Abstract
Background
Acromegaly is characterized by overproduction of growth hormone (GH) by the pituitary gland. GH stimulates the synthesis of insulin-like growth factor-I (IGF-I), and the somatic growth and metabolic dysfunction that characterize acromegaly are a consequence of elevated GH and IGF-I levels. Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are rare, slow-growing neoplasms that have usually metastasized by the time of diagnosis. The majority of GEP-NETs are carcinoid tumors whose syndrome is caused by the hypersecretion of biogenic amines, peptides and polypeptides responsible for the principal symptoms of diarrhea and flushing.
Methods
The MEDLINE and EMBASE databases were searched for preclinical and clinical studies of octreotide (Sandostatin*), a potent synthetic somatostatin analogue, in patients with acromegaly or GEP-NETs.
Objective
This article reviews the 20 years of clinical experience with octreotide and the impact it has made in patients with acromegaly or GEP-NETs.
Results
Octreotide has proven to be an essential component in the management strategy of acromegaly and GEP-NETs over the past 20 years. The multiple beneficial effects of octreotide throughout the body, combined with its established safety profile (the most common adverse effects are injection-site pain and gastrointestinal events), have made it an appealing option for clinicians. The advent of the long-acting release (LAR) formulation of octreotide provided additional benefits to patients through monthly administration, while maintaining the efficacy and tolerability profile of the daily subcutaneous formulation.
Conclusions
Octreotide is a potent synthetic somatostatin analogue that has become the mainstay of medical therapy for tumor control in neuroendocrine disorders such as acromegaly and GEP-NETs. The development of octreotide LAR offered a further advancement; less frequent dosing provided valuable benefits in quality of life to patients, with equivalent efficacy and tolerability. Moreover, recent results from the PROMID study have confirmed the antiproliferative effect of octreotide LAR in patients with well-differentiated metastatic GEP-NETs of the midgut. New therapeutic uses of octreotide are currently under investigation in a variety of clinical settings.
Keywords: Acromegaly, Carcinoid, NETs, Neuroendocrine tumors, Octreotide, Sandostatin, Somatostatin analogue
Introduction
Since its development 20 years ago, octreotide (Sandostatin*), a potent synthetic somatostatin analogue, has emerged as the foremost medical therapy for endocrine disorders such as acromegaly and carcinoid syndrome associated with gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Before the introduction of octreotide, the negative impact of these rare hormonal disorders on patients' quality of life was considerable, and treatment options beyond surgery were limited1–3. The development of subcutaneous octreotide, and later its long-acting release (LAR) formulation, represented major clinical breakthroughs. With octreotide therapy, excessive hormone secretion could be controlled effectively, resulting in a substantial improvement in quality of life for patients1,2.
Acromegaly is a rare, chronic hormonal disorder characterized by overproduction of growth hormone (GH) by the pituitary gland, which in turn results in excessive production of insulin-like growth factor-I (IGF-I) in the liver4,5.In>90% of cases, GH hypersecretion is caused by a benign GH-secreting pituitary adenoma6. Acromegaly not only increases patients' risk of severe comorbidities, including diabetes mellitus, hypertension and cardiovascular disease, but patients often suffer from physical deformities, such as enlarged hands and feet, frontal bone bossing and coarse facial features1,3,5. Patients with active acromegaly also have a two- to three-fold increased mortality risk7. Until the 1980s, surgery, radiation therapy and dopamine agonists were the only treatments available to patients with acromegaly8. As many tumors were too large or invasive to be completely resected, only a minority of patients could be cured with surgery alone8. As dopamine agonists are minimally effective and radiation therapy takes many years to normalize hormone levels, disease in these patients remained uncontrolled for years. The introduction of octreotide in the early 1980s changed the course of acromegaly treatment, and the disease became controllable in two-thirds of patients8,9.
The treatment of patients with GEP-NETs, in particular those suffering from carcinoid syndrome, was also substantially advanced upon the introduction of octreotide9,10. GEP-NETs are rare neoplasms that originate from neuroendocrine cells in the digestive tract, pancreas, lungs and liver11. GEP-NETs are characterized by the production and secretion of excessive amounts of peptide hormones and biogenic amines that are normally regulated in the body in smaller amounts11. Due to the indolent nature of GEP-NETs, patients may be asymptomatic for years or present with only vague symptoms of abdominal pain which are often confused with other disorders such as irritable bowel syndrome. As such, treatment is often considered only when well-differentiated nonpancreatic GEP-NETs (often referred to as carcinoid tumors), the most common type of GEP-NETs, metastasize to the liver, and the clinical manifestations of carcinoid syndrome develop, resulting in more severe symptoms of flushing, debilitating diarrhea, and cramping in the lower abdomen10,12. Although a number of therapeutic strategies, including chemotherapy, radiotherapy and surgery, have been historically utilized in the management of GEP-NETs, the overall clinical results were disappointing until the development of octreotide2. By inhibiting hormonal hypersecretion of GEP-NETs, octreotide reduces circulating hormone levels, stabilizes tumor growth and significantly ameliorates symptoms in patients10,12. Moreover, the introduction of octreotide LAR has led to a substantial improvement in survival in patients with GEP-NETs13.
Octreotide is the most prescribed and most studied somatostatin analogue for acromegaly and GEP-NETs. Its development is considered a milestone in the treatment of patients with these rare, serious hormonal disorders. This article reflects on the 20 years of clinical experience with octreotide and its impact on quality of life in patients with acromegaly and GEP-NETs, beginning with its development in the early 1980s to the launch of a long-acting formulation in 1997. Future therapeutic applications for octreotide are continuing to grow and will be discussed.
MEDLINE and EMBASE were searched using the terms `Sandostatin', `octreotide', `Somatuline', `lanreotide', `acromegaly', `neuroendocrine tumors', and `NETs'. Searches were performed for publications from January 1 1980 to July 13 2009. When available, large, well-controlled trials with appropriate statistical methodology were preferred.
