Abstract
Objective
Overcoming racial differences in acute coronary syndrome (ACS) outcomes is a strategic goal for US healthcare. Genetic polymorphisms in the adrenergic pathway appear to explain some outcome differences by race in other cardiovascular diseases treated with β-adrenergic receptor-blockade (BB). Whether these genetic variants are associated with survival among ACS patients treated with BB, and if this differs by race, is unknown.
Background
BB after ACS is a measure of quality care, but the effectiveness across racial groups, is less clear.
Methods
A prospective cohort of 2,673 ACS patients (2,072 Caucasian; 601 African Americans) discharged on BB from 22 U.S. hospitals were followed for 2 years. Subjects were genotyped for polymorphisms in ADRB1, ADRB2, ADRA2C, and GRK5. We used proportional hazards regression to model the effect of genotype on mortality, stratified by race and adjusted for baseline factors.
Results
The overall 2-year mortality rate was 7.5% for Caucasians and 16.7% for African Americans. The prognosis associated with different genotypes in these BB-treated patients differed by race. In Caucasians, ADRA2C 322-325 deletion (D) carriers had significantly lower mortality as compared with homozygous individuals lacking the deletion (HR 0.46; CI 0.21, 0.99; p=0.047; race-by-genotype interaction p= 0.053). In African Americans, the ADRB2 16R allele was associated with significantly increased mortality (HR for RG vs. GG =2.10; CI 1.14, 3.86; RR vs. GG =2.65; CI 1.38, 5.08; p=0.013; race-by-genotype interaction p=0.096).
Conclusions
Adrenergic pathway polymorphisms are associated with mortality in ACS patients receiving BB in a race-specific manner. Understanding the mechanism by which different genes impact post-ACS mortality differently in Caucasian and African Americans may illuminate opportunities to improve BB therapy in these groups.
More than 1.6 million patients are hospitalized in North America each year with acute coronary syndrome (ACS; acute myocardial infarction (MI) or unstable angina) (1). Evidence-based practice guidelines, developed jointly by the American Heart Association and American College of Cardiology, recommend that β-blocker (BB) therapy be initiated in all patients without a contraindication (2, 3) and administration of BB after MI is an accepted performance measure of high-quality healthcare (4). These recommendations are based on results of older trials with less diverse cohorts, while subsequent larger studies in other racial groups,(5) as well as meta-analyses,(6) have questioned the consistency of BB benefit across the spectrum of MI patients, with the potential for hazard observed in at least one study (5). It has been hypothesized that a portion of this variability may be due to genetic variation, which is of particular interest given the intersection of race with genetics and the disparities in ACS outcomes in the United States (7). Identifying genetic predictors of BB-related outcomes in ACS patients could allow more targeted prognostic risk estimates and, hopefully, support more appropriate use of therapy to reduce the public health burden of ACS and heterogeneity of outcomes.
Support for genetic variation contributing to differences in BB response is available in the case of systolic heart failure, where despite the fact that BBs are a cornerstone of standard therapy due to their overall mortality benefit,(8–10) substantial heterogeneity of individual responses, related in-part to genetic sequence variants in the adrenergic pathway, have been documented (11–13). For example, functional genetic variants within genes encoding the β-adrenergic receptor 1 (ADRB1 R389G; rs1801253), the β-adrenergic receptor 2 (ADRB2 G16R (rs1042713) and ADRB2 Q27E (rs1042714)), the α-2c receptor (ADRA2C 322-325 deletion; rs2234888), and, more recently, G-protein receptor kinase 5 (GRK5 L41Q; rs17098707), a critical regulator of β-receptor function, are associated with BB response in heart failure (13–18). These data raise the question as to whether functional adrenergic genetic variants may influence BB-related outcomes after ACS. Consistent with this notion is our previous work, from an exploratory analysis of a small ACS cohort, which suggested an association of ADRB2 genotypes with survival in patients treated with BB (19). However, this study was inadequately powered to accurately define the importance of each variant within racial subgroups. The fact that most of the candidate variants differ in allele frequency in Caucasians, as compared with African Americans, underscores the importance of defining race-specific associations between genetic variants and outcomes. To address this existing gap in knowledge, we report the results of a large, prospective, longitudinal analysis of the effect of genetic variants on mortality in patients who suffered an ACS and were treated with BB, stratified by race. This analysis represents the first comprehensive study of previously-reported functional adrenergic pathway genetic variants in an ACS cohort.
METHODS
Subjects
Between 3/2001 and 12/2008, 8,037 patients with acute coronary syndromes (7,517 acute myocardial infarction (MI), 520 unstable angina (UA)) from 31 U.S. hospitals were prospectively screened and enrolled into 3 consecutive observational cohort studies (Appendix): 1) INvestigation oF Outcomes from acute coronary syndRoMes (INFORM, N=1,199) from 3/2001 to 10/2002, 2) Prospective Registry Evaluating outcomes after Myocardial Infarction: Events and Recovery (PREMIER N=2,498) from 1/2003 to 6/2004 and 3) Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status (TRIUMPH, N=4,340) from 4/2005 to 12/2008, as previously described (19–22). MI patients were identified by an elevated troponin blood test and either diagnostic electrocardiogram (EKG) changes or ischemic symptoms. UA patients had a normal troponin, but presented with EKG changes (LBBB 4%, ST-elevations 9%, ST-depressions 12%, T-wave inversions 22%) and cardiac symptoms, defined as new onset angina (<2 months) of at least Canadian Cardiovascular Society Classification (CCSC) class III, prolonged (>20 minutes) rest angina, recent (<2 months) worsening of angina, or angina that occurred within 2 weeks of a previous MI.(23) To further increase the specificity of the unstable angina diagnosis, those patients with a diagnostic study that excluded obstructive coronary disease, (e.g. coronary angiography, nuclear or echocardiographic stress testing) (n=125) or confirmed an alternative explanation for their presentation (e.g. esophagogastroduodenoscopy) were excluded. Three physicians adjudicated the charts of those patients with diagnostic uncertainty (n=45) and attained consensus on the final diagnosis.
