean% Males | 53 | |
| Mean%Current Smokers | 49 | |
| Age (years) | 49.5 | 14.6 |
| Analytes | ||
|---|---|---|
| Adiponectin (μg/mL) | 3.9 | 3.5 |
| Leptin (ng/mL) | 7.7 | 10.7 |
| Resistin (ng/mL) | 7.3 | 4.7 |
| IL-6 (pg/mL) | 2.3 | 1.8 |
| TNF-α (pg/mL) | 1.0 | 1.2 |
| Vitamin D (ng/mL) | 26.0 | 15.0 |
Note: Levels of vitamin D represent samples from 21 subjects. Four of the subjects presented amounts of TNF-α were below the detection level. In these subjects TNF-α levels were recorded as zero.
Reference values for serum analytes: Adiponectin: unknown; Leptin: 2.3 – 11.1 ng/mL ( for BMI 18-25), Resistin: 6.39 – 26.4 ng/mL; IL-6: : 0.5-1500pg/mL; TNF-α: : 0.55 – 2.816 pg/mL; Vitamin D: 9-37.6 ng/mL (data provided by HCCL and the assays’ manufacturers):
IQR: Interquartile range
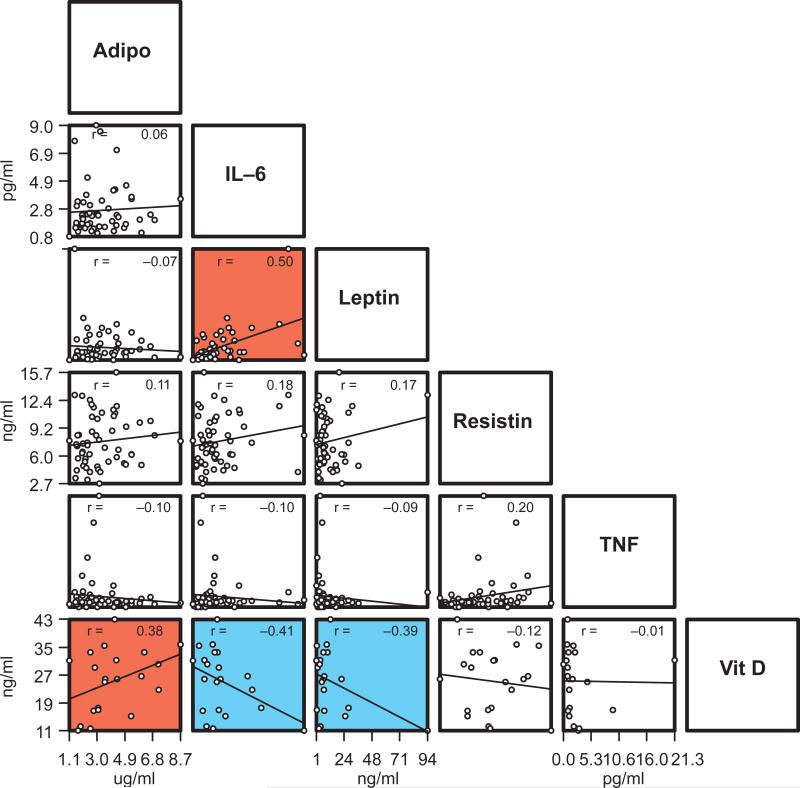
Figure 1 shows that positive correlations were observed between IL-6 and leptin (r=0.50, p<0.001) and adiponectin and vitamin D (r=0.38, p<0.01). Negative correlations were observed between IL-6 and vitamin D (p=-0.41, p<0.01) and leptin and vitamin D (p=-0.39, p<0.01). Weak correlations were observed between serum analytes and mean clinical parameters (-0.25.>r <0.19; p>0.05; data not shown).
Figure 1.
Plot of mean levels of adiponectin, leptin, resistin, IL-6, TNF-α and vitamin D in 56 chronic periodontitis patients (Vitamin D: n=21 patients). Each circle represents values for a single subject. Regression lines were fit to pairs of serum analytes and correlation coefficients are indicated in the panel (r). Red panels represent statistically significant positive correlations and turquoise panels represent statistically significant negative correlations (p<0.01, t>2.678 and p<0.001 t>3.496). Amounts of TNF-α were below the detection level in 4 of the subjects and were recorded as zero.