Development of octreotide
The discovery of somatostatin in 1973, for which Roger Guillemin and Andrew Schally shared the Nobel Prize, provided a new approach to investigating disease states associated with endocrine hypersecretion such as acromegaly and GEP-NETs14,15. Somatostatin is an inhibitory hormone that is widely distributed throughout the central nervous system (CNS) and peripheral tissues. Native somatostatin plays an important regulatory role in neuro-transmission and secretion, preventing the release of GH, thyroid-stimulating hormone, GI hormones, pancreatic enzymes and neuropeptides14,15. The rate of gastric emptying, smooth muscle contractions and blood flow within the intestine are also modulated by somatostatin.
Endogenous somatostatin exerts its biological effects via activation of somatostatin receptors expressed in the CNS, hypothalamus, GI tract and pancreas14–16. Five somatostatin receptor subtypes (sst1–5), each with distinct signaling pathways and tissue distribution, have been identified, cloned and characterized with respect to binding properties14–16. Pituitary tumors found in patients with acromegaly mainly express sst2 and sst5, while GEP-NETs express multiple sst, although predominantly sst214. When it became apparent that the pharmacological properties of native somatostatin limited its use in clinical practice, the search began for analogues of somatostatin to provide a more clinically useful molecule with strong affinities for selected receptor subtypes.
Octreotide is a synthetic octapeptide analogue of somatostatin with more prolonged pharmacological actions than the endogenous hormone. Native somatostatin has a half-life of 2–3 minutes; octreotide has a half-life of 90–120 minutes when administered subcutaneously, and a pharmacodynamic action lasting up to 8–12 hours15,17. Moreover, octreotide selectively binds to sst2 and to a lesser extent sst5 (Table 1), providing a high ratio of therapeutic benefit over adverse effects9,14,15,17. In the pituitary gland, octreotide has an approximately 40-fold greater potency than native somatostatin in inhibiting GH secretion15,18. In the pancreas, octreotide has been shown to inhibit insulin, glucagon, pancreatic polypeptides and bicarbonate secretion. The pharmacological actions of octreotide in the GI tract are numerous, and include inhibition of gastrin, motilin, secretin and vasoactive intestinal polypeptides, as well as decreased blood flow to the gut, intestinal motility and carbohydrate absorption. Octreotide also increases water and electrolyte absorption in the GI tract, which is essential in the treatment of GEP-NETs15,18. Importantly, treatment with octreotide does not result in rebound hypersecretion of hormones.
Table 1.
IC50 values of native somatostatin and octreotide for the different somatostatin receptor subtypes. Adapted from Hofland and Lamberts 200314.
| sst1 | sst2 | sst3 | sst4 | sst5 | |
|---|---|---|---|---|---|
| Somatostatin, nmol/L | 2.3 | 0.2 | 1.4 | 1.8 | 0.9 |
| Octreotide, nmol/L | >1000 | 0.6 | 34.5 | >1000 | 7 |
sst; somatostatin receptor subtype.
The development of the long-acting release (LAR) formulation of octreotide in 1997 further improved the clinical application of this compound. Octreotide LAR is a long-acting release formulation in which octreotide is encapsulated in microspheres of a slowly dissolving polymer, providing a predictable pharmacokinetic profile and steady-state kinetics when administered intramuscularly once every 28 days19. The pharmacokinetic profile of octreotide after a single dose of octreotide LAR 20 mg exhibits three distinct phases: following a transient increase in concentration after administration on day 1, there is a lag phase for about 5 days, during which octreotide concentrations decrease, followed by a new increase in drug levels and a plateau phase for about 30 days19. Octreotide LAR retains the pharmacological characteristics of subcutaneous octreotide, and reaches steady-state concentrations within three injections20. The short-acting formulation is rarely used for long-term therapy, but can be particularly effective for control of headaches in some patients with acromegaly, and for use as `rescue' therapy in patients with carcinoid syndrome.
Clinical experience with octreotide
Acromegaly
The goals of treatment for patients with acromegaly include controlling hormone hypersecretion from the tumor, normalizing circulating GH and IGF-I levels, controlling tumor growth whilst preserving normal pituitary function, and controlling or eliminating comorbidities and symptoms21,22. The use of octreotide in the treatment of acromegaly is supported by more than 20 years of clinical research and experience.
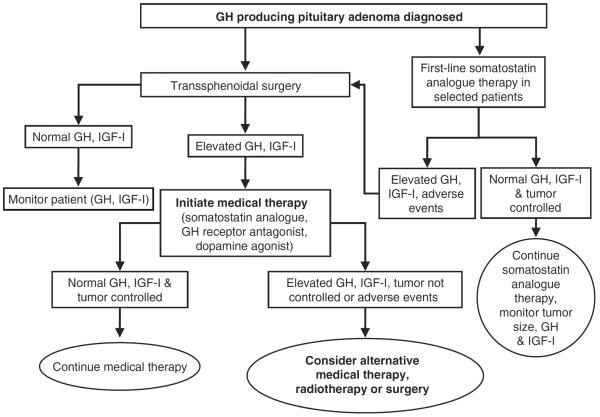
Octreotide is typically used as a post-operative therapy to control GH secretion after debulking a tumor or as first-line therapy for patients who are unsuitable for neurosurgery (Figure 1)21,22. Since the pharmacological properties of octreotide were first described in 1982 by Bauer et al.9, clinical evaluation has consistently demonstrated that both the subcutaneous and long-acting release formulations of octreotide effectively control GH and IGF-I levels and reduce the incidence of comorbid symptoms in most patients with acromegaly. Early studies of subcutaneous octreotide showed that up to 90% of patients with acromegaly experienced some fall in GH and IGF-I serum levels during treatment with octreotide23. Longer-term studies (up to 4 years) published in the 1990s demonstrated that subcutaneous octreotide maintains GH suppression (≤5 mg/L) and normalizes IGF-I levels in up to 65% and 68% of patients with acromegaly, respectively, with no reported tachyphylaxis23–25.
Figure 1.
Acromegaly treatment algorithm.