Within these combined cohorts, a subset of 3,373 patients were approached for, and consented to, genetic testing and were genotyped for adrenergic pathway polymorphisms. The genetics cohort was representative of the entire cohort (24). Of these, 2,946 (87%) were discharged alive on beta-blockers and were included in the present analyses. We further excluded 94 patients who were either transferred to other hospitals or who left against medical advice because their use of BB at discharge could not be definitively ascertained. Finally, given the large frequency differences for several genotypes of interest across race, we restricted the analyses to self-identified Caucasian and African-American patients, yielding a final sample size of 2,673 patients (2,469 MI; 204 UA; 2,072 Caucasian; 601 African Americans) from 22 centers.
Each patient was prospectively interviewed during hospitalization to ascertain their socio-demographic (including self-identified race), economic and health status characteristics. Detailed chart abstractions were performed to obtain patients’ medical history, laboratory results, disease severity and the processes of inpatient care. All three studies received Institutional Review Board approval at all participating sites and written informed consent was obtained from each participant.
Mortality Assessment
The Social Security Administration Death Master File was queried to determine patients’ vital status as of 12/30/2009 (http://www.ntis.gov/products/ssa-dmf.asp) and was available for all patients in this study.
Genotyping
DNA was isolated and purified from whole blood using the Qiagen QIAamp DNA purification kit (Quiagen, Germantown, MD). The DNA segments containing the region of interest were amplified with the polymerase chain reaction (PCR). PCR primers were designed using Primer3 online software (http://fokker.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi),(25) and pyrosequencing primers were designed using the Pyrosequencing SNP Primer Design Version 1.01 software (http://www.pyrosequencing.com). Before use, PCR primer sequences were screened across the human genome using the NCBI Blast program to ensure their specificity for the gene of interest. PCR was carried out using Amplitaq Gold PCR master mix (ABI, Foster City, CA), 1 pmole of each primer (IDT, Coralville, IA), and 1ng DNA. The PCR primers and conditions are listed in Supplemental Table S1. Pyrosequencing was performed using the PSQ HS 96A system with MA v2.0 software, as previously described.(26) Data were automatically transferred from the PSQ HS 96A to a Microsoft Access database for permanent storage and merging with the clinical datasets through SAS v9.1.
Statistical Analyses
Baseline and follow-up characteristics were compared by genotype. Categorical data are reported as frequencies and differences between groups were compared with chi-square or Fisher’s exact tests, as appropriate. Continuous data are reported as mean ± standard deviation (SD) and differences between groups were tested using one-way analysis of variance. Hardy-Weinberg equilibrium was assessed with chi-square test or Monte Carlo permutation with 10,000 iterations, as appropriate. Kaplan-Meier estimates and Cox proportional hazards models were used to describe the effect of genotype on patients’ survival. A dominant model was used for ADRA2C 322-325 deletion and GRK5 L41Q genotypes due to the low MAF of these variants. For all other genotypes, we used an additive model. Follow-up began at the time of discharge from the index hospitalization. Stratified proportional hazards models were used, adjusting for study and site. To estimate the independent contribution of genotype, after adjusting for potential confounders and other clinical predictors, the proportional hazards models included covariates were that were thought to be clinically important (age, sex, type of ACS, hypertension, diabetes, heart failure, chronic obstructive pulmonary disease, coronary angiography, and coronary revascularization) as well as those that differed significantly by genotype (Supplemental Tables S1–S4). To mitigate the possibility of “over-fitting”, we also tested models adjusted for age and sex only, for the variants which had significant associations (minimally-adjusted model, Supplemental Table S6); these were consistent with the results of the primary analysis.
Analyses were performed separately in Caucasians and African-Americans to minimize the risk of false positive findings due to population stratification. We further assessed the issue of population stratification using a subgroup of 492 TRIUMPH subjects (382 Caucasian; 110 African American) who were genotyped as part of a separate project with a custom array containing 3,351 SNPs and 100 ancestry informative markers (27, 28). Using these SNPs, we estimated 10 principal components with Eigenstrat and repeated our analyses in this subgroup to assess whether population stratification impacted our findings. Including principal components in the model did not meaningfully alter the findings, providing support for our categorization of race. For primary effects, p-values <0.05 were considered statistically significant(29). Analyses were performed with SAS version 9.2 (SAS Institute, Inc., Cary, NC) and R version 2.11.1 (13, 30).