In order to explore relationships between serum biomarkers and the periodontal condition, periodontitis subjects were divided into tertiles according to mean PD (Table 2). There was an increase in bleeding on probing (p=0.0001) and attachment level (p=0.0001) with greater pocket depth A similar pattern was observed for levels of pathogenic bacterial species including members of the “red complex”35, Tannerella forsythia (p=0.003), Porphyromonas gingivalis (p=0.012) and Treponema denticola (p=0.05), as well as levels of Prevotella nigrescens (p=0.005) and Eubacterium nodatum (p=0.003), members of the “orange complex”. When levels of serum analytes were compared among the three groups, difference in the serum factors were of smaller magnitude. The same analysis was performed using CAL and % BOP as measures of disease severity. A similar lack of relationship with serum factors was observed (data not shown).
Table 2.
Median and interquartile ranges (IQR) for serum analytes, clinical and microbial parameters subset according to pocket depth.
| Median | IQR | Median | IQR | Median | IQR | Kruskall-Wallis | |
|---|---|---|---|---|---|---|---|
| Pocket Depth (mm) | <2.8 | <3.4 | ≥3.4 | p value | |||
| N= | 18 | 19 | 19 | ||||
| % sites with | |||||||
| Plaque | 49 | 23 | 66 | 29 | 81 | 25 | 0.003 |
| Gingival Redness | 57 | 30 | 68 | 34 | 69 | 15 | 0.097 |
| BOP | 20 | 21 | 30 | 20 | 48 | 29 | 0.0001 |
| Suppuration | 0 | 0 | 0 | 2 | 0 | 2 | 0.125 |
| PD (mm) | 2.6 | 0.2 | 3.1 | 0.3 | 3.9 | 0.7 | 0.0001 |
| AL (mm) | 2.6 | 1.1 | 3.4 | 1.0 | 4.7 | 1.2 | 0.0001 |
| N missing teeth | 1.5 | 5 | 2 | 4 | 4 | 5 | 0.266 |
| BMI (kg/m^2) | 26.5 | 8.1 | 26.5 | 5.3 | 25.9 | 4.6 | 0.892 |
| Mean % Males | 44 | 50 | 70 | 0.353** | |||
| Mean %Current Smokers | 28 | 40 | 80 | 0.009** | |||
| Age (years) | 52.4 | 15.9 | 52.4 | 13.9 | 46.8 | 11.1 | 0.348 |
| Analytes | p value | ||||||
|---|---|---|---|---|---|---|---|
| Adiponectin (μg/mL) | 3.6 | 3.3 | 4.1 | 3.5 | 3.7 | 3.8 | 0.821 |
| Leptin (ng/mL) | 7.9 | 10.9 | 9 | 14.4 | 6.4 | 4.5 | 0.337 |
| Resistin (ng/mL) | 7.6 | 5.6 | 7.2 | 3.4 | 7.4 | 4.2 | 0.923 |
| IL-6 (pg/mL) | 2.5 | 2.0 | 2.3 | 1.2 | 2.1 | 1.7 | 0.507 |
| TNF-α (pg/mL) | 0.9 | 1.3 | 1 | 1.2 | 1.0 | 0.6 | 0.755 |
| Vitamin D (ng/mL) | 29.8 | 5.5 | 25.2 | 17.4 | 25 | 17 | 0.577 |
| Bacterial Species (× 10^5) | p value | ||||||
|---|---|---|---|---|---|---|---|
| T. forsythia | 0.5 | 0.8 | 1.5 | 1.9 | 2.2 | 3.3 | 0.003 |
| P. gingivalis | 0.7 | 0.8 | 1.7 | 1.4 | 0.9 | 4.2 | 0.012 |
| T. denticola | 0.5 | 0.6 | 0.8 | 1.9 | 1.2 | 4.2 | 0.05 |
| P. intermedia | 0.5 | 0.2 | 1.5 | 1.6 | 2.3 | 2.2 | 0.0003 |
| P. nigrescens | 0.5 | 0.9 | 1.1 | 2.0 | 1.5 | 2.1 | 0.005 |
| E. nodatum | 0.1 | 0.3 | 0.5 | 0.8 | 0.7 | 1.0 | 0.0003 |
Note: Levels of vitamin D represent samples from 21 subjects. Four of the subjects presented amounts of TNF-α were below the detection level. In these subjects, TNF-α levels were recorded as zero. Due to the number of bacterial species evaluated, only those that presented differences with associated p values<0.05 were included in the table.