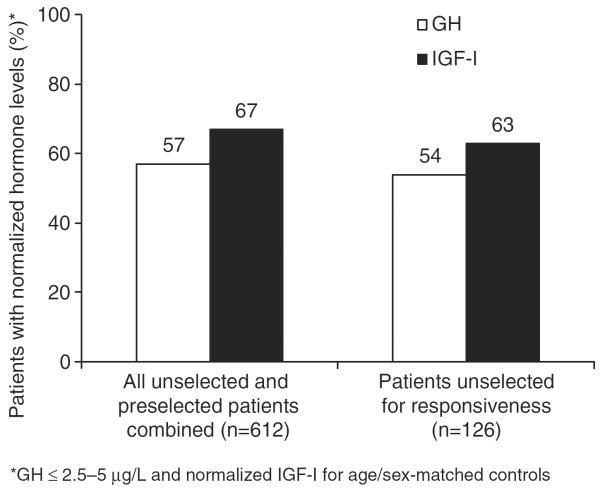
Octreotide LAR offers the convenience of once-monthly administration compared with daily subcutaneous drug administration. The efficacy profile of octreotide LAR was quickly established as similar to that of subcutaneous octreotide in patients with acromegaly, with the potential for improved patient compliance26. Several long-term studies of octreotide LAR (up to 9 years) have demonstrated that around 70% of patients achieve GH levels ≤2.5 μg/L and up to 75% of patients achieve age-matched normalized IGF-I levels27–29. Results from a recent meta-analysis of octreotide LAR in 44 trials that enrolled over 600 patients show that octreotide controls GH and IGF-I in 57% and 67% of patients, respectively30. Interestingly, this meta-analysis also compared the efficacy of octreotide LAR in patients who were and were not preselected for therapy based on responsiveness to octreotide. Preselection did not influence GH normalization rate, but was a positive predictor of IGF-I normalization (Figure 2)30. Importantly, no tachyphylaxis has been reported in the treatment of patients with acromegaly over the long term (≥10 years) for either formulation of octreotide15.
Figure 2.
Octreotide LAR suppresses GH and IGF-I levels in patients with acromegaly (Freda et al. 2005).
Although there have been no large head-to-head studies comparing octreotide LAR with lanreotide Autogel, the only other long-acting somatostatin analogue available for the treatment of acromegaly, several small studies, most of which switched patients well-controlled on octreotide LAR therapy to treatment with lanreotide Autogel, have shown that lanreotide Autogel is noninferior to octreotide LAR with regards to biochemical control31.
Octreotide markedly reduces the clinical symptoms of acromegaly, including debilitating headaches, amount of perspiration, paresthesia, fatigue, joint pain and carpal tunnel syndrome in 70% of patients with acromegaly23–25,27–29. Even if biochemical control is not achieved, results from several studies show that symptomatic improvement occurs in most patients with acromegaly treated with octreotide. Long-term treatment with octreotide also improves cardiac performance in patients with normalized GH and IGF-I levels32,33, although whether or not octreotide improves cardiovascular mortality is yet to be demonstrated.
In selected patients, such as elderly patients or those in whom the surgical risk is high, first-line medical therapy with octreotide LAR should be strongly considered. There is some evidence to suggest that first-line medical therapy should also be favored in patients with macroadenomas without visual or neurological compromise, but for whom the chance of surgical cure is low (Figure 1)28,34,35. A recent study suggested that the biochemical control achieved with first-line octreotide LAR therapy is similar to that expected for surgical therapy28. In this 9-year, long-term study in 67 patients with acromegaly who received octreotide LAR as first-line therapy, control of GH and IGF-I levels was achieved in 68.7% and 70.1% of patients, respectively. Tumor shrinkage occurred in 82.1% of patients, with an average reduction in tumor volume of 62%. Furthermore, the percentage of patients achieving biochemical control and tumor shrinkage increased with time28. Another recent study that randomized newly diagnosed patients with acromegaly to surgery or octreotide LAR showed that the 48-week treatment outcome seen with octreotide LAR was not statistically significantly different from that seen in patients who underwent surgery35.
There are other data to suggest that surgical debulking prior to somatostatin analogue use improves efficacy36,37. There is also debate as to whether preoperative octreotide use improves surgical outcome. Although some studies have not shown a benefit to long-term outcome with preoperative octreotide therapy38–40, two studies have shown improved post-operative cure rates in patients with macroadenomas pretreated for 3–6 months with octreotide sc41 or octreotide LAR42. Finally, 3–6 months of preoperative octreotide may be useful in improving patients' clinical status prior to surgery, in terms of cardiac function, blood pressure, and glucose and lipid metabolism, thus facilitating anesthetic and surgical management41.
The use of first-line medical therapy should be considered on an individual patient basis. The surgical risks for the patient, the experience of the surgeon available, the tumor size and location, and patient preference should be taken into account. Failure to debulk a large tumor that is found to be incompletely somatostatin analogue responsive may limit additional options for therapy, such as radiotherapy or GH receptor antagonist therapy, due to tumor location and size constraints. Even some small tumors that clearly cannot be removed because of their location (such as in the cavernous sinus) are candidates for first-line medical therapy.
In patients receiving long-term octreotide LAR and who have well-controlled disease, individual tailoring of the dose, for example a reduction in the dose or an extension of the interval between doses to more than 4 weeks, may be considered in order to provide maximal benefit to the patient while maintaining adequate disease control43–45. Furthermore, Ronchi et al. recently showed that somatostatin analogues may be successfully withdrawn in a subset of patients who respond well to treatment46. Regular biochemical monitoring and neuroradiological imaging is mandatory in patients with acromegaly withdrawn from medical therapy.
The costs of different medical therapies for acromegaly varies depending on the treatment, for example dopamine agonists (cabergoline), somatostatin analogues (octreotide LAR, lanreotide Autogel) and growth hormone antagonists (pegvisomant), the country and the method of healthcare payment. It is important to consider cost effectiveness and cost benefit in the treatment of patients with acromegaly, and these considerations require therapy to be individualized22. To date, there have been no reported cost-effectiveness studies comparing the different medical treatments available for acromegaly. Although the costs of medical therapy for acromegaly are relatively high and some treatments are more expensive than others, these costs may be acceptable because the overall disease burden on the health system is low due to the rarity of acromegaly. Furthermore, determination of the cost/benefit ratio needs to include the consequences of long-term outcomes of poor disease control, as well as the occurrence of subsequent complications22.