RESULTS
Baseline characteristics of the cohort, stratified by race, are shown in Table 1. Types of BBs prescribed at discharge are shown in Table 2. 91.6% of the cohort was discharged on either Metoprolol (82%; mean dose 73 +/− 58 mg/day) or Carvedilol (9.6% mean dose 18 +/− 14 mg/day); dose distribution in Table 3. The 2-year mortality rate was 7.5% for Caucasians (N=152) and 16.7% for African Americans (N=96). We genotyped the study subjects for functionally significant polymorphisms within the adrenergic pathway that have been previously linked to outcomes after BB use, specifically variants within the genes encoding β-adrenergic receptors 1 (ADRB1) and 2 (ADRB2), α-2c adrenergic receptor (ADRA2C), and G-protein receptor kinase 5 (GRK5). For all variants, genotype call rates were greater than 96%. Variants, genotype frequencies in Caucasians and African Americans, and the test of HWE are shown in Table 4. Consistent with prior reports, GRK5 L41 and ADRA2C deletion allele frequencies were much higher among African Americans as compared with Caucasians (15). The frequencies of the other genotypes examined were similar to those previously reported (31).
Table 1.
Patient Characteristics by Race
| White/Caucasian n = 2072 | Black/African American n = 601 | P-Value | |
|---|---|---|---|
| Age | 59.8 ± 12.3 | 56.9 ± 11.9 | < 0.001 |
| Sex | < 0.001 | ||
| Male | 1476 (71.2%) | 324 (53.9%) | |
| Female | 596 (28.8%) | 277 (46.1%) | |
| BMI | 29.4 ± 6.0 | 30.0 ± 7.3 | 0.043 |
| Smoking Status | < 0.001 | ||
| Current (<30d) | 782 (37.9%) | 273 (45.9%) | |
| Former (>=30d) | 702 (34.0%) | 148 (24.9%) | |
| Never (or <100 total) | 580 (28.1%) | 174 (29.2%) | |
| Dyslipidemia | 1072 (51.7%) | 262 (43.6%) | < 0.001 |
| Hypertension | 1255 (60.6%) | 463 (77.0%) | < 0.001 |
| Diabetes | 518 (25.0%) | 246 (40.9%) | < 0.001 |
| Prior PCI | 457 (22.1%) | 94 (15.6%) | < 0.001 |
| Prior CABG | 275 (13.3%) | 52 (8.7%) | 0.002 |
| Prior MI | 414 (20.0%) | 147 (24.5%) | 0.018 |
| Peripheral Arterial Disease | 109 (5.3%) | 29 (4.8%) | 0.671 |
| Prior CVA | 67 (3.2%) | 44 (7.3%) | < 0.001 |
| Prior TIA | 47 (2.3%) | 9 (1.5%) | 0.245 |
| Chronic Heart Failure | 109 (5.3%) | 110 (18.3%) | < 0.001 |
| Chronic Renal Failure | 74 (3.6%) | 106 (17.6%) | < 0.001 |
| Chronic Lung Disease | 154 (7.4%) | 59 (9.8%) | 0.057 |
| LV Systolic Function | < 0.001 | ||
| Normal | 1178 (57.7%) | 367 (61.8%) | |
| Mild | 458 (22.4%) | 89 (15.0%) | |
| Moderate | 288 (14.1%) | 51 (8.6%) | |
| Severe | 117 (5.7%) | 87 (14.6%) | |
| Diseased Vessels | < 0.001 | ||
| 0 | 101 (5.1%) | 82 (17.5%) | |
| 1 | 818 (41.6%) | 192 (41.0%) | |
| 2 | 562 (28.6%) | 96 (20.5%) | |
| 3 | 485 (24.7%) | 98 (20.9%) | |
| ACS Type | < 0.001 | ||
| STEMI | 1006 (48.6%) | 166 (27.6%) | |
| NSTEMI | 912 (44.0%) | 385 (64.1%) | |
| UA | 154 (7.4%) | 50 (8.3%) | |
| Beta Blocker on Arrival | 638 (30.8%) | 224 (37.3%) | 0.003 |
| GRACE Risk Score | 97.9 ± 29.1 | 100.7 ± 30.1 | 0.043 |
| Revascularization Type | < 0.001 | ||
| None | 377 (18.2%) | 308 (51.2%) | |
| PCI | 1513 (73.0%) | 254 (42.3%) | |
| CABG | 182 (8.8%) | 39 (6.5%) |
Table 2.
BB on Discharge
| Total | |
|---|---|
| n = 2673 | |
| METOPROLOL | 2193 (82.0%) |
| CARVEDILOL | 256 (9.6%) |
| ATENOLOL | 191 (7.1%) |
| LABETALOL | 19 (0.7%) |
| SOTALOL | 8 (0.3%) |
| NADOLOL | 3 (0.1%) |
| PROPRANOLOL | 3 (0.1%) |
| PINDOLOL | 2 (0.1%) |
| BETAXOLOL | 1 (0.0%) |
| BISOPROLOL | 1 (0.0%) |
| BB NOT RECORDED | 14 (0.5%) |
Table 3.
Dose Distribution of Metoprolol and Carvedilol on Discharge
| Carvedilol | |
|---|---|
| Dose (mg/day) | Percent |
| 3.125 | 6.25 |
| 6.25 | 23.75 |
| 12.5 | 32.08 |
| 25 | 25.42 |
| 50 | 12.08 |
| 62.5 | 0.42 |
| Metoprolol | |
| Dose (mg/day) | Percent |
| 12.5 | 2.49 |
| 25 | 22.06 |
| 37.5 | 0.23 |
| 50 | 39.52 |
| 75 | 1.93 |
| 100 | 21.97 |
| 112.5 | 0.05 |
| 125 | 0.05 |
| 150 | 3.36 |
| 200 | 6.86 |
| 300 | 0.83 |
| 400 | 0.64 |
Table 4.