*Chi-square analysis.
Using the same approach to seek associations between increasing levels of serum factors or BMI and clinical and microbial parameters, the study population was subset into tertiles according to levels of adiponectin, resistin, leptin, IL-6, TNF-α and vitamin D, as well as BMI. None of the clinical or microbial parameters were associated with any of the serum analytes (p>0.05, data not shown). Associations were observed for adiponectin, leptin and IL-6 with being female. Leptin was associated with increased levels of IL-6 and BMI. Finally, increased BMI was associated with higher levels of leptin (Table 3).
Table 3.
Mean (± SD) serum analytes, BMI and percentage of males, according to levels of serum analytes.
| Mean | SD | Mean | SD | Mean | SD | p value | |
|---|---|---|---|---|---|---|---|
| Adiponectin (μg/mL) | < 3.1 | <5.4 | <13.2 | ||||
| N= | 18 | 20 | 18 | ||||
| %Male | 72 | 58 | 28 | 0.024* | |||
| Leptin (ng/mL) | <5.4 | <10.6 | <95.2 | ||||
| N= | 18 | 19 | 19 | ||||
| IL-6 | 2.0 | 0.9 | 2.6 | 1.8 | 3.6 | 2.1 | 0.02‡ |
| %Male | 83 | 47 | 28 | 0.003* | |||
| BMI | 23.7 | 3.5 | 25.6 | 3.5 | 32.7 | 8.9 | 0.0001‡ |
| IL-6 (pg/mL) | <1.7 | <2.7 | <9.0 | ||||
| N= | 18 | 20 | 18 | ||||
| Leptin | 7.5 | 6.0 | 8 | 5.5 | 20.2 | 20.7 | 0.003‡ |
| %Male | 67 | 67 | 26 | 0.017* | |||
| BMI | <24.4 | <27.9 | <63.1 | ||||
| N= | 20 | 18 | 18 | ||||
| Leptin | 6 | 4.4 | 8.3 | 21.9 | 20.4 | 0.0005‡ |
Note: All serum, clinical and microbial parameters were included in the analysis. Due to the high number of variables evaluated, only those that presented differences with associated p values<0.05 were included in the table.
Chi-square analysis
Kruskall-Wallis analysis.
Using multiple linear regression, it was observed that the best predictors for adiponectin levels were gender (female), BMI (lower) and increasing age (p=0.000078, r2= 0.278). For leptin levels, the best predictors were gender (female) and BMI (higher) (p=0.000001, r2= 0.789). BMI was the only significant predictor for IL-6 (p=0.00074, r2= 0.195) and Vitamin D (p=0.03735, r2= 0.209).
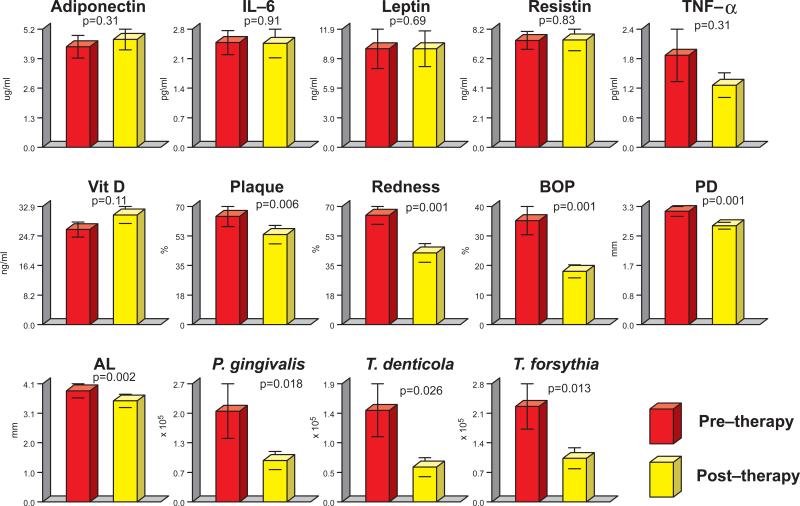
The effects of periodontal therapy on serum analytes, clinical parameters and the subgingival microbiota are shown in Fig. 3. Seventeen patients were examined at 6 months post therapy. There were essentially no differences between pre and post treatment levels of any of the serum analytes, although a trend toward the reduction of TNF-α and increase in vitamin D and adiponectin was detected. All clinical parameters of periodontitis showed improvement after periodontal therapy. In addition, levels of the periodontal pathogens T. forsythia, T. denticola and P. gingivalis were considerably reduced after treatment. Although the periodontitis population in the present study included smokers and smoking is a recognized risk factor for periodontitis, clinical and microbiological improvements could be achieved.