GEP-NETs
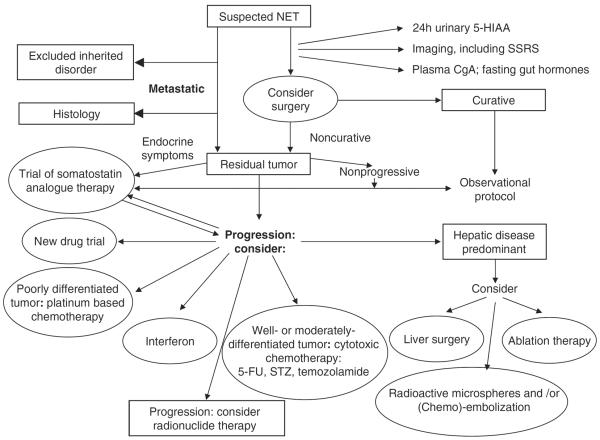
Although surgery is considered the first-line therapy for patients with GEP-NETs, a cure is not possible in 80% of cases as most patients present at the advanced disease stage47. Surgical debulking can reduce the extent of hormone production and relieve symptoms, but because the disease course of carcinoid tumors is often long, palliative care to maintain quality of life is particularly important (Figure 3). Relief from diarrhea and flushing, as well as biochemical control, are fundamental to improving quality of life in patients with symptoms of functioning GEP-NETs.
Figure 3.
Algorithm for the investigation and management of NET. Reproduced from Ramage JK et al. Gut 2005;54(Suppl 4):iv116, with permission.
Octreotide was among the first biotherapeutic agents used in the management of GEP-NETs and continues to be a mainstay of therapy today, although it is rarely curative2. In a review by Modlin et al. (2006), pooled data from more than 14 trials spanning the past two decades and including almost 400 patients revealed that 71% of patients with GEP-NETs experience resolution or improvement of diarrhea (range: 40–88%) and flushing (range: 48–100%) during treatment with octreotide2. Diarrhea is the primary reason for patients to seek medical help, making effective long-term treatment essential. Biochemical responses to octreotide were seen in up to 77% of patients with GEP-NETs, demonstrating that octreotide effectively inhibits hormonal hypersecretion. Octreotide also has antiproliferative activity; although objective tumor responses are uncommon, 55% (range: 48–75%) of patients experience stable disease with octreotide therapy2.
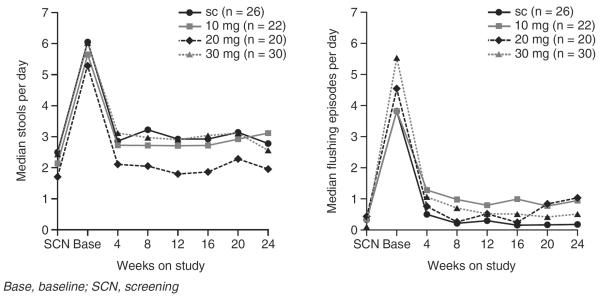
The effects of octreotide LAR have been evaluated in a study of 93 patients with carcinoid tumors and a confirmed diagnosis of carcinoid syndrome48. Suppressing 5-HIAA levels, a metabolite of serotonin, is key for assessing and managing patients with carcinoid syndrome. Octreotide LAR rapidly reduces 5-HIAA levels by up to 50% in patients with carcinoid syndrome48. Uncontrolled diarrhea due to carcinoid syndrome leaves patients at risk of serious comorbidities such as opportunistic intestinal infections and dehydration. By working at the site of carcinoid tumors, octreotide reduces bioactive secretions and reduces diarrhea frequency by 42% in patients with carcinoid syndrome (Figure 4)48. The precise mechanisms by which octreotide exerts its effects on the GI tract in patients with GEP-NETs have not been completely elucidated, but in addition to controlling diarrhea and reducing secretions, octreotide promotes water and electrolyte absorption, decreases splanchnic blood flow and prolongs GI transit time48–50. Flushing episodes in patients with carcinoid syndrome are also reduced by 84%, from 4.5 episodes/day to 0.7 episodes/day (Figure 4)48.
Figure 4.
Octreotide LAR for control of diarrhea and flushing in patients with confirmed carcinoid syndrome. Reproduced from Rubin J et al. J Clin Oncol 1999;17:600–6, with permission.
Interestingly, octreotide LAR appears to improve survival in patients with carcinoid syndrome. In a retrospective analysis, survival was compared in 145 patients with carcinoid syndrome who were receiving octreotide LAR to 90 patients who had received subcutaneous octreotide13. Baseline characteristics were similar between the two groups. Survival in the octreotide LAR group was significantly higher: 229 months compared with 143 months in the control group (p <0.0001)13. Thus, patients with carcinoid syndrome who received treatment with octreotide LAR had a 66% lower risk of death than patients receiving therapy with subcutaneous octreotide in this study13.
Octreotide LAR has also recently been evaluated as first-line therapy in difficult-to-treat nonfunctioning GEP-NETs. Approximately 40% of nonfunctioning pancreatic endocrine carcinomas cannot be cured by surgery because of advanced-stage disease. In a prospective phase IV study, 21 patients with advanced-stage, well-differentiated nonfunctioning pancreatic endocrine carcinomas were treated with octreotide LAR 20 mg. At a median follow-up of 49.5 months, eight (38%) patients had stable disease51. Thirteen patients (62%) experienced progressive disease after a median of 18 months. Notably, tumor progression correlated with a proliferative index (Ki-67) ≥5% (p=0.016), weight loss (p=0.006) and absence of abdominal pain (p=0.003) at diagnosis. Thus, treatment with octreotide LAR was associated with stabilization of disease and a better quality of life in 38% of patients51.