Genotype frequencies and H-W p-values
| SNP | Genotype | % Frequency Caucasian | HW P-value | % Frequency African Americans | HW P-value |
|---|---|---|---|---|---|
| ADRA2C INDEL | II | 90.46 | 0.2033 | 38.63 | 0.0334 |
| ID | 8.99 | 42.51 | |||
| DD | 0.55 | 18.85 | |||
| ADRB1 R389G | RR | 52.85 | 0.1747 | 34.77 | 0.2237 |
| RG | 38.63 | 50.43 | |||
| GG | 8.52 | 14.80 | |||
| ADRB2 G16R | GG | 37.01 | 0.5009 | 23.84 | 0.6096 |
| GR | 48.24 | 48.89 | |||
| RR | 14.75 | 27.27 | |||
| ADRB2 Q27E | 34.39 | 0.3663 | 67.98 | 0.3525 | |
| QE | 49.33 | 28.25 | |||
| EE | 16.28 | 3.77 | |||
| GRK5 Q41L | 96.50 | 0.3427 | 54.99 | 0.2403 | |
| QL | 3.35 | 37.06 | |||
| LL | 0.15 | 7.95 |
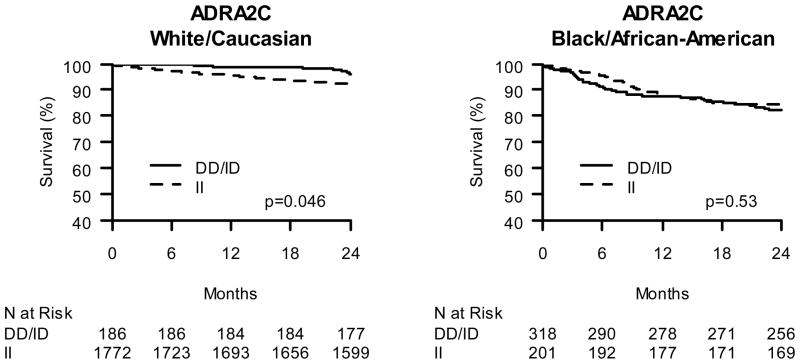
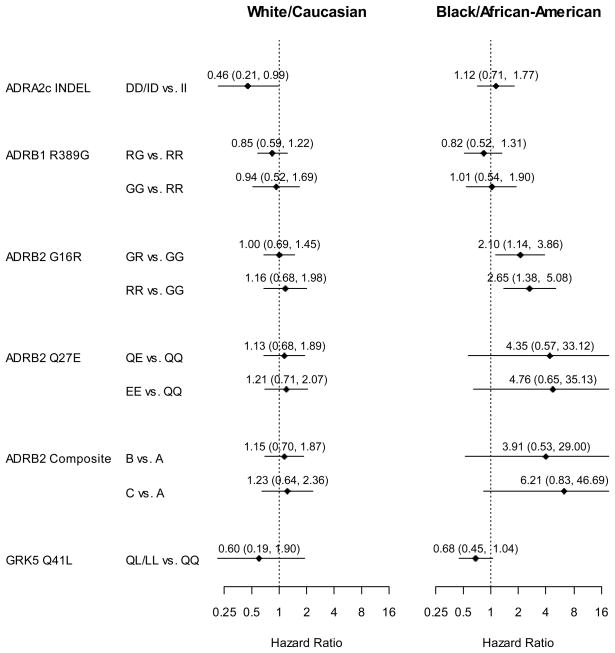
Among the 2,072 Caucasian subjects, 2-year mortality differences across genotypes were assessed Kaplan-Meier estimates (Figures 1 to 4A) and proportional hazards regression modeling (Figure 5) with adjustment for a priori selected baseline characteristics and clinical characteristics that differed significantly between genotype groups (Supplemental tables S1 to S5). The ADRA2C 322-325 insertion/deletion (I/D) genotype was significantly associated with 2-year mortality among Caucasians in the adjusted analyses (HR 0.46; CI 0.21, 0.99; p=0.047; Figure 5) with ADRA2C ID and ADRA2C DD (i.e. deletion carriers) subjects having greater survival compared with ADRA2C II homozygous individuals. In contrast, none of the other genetic variants tested were significantly associated with 2-year mortality among Caucasians in either adjusted or unadjusted analyses (all p>0.3).
Figure 1.
Kaplan-Meier Curves of ADRA2C insertion/deletion effect on post-ACS 2-year survival. A. Caucasian β-blocker treated ACS subjects stratified by ADRA2C deletion carrier status. B. African American β-blocker treated ACS subjects stratified by ADRA2C deletion carrier status.
Figure 4.
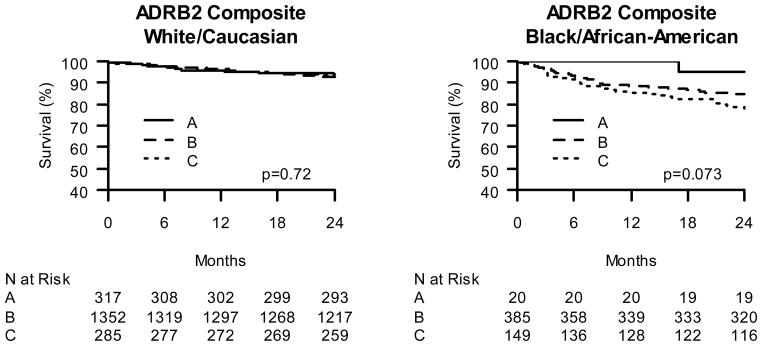
Kaplan-Meier Curves of ADRB2 composite genotype effect on post-ACS 2-year survival. A. Caucasian β-blocker treated ACS subjects stratified by ADRB2 composite genotype status. B. African American β-blocker treated ACS subjects stratified by ADRB2 composite genotype status. ADRB2 composite genotype as follows: A: subjects homozygous for both ADRB2 G16 allele and ADRB2 E27 allele; C: subjects homozygous for both ADRB2 R16 allele and ADRB2 Q27 allele; B: subjects heterozygous for ADRB2 G16R and/or ADRB2 Q27E.