DISCUSSION
The primary goal of this exploratory study was to examine relationships among serum levels of IL6, TNF-α, adipokines, vitamin D and clinical and microbial parameters of chronic periodontitis. The secondary objective of the present investigation was to assess the effects of therapy on the levels of those serum analytes before and after non-surgical periodontal therapy. We hypothesized that periodontal infection and inflammation might affect systemic levels of adiponectin, leptin, resistin, IL-6 and TNF-α, which can influence and/or promote other systemic conditions, such as insulin resistance and metabolic syndrome. In that case, periodontal treatment could directly affect not only the oral cavity, but systemic health as well. In addition, clinical and microbial periodontal parameters and the serum analytes selected for study could be influenced by systemic levels of vitamin D due to its proposed anti-inflammatory properties
Serum Levels of Adiponectin, Leptin, Resistin, IL-6 and TNF-α at Baseline
Even though elevated levels of IL-6 and TNF-α have been reported in periodontitis patients, both locally 36, 37 and systemically 8, 9, 12-15, we did not find a dose-response association between serum levels of analytes and severity of periodontitis (Table 2). Although no relationship was observed among periodontal parameters and serum analytes, we observed a strong positive correlation between IL-6 and leptin (Fig. 1). This relationship has been reported previously 30 and supports the pro-inflammatory role of those serum analytes. We also found a positive correlation between adiponectin and vitamin D, two proposed anti-inflammatory mediators. In addition, negative correlations were found between vitamin D and both IL-6 and leptin, supporting their proposed antagonistic roles in inflammation.
Few studies have reported on the association between adipokines and periodontal diseases. Saito et al (2008)23 observed higher serum levels of resistin, but not adiponectin in Japanese women with periodontitis. The authors proposed that the increase in circulating levels of resistin might be due to the local involvement of monocytes and macrophages in periodontal inflammation. However, in our study, patients presented even higher mean % BOP and resistin was not associated with any of the periodontal parameters. Furugen et al (2008)24 observed higher levels of resistin in Japanese elders with periodontitis, when compared with periodontally healthy elders. This finding is in contrast with our results, possibly due to differences in age, BMI and ethnic characteristics. Similar to our results, those authors found that circulating adiponectin was associated with gender, as higher levels of adiponectin were observed in females, but was not associated with periodontal parameters. They suggested that different isoforms of adiponectin - low, middle and high molecular weight - might exert different effects, which would not have been detected in previous studies, as well as in ours, since only total adiponectin was evaluated. Even though the role of those isoforms is not fully understood, Nagano et al (2011)38 observed that the high molecular weight adiponectin to total adiponectin ratio was significantly lower in subjects with periodontal pockets.
Infections lead to increased circulating leptin 39, a suggested risk factor for cardiovascular disease 40. Karthikeyan & Pradeep (2007)25 found an association between high serum leptin and severity of periodontitis and detected mean serum leptin levels of 12,082 pg/ml in periodontitis patients, which was above the level that is considered a risk factor for cardiovascular disease (10,000 pg/ml).41 In the present study, we did not find an association between serum leptin and severity of periodontitis. At baseline, we detected a mean leptin level of 11,970 pg/ml, which is comparable to levels from the Karthikeyan & Pradeep study.25 This mean level remained virtually unchanged after periodontal treatment, even though periodontal infection and inflammation were successfully controlled.
A positive association between systemic levels of TNF-α and periodontitis has been reported.9, 12, 14, 15, 28, 42 Duarte et al (2010)28 found higher TNF-α in generalized aggressive periodontitis (GAgP) patients than in generalized chronic periodontitis (GCP) patients and healthy subjects (H), although no differences were observed between GCP and H. Passoja et al 14 showed that chronic periodontitis patients presented higher circulating levels of TNF-α than periodontally healthy subjects. However, when subjects were categorized into tertiles according to severity of periodontitis, no significant differences were observed. Conversely, other authors have failed to demonstrate this association. 8, 9, 23, 24, 43 A similar scenario exists for IL-6, in which a positive association with periodontitis has been shown by some investigators 8, 9, 12, 15, 28, but not others.23, 24, 27, 30
In the present study, we did not find associations between circulating TNF-α and IL-6 and any of the periodontal parameters (Fig. 2, Table 2), including the subgingival microbiota. This is in accord with Bretz et al (2005)12 who did not find an association between periodontal pathogens assessed by BANA and levels of IL-6 and TNF-α.