Recently, the antiproliferative effect of octreotide LAR has been demonstrated in a randomized, double-blind, placebo-controlled phase IIIb study (PROMID trial) in 85 patients with functioning or nonfunctioning metastatic GEP-NETs of the midgut52. At the time of the preplanned confirmatory interim analysis after 67 tumor progressions, octreotide LAR 30 mg/28 days led to a significant increase in time to tumor progression (TTP) compared with placebo (14.3 vs. 6.0 months; p=0.000072), regardless of whether patients had a functioning or nonfunctioning tumor52,53. A further ad hoc analysis performed 11 months later once all patients had been unblinded from treatment showed a further improvement in TTP with octreotide therapy versus placebo (15.6 vs. 5.9 months; p=0.000017)52,54. Patients with nonfunctioning tumors (n=52) achieved a TTP of 27.14 months with octreotide LAR versus 7.21 months with placebo (p=0.0008)54. Octreotide LAR also significantly extended TTP in patients with functioning tumors (n=33) compared with placebo (10.35 months vs. 5.45 months; p=0.0007)54.
In response to results from the PROMID study, guidelines from the National Comprehensive Cancer Network (NCCN) have been updated to recommend octreotide LAR 20–30 mg as a management option in patients with recurrent or unresectable metastatic carcinoid tumors originating from any primary site of disease (small bowel, colon, rectum, appendix, lung, thymus, stomach) irrespective of functional status, symptomatology and progression status55.
There are fewer data available for lanreotide Autogel in the treatment of patients with GEP-NETs. In 75 patients with symptoms of metastatic GEP-NETs who received dose-titrated lanreotide Autogel for 6 months, 65% and 18% of patients with flushing or diarrhea, respectively, at baseline achieved a≥50% reduction in frequency of symptoms56.
Although octreotide quickly and effectively ameliorates symptoms in patients with carcinoid syndrome, the duration of effective treatment varies widely because of the development of escape from response57–64. This loss of response to octreotide LAR in patients with GEP-NETs is in contrast to the durable response seen in patients with GH-secreting pituitary adenomas. The mechanisms underlying this phenomenon in patients with GEP-NETs remain unclear. However, it has been suggested that the loss of sensitivity may be due to either the outgrowth of sst2-deficient tumor cell clones or a downregulation of sst2 after prolonged exposure to agonists62,65.
Safety and tolerability of octreotide
Octreotide has a well-established safety profile based on over 20 years of clinical experience. Both formulations are well-tolerated in most patients. Treatment discontinuations due to adverse events are rare.
The most frequent adverse events in patients receiving octreotide include pain at the injection site (10–20% of patients) and mild-to-moderate GI disturbances, such as loose stools, abdominal cramping, nausea and flatulence, which persist during therapy in only 5–15% of patients15,23,28,29,66–68. Continuing treatment will often lead to a resolution of GI complications15,25,29. No significant changes in routine biochemical and hematological variables have been reported. However, treatment with octreotide has the potential to alter glucose metabolism and patients with acromegaly and comorbid diabetes mellitus should be monitored carefully15.
Octreotide therapy also poses a risk of cholelithiasis, which may increase with longer treatment periods. Octreotide-associated biliary tract alterations, such as gallstones, sediment and sludge, have been variably reported in up to 20% of patients during octreotide treatment, but are usually asymptomatic and do not require surgical or medical therapy69. The mechanism involved in the development of gallstones during therapy with somatostatin analogues is complex, although the main cause is thought to be the inhibition of cholecystokinin release from the small intestine, which results in reduced gallbladder emptying70. Other mechanisms contributing to possible gallstone formation during somatostatin analogue therapy are increases in deoxycholic acid conjugates and cholesterol saturation, and inhibition of the usual prandial relaxation of the sphincter of Oddi71. It has been suggested recently that patients who develop asymptomatic gallstones during therapy with somatostatin analogues are at high risk of developing symptoms if that therapy is discontinued71.
The next 20 years
Octreotide LAR is currently the leading medical therapy in acromegaly and GEP-NETs and will continue to be the mainstay for tumor control in these indications for the foreseeable future. It is effective in the majority of patients in controlling the biochemical and physiological symptoms of acromegaly and GEP-NETs, has proven antiproliferative effects in GH-secreting pituitary adenomas and metastatic functioning or nonfunctioning midgut NETs, and is well-tolerated, with an established safety profile. In addition, the less frequent administration regimen of octreotide LAR compared with subcutaneous octreotide offers benefits in terms of patient satisfaction and compliance. Due to its multiple mechanisms of action, clinical evaluation of octreotide is ongoing in a range of therapy areas, including oncology, gastroenterology and obesity.
Oncology
Antineoplastic effects observed in patients with acromegaly and GEP-NETs make octreotide an attractive candidate for the treatment of refractory solid tumors. Preclinical studies indicate that somatostatin analogues exert inhibitory and suppressive effects in prostate, gastric, lung, colorectal, mammary, thyroid, and pancreatic cell lines72,73. When octreotide binds to tumors expressing somatostatin receptors, direct effects such as inhibition of the cell cycle and growth factors, and apoptosis occur74. In addition, indirect antiproliferative effects include inhibition of growth factor and hormone release and angiogenesis, with modulation of the immune system74. A range of clinical evaluations have shown mixed outcomes, but the therapeutic potential warrants further evaluation, possibly in combination with hormonal or cytotoxic therapies and in both adjuvant and neoadjuvant settings74,75.
Advanced hepatocellular carcinoma is difficult to treat and survival is poor. In a pilot, 2-year study, octreotide LAR was evaluated in 30 patients; although the effect on survival appeared to be limited, 29% of patients did exhibit stable disease76. Moreover, in a study by Dimitroulopoulos et al. (2007), survival was significantly improved in 31 patients who received octreotide for advanced hepatocellular carcinoma compared with placebo (49 vs. 28 weeks; p<0.01)77.
Treatment with radio-labeled octreotide in patients with inoperable or metastasized sst2-positive tumors, including GEP-NETs, appears to be a promising new therapy78,79. In a phase I study, therapeutic effects, including one partial remission, six minor remissions, and stable disease in 14 tumors, were seen in 21/40 evaluable patients treated with multiple doses of radio-labeled octreotide, with only mild bone marrow toxicity. In three other phase I trials, 20% of patients experienced a partial response and 60% had stable disease79.