Figure 5. Model-Adjusted Hazard Ratios for 2-Year All Cause Mortality.
ADRB2 composite genotype as in Figure 4.
In similarly constructed models, the association of each polymorphism with mortality was tested in a total of 601 African Americans (Figures 1–4B and Figure 5). While neither the ADRB1 nor ADRA2C variants were associated with mortality (p>0.5), ADRB2 variants were. The ADRB2 G16R variant was significantly associated with mortality among BB-treated African Americans in both unadjusted and adjusted analyses. Compared to African American ADRB2 G16 allele homozygous individuals treated with BB, ADRB2 R16 allele carriers had greater mortality in both unadjusted (RG vs. GG HR=1.74; CI 0.96, 3.16; RR vs. GG HR= 2.25; CI1.21, 4.21; p=0.039; Figure 3B) and fully-adjusted analyses (RG vs. GG HR=2.10; CI1.14, 3.86; RR vs. GG HR=2.65; CI 1.38, 5.08; p=0.013; Figure 5). Notably, patients homozygous for the R allele had the greatest risk and heterozygous patients had an intermediate risk, consistent with a gene-dose effect.
Figure 3.
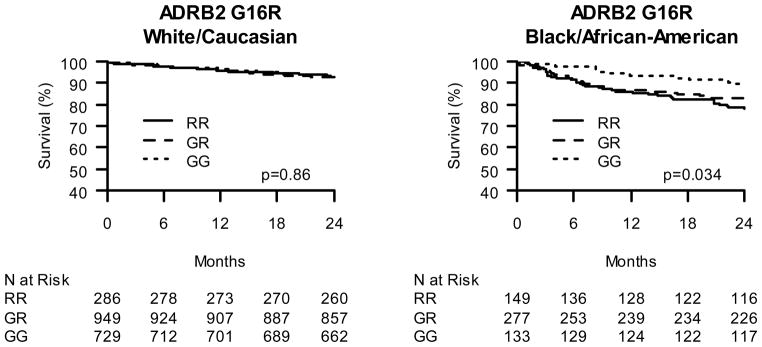
Kaplan-Meier Curves of ADRB2 G16R effect on post-ACS 2-year survival. A. Caucasian β-blocker treated ACS subjects stratified by ADRB2 G16R status. B. African American β-blocker treated ACS subjects stratified by ADRB2 G16R status.
Since our pilot data suggested that both of the ADRB2 variants (ADRB2 G16R and ADRB2 Q27E), which are in linkage disequilibrium, may contribute to BB related outcomes, we re-tested the mortality association of the compound genotype that most strongly correlated with risk in this earlier study. Among African Americans treated with BB, the results were similar to our pilot data, whereas this was not the case in Caucasians (Figure 4). In adjusted analyses among African Americans, the compound genotype was associated with a 6-fold risk of mortality in the high-risk group and approximately a 4-fold risk in the intermediate group (Figure 5, p=0.048).
In African Americans, the GRK5 Q41L variant had a borderline-significant association with mortality in adjusted analyses. While not achieving statistical significance, the GRK5 L41 African American patients treated with BB trended toward lower mortality as compared with homozygous GRK5 Q41 patients (adjusted HR 0.68; CI 0.45–1.04; p= 0.07). We also tested for, but found no significant, gene-gene interaction between ADRB2 and GRK5 and outcomes in African American ACS patients (interaction p-value between ADRB2 G16R and GRK5 Q41L = 0.86).
Since each of the two sequence variants that showed a significant survival association did so in only one racial group, we performed formal interaction tests to further assess the significance of the racial distinction. The race-by-genotype interaction approached significance for both ADRA2C I/D (p = 0.053) and ADRB2 G16R (p= 0.096).
DISCUSSION
In accordance with consensus guidelines and quality performance measures, it is recommended that all patients without contra-indications be discharged on BB therapy after MI (4, 32–34). However, the consistent efficacy of BB in all groups has been questioned, and this variability may be related, in part, to genetic heterogeneity (5), (6). Our data suggest that variability in post-ACS outcomes among BB-treated patients is associated with genetic variation in the adrenergic pathway. Importantly, we identified different prognostically-important genes in African Americans, as compared with Caucasians. Specifically, the ADRA2C I/D genotype was significantly associated with 2-year mortality in post-ACS Caucasians treated with BB while the ADRB2 G16R and GRK5 Q41L genotypes were associated with 2-year mortality in post-ACS African Americans treated with BB. These findings suggest that race-specific adrenergic-pathway variants may be useful in defining prognosis after BB therapy for Caucasians and African American ACS patients.
It is important to note that the race specificity of our findings is not due to differences in allele frequency. For example, although the ADRA2C D-allele is much more common in African Americans, there was no risk relationship between this gene and mortality in these patients, while it was associated with risk in Caucasians (race-by-genotype interaction p= 0.053). This contrasts with our pre-study hypothesis that this allele likely would have similar effects regardless of race. Our data suggest something more complex; that the allele effects may be specific to the Caucasian genetic environment. This is a novel observation requiring replication. If verified in other populations, then mechanistic studies are needed to understand whether ancestry specific linkage or other genetic modifiers might explain the observed differences in allelic associations across race.