Figure 2.
Bar plots of mean serum analytes, mean periodontal clinical parameters and mean counts of red complex species (Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia) (Socransky et al 1998)35 in subgingival plaque before (red bars) and after periodontal treatment (yellow bars). Due to the number of bacterial species evaluated, only those that presented difference in pre and post- treatment levels with an associated p value<0.05 were included in the figure. The bars represent values for each serum analyte, clinical parameter and bacterial species averaged within each subject and then averaged across subjects at each time point separately. The whiskers represent the standard error of the mean. Note that y axis values differ for each panel. Significance of differences between time points was sought using the Wilcoxon signed ranks test. Amounts of TNF-α were below the detection level in 1 of the subjects at baseline and was recorded as zero.
Because adiponectin, resistin, leptin, IL-6 and TNF-α have such an important role in inflammation, the population was subset into tertiles according to levels of those analytes. We hypothesized that a worse periodontal status would account for the higher levels of a few of those biomarkers, including IL-6, TNF-α and leptin. However, there was no difference among tertiles for any of the analytes or any of the clinical or microbiological parameters analyzed. In fact, analysis showed a greater influence of gender and BMI on the levels of most of the serum analytes than any clinical or microbial parameter of periodontitis (Table 3).
It should be noted that most of the studies cited above compared serum samples from periodontitis and periodontally healthy individuals. In the present study, we compared levels of serum analytes in periodontitis patients with different levels of disease severity and bacterial load. It should also be highlighted that differences in the study population, including the extent and severity of periodontitis, as well as the methods of detection of analytes, and the wide range of levels of the serum markers may contribute to the lack of consensus in the literature cited above.
Levels of Vitamin D at Baseline
Vitamin D has an important role in calcium homeostasis, bone growth and preservation. It has been shown to inhibit antigen-induced T cell proliferation and cytokine production 44, acting as an immunomodulatory agent.45
Recently, vitamin D has been proposed to also have anti-inflammatory properties. Analyzing 6,700 subjects, Dietrich et al (2005)31 found that individuals in the highest quintile of serum vitamin D presented significantly less bleeding, lower mean pocket depth and clinical attachment loss, number of missing teeth and BMI. It has also been suggested that vitamin D (and calcium) supplementation may have a positive effect on periodontal health, particularly on bleeding on probing, gingival index and PD.46, 47 Interestingly, polymorphisms in vitamin D receptors have been linked to generalized aggressive periodontitis (GAgP)48 and severe chronic periodontitis 49. Our results are in accord with those of Dietrich et al (2005)31. The highest levels of circulating vitamin D were detected among the individuals that presented less bleeding on probing, lower mean PD, CAL and number of missing teeth, as well as levels of pathogenic bacteria. In addition, the proposed anti-inflammatory role of vitamin D was supported in this study by its positive correlation with adiponectin and negative correlation with IL-6 and leptin.
Effects of Treatment on Serum Analytes, Clinical Parameters and Subgingival Bacteria
Intervention studies have been performed in order to assess whether periodontal therapy can influence serum levels of pro- and anti-inflammatory mediators, with conflicting results. Marcaccini et al (2009)15 showed significantly lower serum IL-6 in chronic periodontitis patients after non-surgical periodontal treatment. Similar results have been reported by others.9, 26 Duarte et al (2010)28 found that non-surgical periodontal therapy reduced levels of TNF-α in GAgP, but not chronic periodontitis patients. Shimada et al (2010)30 found significant reduction in circulating leptin after the treatment of periodontitis patients. However, others did not find significant differences in post-therapy circulating levels of TNF-α 9, 27 or IL-6 27, despite positive changes in clinical periodontal parameters. In the present study, we demonstrated improvement in all clinical and microbial parameters of periodontitis. However, virtually no differences were detected in the levels of any of the serum analytes post-therapy. The lack of consensus in the current literature regarding the systemic effects of periodontal therapy might be due to factors already discussed above and may also include the presence of systemic risk factors and co-morbidities. Studies that detected effects on serum analytes focused on patients with severe and/or generalized forms of periodontitis 26, 28, on patients at risk for systemic diseases, such as cardiovascular diseases 29 or subjects who presented with other modifying factors, such as diabetes.50 Hence, it is possible that those individuals presented an infectious/inflammatory burden that surpassed the burden elicited solely by moderate chronic periodontitis.