Managing patients with severe GI symptoms
Octreotide may have a therapeutic effect in the management of diarrhea in patients receiving chemotherapy for cancer. In an open-label, randomized, multicenter study designed to assess the effects of two dose levels of octreotide LAR, 147 patients with active or prior chemotherapy-induced diarrhea and scheduled for chemotherapy were randomized to receive up to six doses of either octreotide LAR 30 or 40 mg. Both dose levels provided clinical benefit, although fewer patients in the 40-mg group than the 30-mg group experienced severe diarrhea, required intravenous fluid, and had diarrhea-related unscheduled healthcare visits80. Another study has shown complete resolution of diarrhea in 30 of the 32 patients treated with octreotide81. Patients who suffer diarrhea as a complication of AIDS, graft-versus-host disease and tumor-related diarrhea can also obtain major benefit from treatment with octreotide82.
Symptoms associated with small intestinal involvement in patients with systemic sclerosis (SSc) are usually severe and resistant to treatment. To assess the safety and efficacy of octreotide in refractory small intestinal disease complicating SSc, seven patients with SSc non-responsive to traditional therapies received octreotide83. All patients responded to octreotide, and a significant reduction in symptom severity was noted in the first month. A significant disturbance of defecation in two patients improved dramatically83. These results suggest that long-term treatment with octreotide may be a safe and effective approach in the treatment of small intestinal disease in patients with SSc83.
Obesity and polycystic ovary syndrome
Because octreotide reduces circulating levels of GH and insulin, it is a potential candidate for the treatment of obesity and polycystic ovary syndrome (PCOS), both of which include patients exhibiting insulin hypersecretion84,85. A single-blind, placebo-controlled study in anovulatory abdominally obese women with PCOS has shown significant clinical benefits in patients who received octreotide84. Octreotide produced significant decreases in fasting and glucose-stimulated insulin levels, increases in IGF-binding proteins, and improvements in hirsutism. Moreover, a trend toward greater reductions in testosterone and androstenedione were observed in women treated with octreotide compared with those given placebo. All women treated with octreotide ovulated at the end of the study compared with only one of those receiving placebo (p< 0.001). Based on these results, the authors concluded that octreotide may be useful in hypocalorically dieting, abdominally obese PCOS women to improve hyperandrogenism and the insulin–IGF-I system.
Another randomized, double-blind, placebo-controlled trial has investigated the potential of octreotide LAR to improve weight loss, body mass index, and fasting serum in 172 adults with moderate obesity and evidence of insulin hypersecretion85. After 6 months of treatment, patients receiving octreotide LAR 40 or 60 mg experienced statistically significant weight loss. However, the mean weight loss was modest (approximately 2 kg). A total of 7–21% of the patients taking octreotide LAR achieved a >5% decrease in body weight from baseline, compared with 11% in the placebo group. A post hoc analysis stratifying patients by race indicated that Caucasian patients with the greater degree of insulin hypersecretion appeared to derive the most benefit from treatment.
Conclusions
Over the past 20 years, octreotide has proven to be essential to the successful management of patients with acromegaly and GEP-NETs. The multiple effects of octreotide throughout the body, combined with its established safety profile, make it an appealing and reliable option for clinicians. The development of octreotide LAR offered a further advancement; less frequent dosing provided valuable benefits in quality of life to patients, with equivalent efficacy and tolerability. Moreover, recent results from the PROMID study have confirmed the antiproliferative effect of octreotide LAR in patients with well-differentiated metastatic GEP-NETs of the midgut. Exciting and new therapeutic uses of octreotide are currently under investigation in a variety of clinical settings.
Acknowledgment
The authors thank Diana James and Keri Wellington, PhD, Mudskipper Bioscience, London, UK, for editorial assistance, funded by Novartis.
Declaration of funding This Review was funded by Novartis Pharmaceuticals Corporation.
Declaration of financial/other relationships L.A. has disclosed that he has received grants from Imclone, Pfizer, Novartis and several other pharmaceutical companies, and that he has served as consultant for Pfizer, Novartis, Molecular Insights and Roche. He is also on speakers' bureaus for Amgen, Novartis, Bristol Myers, and several other pharmaceutical companies. P.U.F. has disclosed that Columbia University has received grants for her research from Novartis, Pfizer, Tercica and Ipsen, and that she has served as a consultant to Novartis.
Some peer reviewers receive honoraria from CMRO for their review work. The peer reviewers of this paper have disclosed that they have no relevant financial relationships.
Footnotes
Novartis Pharma AG, Basel, Switzerland.
References
- 1.Webb SM. Quality of life in acromegaly. Neuroendocrinology. 2006;83:224–9. doi: 10.1159/000095532. [DOI] [PubMed] [Google Scholar]