Beyond the prognostic difference between races, the association of the ADRA2C I/D variant with 2-year mortality in Caucasian patients discharged on BB is the first report of this finding in ACS patients. ADRA2C D creates a hypo-functioning form of this presynaptic inhibitory receptor, which has been previously associated with increased catecholemine release in vitro (35) and in vivo (36). Consistent with this, the ADRA2C D allele has also been previously associated with increased risk of heart failure (35). Our findings could be consistent with this line of evidence considering that patients with the ADRA2C D allele may have a higher adrenergic state and thus display enhanced benefit from BB(37), potentially resulting in the lower mortality we observed when comparing genotype groups treated with BB. On the other hand, our findings contrast with recent observations in heart failure patients where the BB bucindolol provided survival benefit in ADRA2C II homozygous individuals, but not in deletion carriers, possibly due to enhanced sympatholysis in ADRA2C D carriers (18). This inconsistency may be attributable to differences between the pathophysiologic role of adrenergic signaling in ACS patients as compared with heart failure. For example, it has been proposed that sympatholysis may be particularly detrimental in heart failure patients (38). Alternatively, the inconsistency could be due to pharmacological differences in the specific agents used (no bucindolol in the present study (Table 2) vs. 100% bucindolol use in BEST); especially given that bucindolol is unique among BB agents for causing sympatholysis. Our findings warrant further investigation of the role of genetic variation in ADRA2C, and the possible variability by type of BB agent, in Caucasian ACS patients.
Beta-adrenergic receptor signaling is downregulated by G-protein receptor kinases (GRKs) (39). GRK 5 phosphorylates, uncouples, and internalizes β1-adrenergic receptors, and the GRK5 L41 variant has greater activity than the GRK5 Q41 variant for all of these functions (15). We previously described a pharmacogenomic effect of GRK5 in subjects with ACS (15). The GRK5 Q41L polymorphism was previously found to be a determinant of beta-blocker responsiveness in heart failure among African Americans, with a protective β-blocker-mimetic effect seen in GRK5 L41 allele carriers (13). Similarly, we found a strong trend toward improved outcomes in post-ACS African American patients carrying this allele. Our findings are also consistent with our previous observations in heart failure patients in terms of the magnitude of effect; roughly one-half the mortality risk as compared to the GRK5 Q41 allele homozygous patients. The borderline statistical significance, with a similar effect size, likely reflects suboptimal power for this particular analysis, as well as our inability to contrast BB-treated patients with those not treated with BB (due to the near universal compliance with BB as a performance measure of quality in ACS).
In this study, the ADRB2 G16R genotype was significantly associated with mortality in African American ACS patients, such that patients homozygous for the R allele had greatest risk and heterozygous patients had an intermediate risk, consistent with a gene-dose effect and our previous findings (19). There was an even greater stratification in risk when both functional G16R and Q27E ADRB2 variants were accounted for, showing approximately a 4-fold and 6-fold risk in the intermediate and high-risk groups, respectively. However, unlike our pilot data, in which the association was found in a much smaller cohort that included both African American and Caucasian patients, our current analysis suggests that the association is specific to African Americans. Due to this distinction, the ADRB2 association is not a strict validation and requires confirmation in additional, adequately sized, cohort(s) of African American ACS patients. Nonetheless, these data extend previous reports and are the first description, to our knowledge, of an African American-specific effect of ADRB2 genotype in ACS patients. Conversely, the lack of association in Caucasians is strong, suggesting that additional inquiry into this association is unlikely to reveal a strong effect of ADRB2 genotype among Caucasian ACS patients treated with BB.
Our study should be interpreted in the context of several potential limitations. First, due to the high level of adherence to guidelines recommendations, only a small number of subjects were not treated with BB. This precluded a formal analysis to assess the interaction of genotype with BB treatment. Thus we cannot specifically comment on BB efficacy, and have focused solely on the genetic association with outcomes in BB-treated patients. In light of these data, however, it may be worthwhile to consider a randomized trial of BB in the genetic subgroups that show poor outcomes with BB therapy in order to exclude the possibility of harm from BB treatment. Alternatively, new study approaches such as utilizing quantified drug exposure metrics (as opposed to simply present vs. absent), or identification of a non-US cohort of ACS patients where BB treatment is less common, might show greater variability in BB use (i.e. more subjects off BB) and allow drug-by-genotype interaction analyses. Despite this inherent limitation, our findings are important as they identify genetic subgroups with worse outcomes despite BB treatment, and in whom more aggressive interventions to improve mortality might be warranted. An additional limitation is that we had insufficient sample size to differentiate between BB agents and thus agent-specific effects are not obtainable from this study. A third limitation is that the current study includes 735 subjects previously described in our preliminary analysis testing the hypothesis that ADRB1 and ADRB2 polymorphisms would be associated with mortality in BB-treated patients (19). However, in the current study, we have increased the total cohort by more than 4-fold (including a quadrupling of the African Americans population), enabling us to examine race-specific associations between genetic variation and mortality. We have also included additional published variants in our analysis (GRK5 and ADRA2C), enabling a more complete analysis of genes within the adrenergic pathway and have performed analyses that provide evidence that population stratification is not confounding our findings. Finally, we were not able to account for changes in medications over time. However classifying patients by discharge medication status is a well recognized and often used approach, since most patients remain on their discharge regimen after hospitalization (40).