Our findings suggest that chronic periodontitis might play a minor role, if any, in serum levels of inflammatory mediators in systemically healthy subjects. The influence of gender and BMI outweighted any potential role that local periodontal infection and inflammation may have on systemic markers of inflammation in the absence of generalized periodontitis or systemic modifying factors. Even though there is controversy on the direct impact of periodontal therapy on systemic inflammation, there is consensus regarding the clinical and microbiological benefits of periodontal therapy. Therefore, any positive systemic effect that might occur as consequence of periodontal treatment would be a welcome bonus to the essential role of treatment in maintaining the periodontal tissues.
Summary sentence.
Chronic periodontitis might play a minor role in serum levels of inflammatory mediators in systemically healthy subjects. The influence of gender and BMI seems to outweighted the potential role that local periodontal infection and inflammation may have on systemic markers of inflammation in the absence of generalized periodontitis or systemic modifying factors.
Achnowledgements
This work was supported in part by NIH/NIDCR R01-DE014242 (A.H.), R03 DE021742 (F.T.), U01-DE021127 (R.T.), the PCIR Junior Investigator Laboratory Support Award (Harvard Medical School) (F.T.) and the Eleanor and Miles Shore Fellowship Program for Scholars in Medicine (The Forsyth Institute/Harvard Medical School) (F.T.).
Footnotes
Immunetics, Cambridge MA.
Roche, Indianapolis, IN.
ALPCO Diagnostics Inc, Salem, NH.
R & D Systems, Minneapolis, MN.
LINCO Research, St. Charles, MS.
Diasorin, Inc., Stillwater, MN.
Beckman Coulter, Fullerton, CA.
The authors have no conflict of interest.
References
- 1.Cochran DL. Inflammation and bone loss in periodontal disease. J Periodontol. 2008;79:1569–1576. doi: 10.1902/jop.2008.080233. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey LL, Fu R, Buckley DI, Freeman M, Helfand M. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med. 2008;23:2079–2086. doi: 10.1007/s11606-008-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novak MJ, Potter RM, Blodgett J, Ebersole JL. Periodontal disease in Hispanic Americans with type 2 diabetes. J Periodontol. 2008;79:629–636. doi: 10.1902/jop.2008.070442. [DOI] [PubMed] [Google Scholar]
- 4.Morita I, Okamoto Y, Yoshii S, et al. Five-year incidence of periodontal disease is related to body mass index. J Dent Res. 2011;90:199–202. doi: 10.1177/0022034510382548. [DOI] [PubMed] [Google Scholar]
- 5.Benguigui C, Bongard V, Ruidavets JB, et al. Metabolic syndrome, insulin resistance, and periodontitis: a cross-sectional study in a middle-aged French population. J Clin Periodontol. 2010;37:601–608. doi: 10.1111/j.1600-051X.2010.01571.x. [DOI] [PubMed] [Google Scholar]
- 6.Nibali L, D'Aiuto F, Griffiths G, Patel K, Suvan J, Tonetti MS. Severe periodontitis is associated with systemic inflammation and a dysmetabolic status: a case-control study. J Clin Periodontol. 2007;34:931–937. doi: 10.1111/j.1600-051X.2007.01133.x. [DOI] [PubMed] [Google Scholar]
- 7.Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 2008;35:277–290. doi: 10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 8.Saxlin T, Suominen-Taipale L, Leiviska J, Jula A, Knuuttila M, Ylostalo P. Role of serum cytokines tumour necrosis factor-alpha and interleukin-6 in the association between body weight and periodontal infection. J Clin Periodontol. 2009;36:100–105. doi: 10.1111/j.1600-051X.2008.01350.x. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima T, Honda T, Domon H, et al. Periodontitis-associated up-regulation of systemic inflammatory mediator level may increase the risk of coronary heart disease. J Periodontal Res. 45:116–122. doi: 10.1111/j.1600-0765.2009.01209.x. [DOI] [PubMed] [Google Scholar]
- 10.Ejeil AL, Gaultier F, Igondjo-Tchen S, et al. Are cytokines linked to collagen breakdown during periodontal disease progression? J Periodontol. 2003;74:196–201. doi: 10.1902/jop.2003.74.2.196. [DOI] [PubMed] [Google Scholar]