- 2.Modlin IM, Latich I, Kidd M, et al. Therapeutic options for gastrointestinal carcinoids. Clin Gastroenterol Hepatol. 2006;4:526–47. doi: 10.1016/j.cgh.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Colao A, Ferone D, Marzullo P, et al. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004;25:102–52. doi: 10.1210/er.2002-0022. [DOI] [PubMed] [Google Scholar]
- 4.Melmed S. Acromegaly. N Engl J Med. 1990;322:966–77. doi: 10.1056/NEJM199004053221405. [DOI] [PubMed] [Google Scholar]
- 5.Melmed S. Medical progress: Acromegaly. N Engl J Med. 2006;355:2558–73. doi: 10.1056/NEJMra062453. [DOI] [PubMed] [Google Scholar]
- 6.Sanno N, Teramoto A, Osamura RY, et al. Pathology of pituitary tumors. Neurosurg Clin N Am. 2003;14:25–39. vi. doi: 10.1016/s1042-3680(02)00035-9. [DOI] [PubMed] [Google Scholar]
- 7.Biermasz NR, Romijn JA, Pereira AM, et al. Current pharmacotherapy for acromegaly: a review. Expert Opin Pharmacother. 2005;6:2393–405. doi: 10.1517/14656566.6.14.2393. [DOI] [PubMed] [Google Scholar]
- 8.Kleinberg DL. Primary therapy for acromegaly with somatostatin analogs and a discussion of novel peptide analogs. Rev Endocr Metab Disord. 2005;6:29–37. doi: 10.1007/s11154-005-5222-2. [DOI] [PubMed] [Google Scholar]
- 9.Bauer W, Briner U, Doepfner W, et al. SMS 201–995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci. 1982;31:1133–40. doi: 10.1016/0024-3205(82)90087-x. [DOI] [PubMed] [Google Scholar]
- 10.Delaunoit T, Rubin J, Neczyporenko F, et al. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumors. Mayo Clin Proc. 2005;80:502–6. doi: 10.4065/80.4.502. [DOI] [PubMed] [Google Scholar]
- 11.Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev. 2004;25:458–511. doi: 10.1210/er.2003-0014. [DOI] [PubMed] [Google Scholar]
- 12.Robertson RG, Geiger WJ, Davis NB. Carcinoid tumors. Am Fam Physician. 2006;74:429–34. [PubMed] [Google Scholar]
- 13.Anthony LB, Kang T, Shyr Y. Malignant carcinoid syndrome: survival in the octreotide LAR era. Proc Am Soc Clin Oncol. 2005 abst 4084. [Google Scholar]
- 14.Hofland LJ, Lamberts SW. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev. 2003;24:28–47. doi: 10.1210/er.2000-0001. [DOI] [PubMed] [Google Scholar]
- 15.Cozzi R, Attanasio R. Octreotide for acromegaly. Expert Rev Endocrinol Metab. 2007;2:129–45. doi: 10.1586/17446651.2.2.129. [DOI] [PubMed] [Google Scholar]
- 16.Reisine T, Bell GI. Molecular properties of somatostatin receptors. Neuroscience. 1995;67:777–90. doi: 10.1016/0306-4522(95)00072-q. [DOI] [PubMed] [Google Scholar]
- 17.Bronstein MD. Acromegaly: molecular expression of somatostatin receptor subtypes and treatment outcome. Front Horm Res. 2006;35:129–34. doi: 10.1159/000094315. [DOI] [PubMed] [Google Scholar]
- 18.Gillis JC, Noble S, Goa KL. Octreotide long-acting release (LAR). A review of its pharmacological properties and therapeutic use in the management of acromegaly. Drugs. 1997;53:681–99. doi: 10.2165/00003495-199753040-00009. [DOI] [PubMed] [Google Scholar]
- 19.Astruc B, Marbach P, Bouterfa H, et al. Long-acting octreotide and prolonged-release lanreotide formulations have different pharmacokinetic profiles. J Clin Pharmacol. 2005;45:836–44. doi: 10.1177/0091270005277936. [DOI] [PubMed] [Google Scholar]
- 20.Chen T, Miller TF, Prasad P, et al. Pharmacokinetics, pharmacodynamics, and safety of microencapsulated octreotide acetate in healthy subjects. J Clin Pharmacol. 2000;40:475–81. doi: 10.1177/00912700022009242. [DOI] [PubMed] [Google Scholar]
- 21.Melmed S, Vance ML, Barkan AL, et al. Current status and future opportunities for controlling acromegaly. Pituitary. 2002;5:185–96. doi: 10.1023/a:1023369317275. [DOI] [PubMed] [Google Scholar]
- 22.Melmed S, Casanueva F, Cavagnini F, et al. Consensus statement: medical management of acromegaly. Eur J Endocrinol. 2005;153:737–40. doi: 10.1530/eje.1.02036. [DOI] [PubMed] [Google Scholar]
- 23.Vance ML, Harris AG. Long-term treatment of 189 acromegalic patients with the somatostatin analog octreotide. Results of the International Multicenter Acromegaly Study Group. Arch Intern Med. 1991;151:1573–8. [PubMed] [Google Scholar]
- 24.Ezzat S, Snyder PJ, Young WF, et al. Octreotide treatment of acromegaly. A randomized, multicenter study. Ann Intern Med. 1992;117:711–18. doi: 10.7326/0003-4819-117-9-711. [DOI] [PubMed] [Google Scholar]
- 25.Newman CB, Melmed S, Snyder PJ, et al. Safety and efficacy of long-term octreotide therapy of acromegaly: results of a multicenter trial in 103 patients–a clinical research center study. J Clin Endocrinol Metab. 1995;80:2768–75. doi: 10.1210/jcem.80.9.7673422. [DOI] [PubMed] [Google Scholar]
- 26.McKeage K, Cheer S, Wagstaff AJ. Octreotide long-acting release (LAR): a review of its use in the management of acromegaly. Drugs. 2003;63:2473–99. doi: 10.2165/00003495-200363220-00014. [DOI] [PubMed] [Google Scholar]
- 27.Cozzi R, Attanasio R, Montini M, et al. Four-year treatment with octreotide-long-acting repeatable in 110 acromegalic patients: predictive value of short-term results? J Clin Endocrinol Metab. 2003;88:3090–8. doi: 10.1210/jc.2003-030110. [DOI] [PubMed] [Google Scholar]
- 28.Cozzi R, Montini M, Attanasio R, et al. Primary treatment of acromegaly with octreotide LAR: a long-term (up to 9 years) prospective study of its efficacy in the control of disease activity and tumor shrinkage. J Clin Endocrinol Metab. 2006;91:1397–403. doi: 10.1210/jc.2005-2347. [DOI] [PubMed] [Google Scholar]
- 29.Lancranjan I, Atkinson AB. Results of a European multicentre study with Sandostatin LAR in acromegalic patients. Sandostatin LAR Group. Pituitary. 1999;1:105–14. doi: 10.1023/a:1009980404404. [DOI] [PubMed] [Google Scholar]