In summary, our findings represent the first comprehensive analysis of established functional genetic variants within adrenergic pathway genes in an ACS cohort and show that several of these variants are associated with mortality among BB-treated patients and that these associations vary by race. Among Caucasian patients that have suffered an ACS and are treated with BB, ADRA2C II homozygous individuals had increased mortality as compared with ADRA2C ID and ADRA2C DD subjects. In African American ACS patients treated with BB, GRK5 L41 allele carriers trended towards having lower mortality compared to GRK5 Q41 homozygous patients, while ADRB2 16R allele carriers had significantly increased mortality in a gene-dose-response manner. Further study is needed to replicate our findings in African American patients; randomized trials of post-ACS BB treatment in race-specific, genotype-defined subgroups may be warranted to insure the benefits of BB therapy in these high-risk individuals.
Supplementary Material
Figure 2.
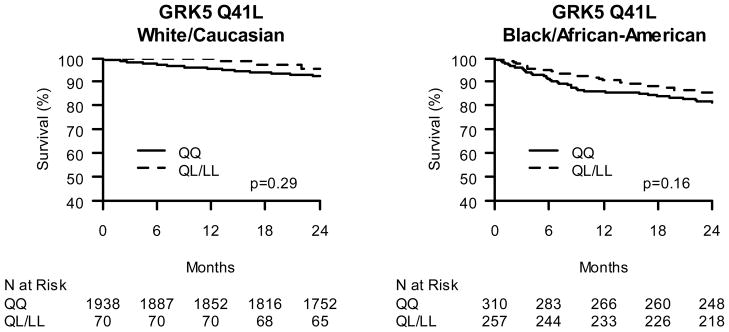
Kaplan-Meier Curves of GRK5 Gln/Leu 41 effect on post-ACS 2-year survival. A. Caucasian β-blocker treated ACS subjects stratified by GRK5 41Leu carrier status. B. African American β-blocker treated ACS subjects stratified by GRK5 41Leu carrier status.
Acknowledgments
Funding Sources:
This work and Dr. Cresci’s effort are supported in part by the National Institutes of Health (Cresci NR013396 and NIH Specialized Center for Clinically-Oriented Research (SCCOR) in Cardiac Dysfunction and Disease P50 HL077113), and The Longer Life Foundation. Dr. Lanfear’s effort is supported in part by the National Institutes of Health (Lanfear HL085124, HL103871).
Reference List
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of patients with unstable angina) Circulation. 2000;102:1193–209. doi: 10.1161/01.cir.102.10.1193. [DOI] [PubMed] [Google Scholar]
- 3.Antman EM, Hand M, Armstrong PW, et al. 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration With the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee. Circulation. 2008;117:296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- 4.Krumholz HM, Anderson JL, Bachelder BL, et al. ACC/AHA 2008 performance measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to develop performance measures for ST-elevation and non-ST-elevation myocardial infarction): developed in collaboration with the American Academy of Family Physicians and the American College of Emergency Physicians: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, Society for Cardiovascular Angiography and Interventions, and Society of Hospital Medicine. Circulation. 2008;118:2596–648. doi: 10.1161/CIRCULATIONAHA.108.191099. [DOI] [PubMed] [Google Scholar]
- 5.Chen ZM, Pan HC, Chen YP, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1622–32. doi: 10.1016/S0140-6736(05)67661-1. [DOI] [PubMed] [Google Scholar]
- 6.Freemantle N, Cleland J, Young P, Mason J, Harrison J. beta Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318:1730–7. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asher CR, Moliterno DJ, Bhapkar MV, et al. Association of race with complications and prognosis following acute coronary syndromes. Am J Cardiol. 2004;94:792–4. doi: 10.1016/j.amjcard.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 8.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- 9.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 10.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–55. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW. Heart failure in African Americans: a cardiovascular engima. J Card Fail. 2000;6:183–6. doi: 10.1054/jcaf.2000.17610. [DOI] [PubMed] [Google Scholar]
- 12.Yancy CW. Heart failure in African Americans. Am J Cardiol. 2005;96:3i–12i. doi: 10.1016/j.amjcard.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Cresci S, Kelly RJ, Cappola TP, et al. Clinical and genetic modifiers of long-term survival in heart failure. J Am Coll Cardiol. 2009;54:432–44. doi: 10.1016/j.jacc.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liggett SB, Mialet-Perez J, Thaneemit-Chen S, et al. A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc Natl Acad Sci U S A. 2006;103:11288–93. doi: 10.1073/pnas.0509937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liggett SB, Cresci S, Kelly RJ, et al. A GRK5 polymorphism that inhibits beta-adrenergic receptor signaling is protective in heart failure. Nat Med. 2008;14:510–7. doi: 10.1038/nm1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bristow MR. beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–69. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 17.Shin J, Lobmeyer MT, Gong Y, et al. Relation of beta(2)-adrenoceptor haplotype to risk of death and heart transplantation in patients with heart failure. Am J Cardiol. 2007;99:250–5. doi: 10.1016/j.amjcard.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Bristow MR, Murphy GA, Krause-Steinrauf H, et al. An alpha2C-adrenergic receptor polymorphism alters the norepinephrine-lowering effects and therapeutic response of the beta-blocker bucindolol in chronic heart failure. Circ Heart Fail. 2010;3:21–8. doi: 10.1161/CIRCHEARTFAILURE.109.885962. [DOI] [PubMed] [Google Scholar]