- 11.Graves DT. The potential role of chemokines and inflammatory cytokines in periodontal disease progression. Clin Infect Dis. 1999;28:482–490. doi: 10.1086/515178. [DOI] [PubMed] [Google Scholar]
- 12.Bretz WA, Weyant RJ, Corby PM, et al. Systemic inflammatory markers, periodontal diseases, and periodontal infections in an elderly population. J Am Geriatr Soc. 2005;53:1532–1537. doi: 10.1111/j.1532-5415.2005.53468.x. [DOI] [PubMed] [Google Scholar]
- 13.Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76:2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 14.Passoja A, Puijola I, Knuuttila M, et al. Serum levels of interleukin-10 and tumour necrosis factor-alpha in chronic periodontitis. J Clin Periodontol. 2010;37:881–887. doi: 10.1111/j.1600-051X.2010.01602.x. [DOI] [PubMed] [Google Scholar]
- 15.Marcaccini AM, Meschiari CA, Sorgi CA, et al. Circulating interleukin-6 and high-sensitivity C-reactive protein decrease after periodontal therapy in otherwise healthy subjects. J Periodontol. 2009;80:594–602. doi: 10.1902/jop.2009.080561. [DOI] [PubMed] [Google Scholar]
- 16.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 17.Maruna P, Gurlich R, Frasko R, Haluzik M. Serum leptin levels in septic men correlate well with C-reactive protein (CRP) and TNF-alpha but not with BMI. Physiol Res. 2001;50:589–594. [PubMed] [Google Scholar]
- 18.Yilmaz Z, Ilcol YO, Ulus IH. Endotoxin increases plasma leptin and ghrelin levels in dogs. Crit Care Med. 2008;36:828–833. doi: 10.1097/01.CCM.0B013E3181611F5AA. [DOI] [PubMed] [Google Scholar]
- 19.Lehrke M, Reilly MP, Millington SC, Iqbal N, Rader DJ, Lazar MA. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med. 2004;1:e45. doi: 10.1371/journal.pmed.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokota T, Oritani K, Takahashi I, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- 21.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 22.Otero M, Lago R, Lago F, et al. Leptin, from fat to inflammation: old questions and new insights. FEBS Lett. 2005;579:295–301. doi: 10.1016/j.febslet.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Saito T, Yamaguchi N, Shimazaki Y, et al. Serum levels of resistin and adiponectin in women with periodontitis: the Hisayama study. J Dent Res. 2008;87:319–322. doi: 10.1177/154405910808700416. [DOI] [PubMed] [Google Scholar]
- 24.Furugen R, Hayashida H, Yamaguchi N, et al. The relationship between periodontal condition and serum levels of resistin and adiponectin in elderly Japanese. J Periodontal Res. 2008;43:556–562. doi: 10.1111/j.1600-0765.2008.01085.x. [DOI] [PubMed] [Google Scholar]
- 25.Karthikeyan BV, Pradeep AR. Gingival crevicular fluid and serum leptin: their relationship to periodontal health and disease. J Clin Periodontol. 2007;34:467–472. doi: 10.1111/j.1600-051X.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- 26.D'Aiuto F, Parkar M, Andreou G, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83:156–160. doi: 10.1177/154405910408300214. [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki K, Honda T, Oda T, et al. Effect of periodontal treatment on the C-reactive protein and proinflammatory cytokine levels in Japanese periodontitis patients. J Periodontal Res. 2005;40:53–58. doi: 10.1111/j.1600-0765.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 28.Duarte PM, da Rocha M, Sampaio E, et al. Serum levels of cytokines in subjects with generalized chronic and aggressive periodontitis before and after non-surgical periodontal therapy: a pilot study. J Periodontol. 81:1056–1063. doi: 10.1902/jop.2010.090732. [DOI] [PubMed] [Google Scholar]
- 29.Iwamoto Y, Nishimura F, Soga Y, et al. Antimicrobial periodontal treatment decreases serum C-reactive protein, tumor necrosis factor-alpha, but not adiponectin levels in patients with chronic periodontitis. J Periodontol. 2003;74:1231–1236. doi: 10.1902/jop.2003.74.8.1231. [DOI] [PubMed] [Google Scholar]
- 30.Shimada Y, Komatsu Y, Ikezawa-Suzuki I, Tai H, Sugita N, Yoshie H. The effect of periodontal treatment on serum leptin, interleukin-6, and C-reactive protein. J Periodontol. 81:1118–1123. doi: 10.1902/jop.2010.090741. [DOI] [PubMed] [Google Scholar]
- 31.Dietrich T, Nunn M, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am J Clin Nutr. 2005;82:575–580. doi: 10.1093/ajcn.82.3.575. [DOI] [PubMed] [Google Scholar]
- 32.Haffajee AD, Socransky SS, Goodson JM. Clinical parameters as predictors of destructive periodontal disease activity. J Clin Periodontol. 1983;10:257–265. doi: 10.1111/j.1600-051x.1983.tb01274.x. [DOI] [PubMed] [Google Scholar]
- 33.Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AE. “Checkerboard” DNA-DNA hybridization. Biotechniques. 1994;17:788–792. [PubMed] [Google Scholar]
- 34.Socransky SS, Haffajee AD, Smith C, et al. Use of checkerboard DNA-DNA hybridization to study complex microbial ecosystems. Oral Microbiol Immunol. 2004;19:352–362. doi: 10.1111/j.1399-302x.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- 35.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 36.Thunell DH, Tymkiw KD, Johnson GK, et al. A multiplex immunoassay demonstrates reductions in gingival crevicular fluid cytokines following initial periodontal therapy. J Periodontal Res. 2010;45:148–152. doi: 10.1111/j.1600-0765.2009.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gamonal J, Sanz M, O'Connor A, et al. Delayed neutrophil apoptosis in chronic periodontitis patients. J Clin Periodontol. 2003;30:616–623. doi: 10.1034/j.1600-051x.2003.00350.x. [DOI] [PubMed] [Google Scholar]
- 38.Nagano Y, Arishiro K, Uene M, et al. A low ratio of high molecular weight adiponectin to total adiponectin associates with periodontal status in middle-aged men. Biomarkers. 16:106–111. doi: 10.3109/1354750X.2010.533286. [DOI] [PubMed] [Google Scholar]
- 39.Sarraf P, Frederich RC, Turner EM, et al. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res. 2001;88:954–960. doi: 10.1161/hh0901.090975. [DOI] [PubMed] [Google Scholar]
- 41.Wallace AM, McMahon AD, Packard CJ, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation. 2001;104:3052–3056. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 42.D'Aiuto F, Nibali L, Parkar M, Suvan J, Tonetti MS. Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res. 2005;84:269–273. doi: 10.1177/154405910508400312. [DOI] [PubMed] [Google Scholar]
- 43.Gorska R, Gregorek H, Kowalski J, Laskus-Perendyk A, Syczewska M, Madalinski K. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J Clin Periodontol. 2003;30:1046–1052. doi: 10.1046/j.0303-6979.2003.00425.x. [DOI] [PubMed] [Google Scholar]
- 44.Bhalla AK, Amento EP, Serog B, Glimcher LH. 1,25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol. 1984;133:1748–1754. [PubMed] [Google Scholar]
- 45.Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med. 2002;8:174–179. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 46.Garcia MN, Hildebolt CF, Miley DD, et al. One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J Periodontol. 82:25–32. doi: 10.1902/jop.2010.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miley DD, Garcia MN, Hildebolt CF, et al. Cross-sectional study of vitamin D and calcium supplementation effects on chronic periodontitis. J Periodontol. 2009;80:1433–1439. doi: 10.1902/jop.2009.090077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park KS, Nam JH, Choi J. The short vitamin D receptor is associated with increased risk for generalized aggressive periodontitis. J Clin Periodontol. 2006;33:524–528. doi: 10.1111/j.1600-051X.2006.00944.x. [DOI] [PubMed] [Google Scholar]
- 49.Wang C, Zhao H, Xiao L, et al. Association between vitamin D receptor gene polymorphisms and severe chronic periodontitis in a Chinese population. J Periodontol. 2009;80:603–608. doi: 10.1902/jop.2009.080465. [DOI] [PubMed] [Google Scholar]
- 50.Kardesler L, Buduneli N, Cetinkalp S, Kinane DF. Adipokines and inflammatory mediators after initial periodontal treatment in patients with type 2 diabetes and chronic periodontitis. J Periodontol. 81:24–33. doi: 10.1902/jop.2009.090267. [DOI] [PubMed] [Google Scholar]