- 19.Lanfear DE, Jones PG, Marsh S, Cresci S, McLeod HL, Spertus JA. Beta2-adrenergic receptor genotype and survival among patients receiving beta-blocker therapy after an acute coronary syndrome. JAMA. 2005;294:1526–33. doi: 10.1001/jama.294.12.1526. [DOI] [PubMed] [Google Scholar]
- 20.Spertus J, Safley D, Garg M, Jones P, Peterson ED. The influence of race on health status outcomes one year after an acute coronary syndrome. J Am Coll Cardiol. 2005;46:1838–44. doi: 10.1016/j.jacc.2005.05.092. [DOI] [PubMed] [Google Scholar]
- 21.Morgan TM, Krumholz HM, Lifton RP, Spertus JA. Nonvalidation of reported genetic risk factors for acute coronary syndrome in a large-scale replication study. JAMA. 2007;297:1551–61. doi: 10.1001/jama.297.14.1551. [DOI] [PubMed] [Google Scholar]
- 22.Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C, Krumholz H. The Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER)--evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151:589–97. doi: 10.1016/j.ahj.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 23.Braunwald E. Unstable angina. A classification. Circulation. 1989;80:410–4. doi: 10.1161/01.cir.80.2.410. [DOI] [PubMed] [Google Scholar]
- 24.Lanfear DE, Jones PG, Cresci S, Tang F, Rathore SS, Spertus JA. Factors influencing patient willingness to participate in genetic research after a myocardial infarction. Genome Med. 2011;3:39. doi: 10.1186/gm255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 26.Marsh S, King CR, Garsa AA, McLeod HL. Pyrosequencing of clinically relevant polymorphisms. Methods Mol Biol. 2005;311:97–114. doi: 10.1385/1-59259-957-5:097. [DOI] [PubMed] [Google Scholar]
- 27.Collins-Schramm HE, Phillips CM, Operario DJ, et al. Ethnic-difference markers for use in mapping by admixture linkage disequilibrium. Am J Hum Genet. 2002;70:737–50. doi: 10.1086/339368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cresci S, Wu J, Province MA, et al. Peroxisome Proliferator-Activated Receptor Pathway Gene Polymorphism Associated With Extent of Coronary Artery Disease in Patients With Type 2 Diabetes in the Bypass Angioplasty Revascularization Investigation 2 Diabetes Trial. Circulation. 2011;124:1426–34. doi: 10.1161/CIRCULATIONAHA.111.029173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleiss JL, Levin BA, Paik MC. Statistical methods for rates and proportions. 3. Hoboken, N.J: J. Wiley; 2003. [Google Scholar]
- 30.R Development Core Team. R Foundation for Statistical Computing. Vienna, Austria: 2008. A language and environment for statistical computing. Ref Type: Internet Communication. [Google Scholar]
- 31.Lanfear DE, Marsh S, Cresci S, Spertus JA, McLeod HL. Frequency of compound genotypes associated with beta-blocker efficacy in congestive heart failure. Pharmacogenomics. 2004;5:553–8. doi: 10.1517/14622416.5.5.553. [DOI] [PubMed] [Google Scholar]
- 32.Krumholz HM, Anderson JL, Brooks NH, et al. ACC/AHA Clinical Performance Measures for Adults With ST-Elevation and Non-ST-Elevation Myocardial Infarction. A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Performance Measures on ST-Elevation and Non-ST-Elevation Myocardial Infarction) Circulation. 2006 doi: 10.1161/CIRCULATIONAHA.106.172860. [DOI] [PubMed] [Google Scholar]
- 33.Spertus JA, Eagle KA, Krumholz HM, Mitchell KR, Normand SL. American College of Cardiology and American Heart Association methodology for the selection and creation of performance measures for quantifying the quality of cardiovascular care. Circulation. 2005;111:1703–12. doi: 10.1161/01.CIR.0000157096.95223.D7. [DOI] [PubMed] [Google Scholar]
- 34.Cannon CP, Battler A, Brindis RG, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) J Am Coll Cardiol. 2001;38:2114–30. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 35.Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med. 2002;347:1135–42. doi: 10.1056/NEJMoa020803. [DOI] [PubMed] [Google Scholar]
- 36.Gerson MC, Wagoner LE, McGuire N, Liggett SB. Activity of the uptake-1 norepinephrine transporter as measured by I-123 MIBG in heart failure patients with a loss-of-function polymorphism of the presynaptic alpha2C-adrenergic receptor. J Nucl Cardiol. 2003;10:583–9. doi: 10.1016/j.nuclcard.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Lobmeyer MT, Gong Y, Terra SG, et al. Synergistic polymorphisms of beta1 and alpha2C-adrenergic receptors and the influence on left ventricular ejection fraction response to beta-blocker therapy in heart failure. Pharmacogenet Genomics. 2007;17:277–82. doi: 10.1097/FPC.0b013e3280105245. [DOI] [PubMed] [Google Scholar]
- 38.Bristow MR, Krause-Steinrauf H, Nuzzo R, et al. Effect of baseline or changes in adrenergic activity on clinical outcomes in the beta-blocker evaluation of survival trial. Circulation. 2004;110:1437–42. doi: 10.1161/01.CIR.0000141297.50027.A4. [DOI] [PubMed] [Google Scholar]
- 39.Kohout TA, Lefkowitz RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- 40.Muhlestein JB, Horne BD, Bair TL, et al. Usefulness of in-hospital prescription of statin agents after angiographic diagnosis of coronary artery disease in improving continued compliance and reduced mortality. Am J Cardiol. 2001;87:257–61. doi: 10.1016/s0002-9149(00)01354-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.