- 30.Freda PU, Katznelson L, van der Lely AJ, et al. Long-acting somatostatin analog therapy of acromegaly: a meta-analysis. J Clin Endocrinol Metab. 2005;90:4465–73. doi: 10.1210/jc.2005-0260. [DOI] [PubMed] [Google Scholar]
- 31.Murray RD, Melmed S. A Critical Analysis of Clinically Available Somatostatin Analog Formulations for Therapy of Acromegaly. J Clin Endocrinol Metab. 2008;93:2957–68. doi: 10.1210/jc.2008-0027. [DOI] [PubMed] [Google Scholar]
- 32.Colao A, Marzullo P, Ferone D, et al. Cardiovascular effects of depot long-acting somatostatin analog Sandostatin LAR in acromegaly. J Clin Endocrinol Metab. 2000;85:3132–40. doi: 10.1210/jcem.85.9.6782. [DOI] [PubMed] [Google Scholar]
- 33.Colao A, Cuocolo A, Marzullo P, et al. Is the acromegalic cardiomyopathy reversible? Effect of 5-year normalization of growth hormone and insulin-like growth factor I levels on cardiac performance. J Clin Endocrinol Metab. 2001;86:1551–7. doi: 10.1210/jcem.86.4.7376. [DOI] [PubMed] [Google Scholar]
- 34.Ferone D, Colao A, van der Lely AJ, et al. Pharmacotherapy or surgery as primary treatment for acromegaly? Drugs Aging. 2000;17:81–92. doi: 10.2165/00002512-200017020-00001. [DOI] [PubMed] [Google Scholar]
- 35.Colao A, Cappabianca P, Caron P, et al. Octreotide LAR vs. surgery in newly diagnosed patients with acromegaly: a randomized, open-label, multicentre study. Clin Endocrinol (Oxf) 2009;70:757–68. doi: 10.1111/j.1365-2265.2008.03441.x. [DOI] [PubMed] [Google Scholar]
- 36.Petrossians P, Borges-Martins L, Espinoza C, et al. Gross total resection or debulking of pituitary adenomas improves hormonal control of acromegaly by somatostatin analogs. Eur J Endocrinol. 2005;152:61–6. doi: 10.1530/eje.1.01824. [DOI] [PubMed] [Google Scholar]
- 37.Colao A, Attanasio R, Pivonello R, et al. Partial surgical removal of GH-secreting pituitary tumors enhances the response to somatostatin analogues in acromegaly. J Clin Endocrinol Metab. 2006;91:85–92. doi: 10.1210/jc.2005-1208. [DOI] [PubMed] [Google Scholar]
- 38.Losa M, Mortini P, Urbaz L, et al. Presurgical treatment with somatostatin analogs in patients with acromegaly: effects on the remission and complication rates. J Neurosurg. 2006;104:899–906. doi: 10.3171/jns.2006.104.6.899. [DOI] [PubMed] [Google Scholar]
- 39.Abe T, Ludecke DK. Effects of preoperative octreotide treatment on different subtypes of 90 GH-secreting pituitary adenomas and outcome in one surgical centre. Eur J Endocrinol. 2001;145:137–45. doi: 10.1530/eje.0.1450137. [DOI] [PubMed] [Google Scholar]
- 40.Biermasz NR, van Dulken H, Roelfsema F. Direct postoperative and follow-up results of transsphenoidal surgery in 19 acromegalic patients pretreated with octreotide compared to those in untreated matched controls. J Clin Endocrinol Metab. 1999;84:3551–5. doi: 10.1210/jcem.84.10.6027. [DOI] [PubMed] [Google Scholar]
- 41.Colao A, Ferone D, Cappabianca P, et al. Effect of octreotide pretreatment on surgical outcome in acromegaly. J Clin Endocrinol Metab. 1997;82:3308–14. doi: 10.1210/jcem.82.10.4283. [DOI] [PubMed] [Google Scholar]
- 42.Carlsen SM, Lund-Johansen M, Schreiner T, et al. Preoperative octreotide treatment in newly diagnosed acromegalic patients with macroadenomas increases cure short-term postoperative rates: a prospective, randomized trial. J Clin Endocrinol Metab. 2008;93:2984–90. doi: 10.1210/jc.2008-0315. [DOI] [PubMed] [Google Scholar]
- 43.Biermasz NR, van den Oever NC, Frolich M, et al. Sandostatin LAR in acromegaly: a 6-week injection interval suppresses GH secretion as effectively as a 4-week interval. Clin Endocrinol (Oxf) 2003;58:288–95. doi: 10.1046/j.1365-2265.2003.01710.x. [DOI] [PubMed] [Google Scholar]
- 44.Jenkins PJ, Akker S, Chew SL, et al. Optimal dosage interval for depot somatostatin analogue therapy in acromegaly requires individual titration. Clin Endocrinol (Oxf) 2000;53:719–24. doi: 10.1046/j.1365-2265.2000.01168.x. [DOI] [PubMed] [Google Scholar]
- 45.Turner HE, Thornton-Jones VA, Wass JA. Systematic dose-extension of octreotide LAR: the importance of individual tailoring of treatment in patients with acromegaly. Clin Endocrinol (Oxf) 2004;61:224–31. doi: 10.1111/j.1365-2265.2004.02084.x. [DOI] [PubMed] [Google Scholar]
- 46.Ronchi CL, Rizzo E, Lania AG, et al. Preliminary data on biochemical remission of acromegaly after somatostatin analogs withdrawal. Eur J Endocrinol. 2008;158:19–25. doi: 10.1530/EJE-07-0488. [DOI] [PubMed] [Google Scholar]
- 47.Lal A, Chen H. Treatment of advanced carcinoid tumors. Curr Opin Oncol. 2006;18:9–15. doi: 10.1097/01.cco.0000198018.53606.62. [DOI] [PubMed] [Google Scholar]
- 48.Rubin J, Ajani J, Schirmer W, et al. Octreotide acetate long-acting formulation versus open-label subcutaneous octreotide acetate in malignant carcinoid syndrome. J Clin Oncol. 1999;17:600–6. doi: 10.1200/JCO.1999.17.2.600. [DOI] [PubMed] [Google Scholar]
- 49.Dueno MI, Bai JC, Santangelo WC, et al. Effect of somatostatin analog on water and electrolyte transport and transit time in human small bowel. Dig Dis Sci. 1987;32:1092–6. doi: 10.1007/BF01300194. [DOI] [PubMed] [Google Scholar]
- 50.Viola KV, Sosa JA. Current advances in the diagnosis and treatment of pancreatic endocrine tumors. Curr Opin Oncol. 2005;17:24–7. doi: 10.1097/01.cco.0000147902.50442.28. [DOI] [PubMed] [Google Scholar]
- 51.Butturini G, Bettini R, Missiaglia E, et al. Predictive factors of efficacy of the somatostatin analogue octreotide as first line therapy for advanced pancreatic endocrine carcinoma. Endocr Relat Cancer. 2006;13:1213–21. doi: 10.1677/erc.1.01200. [DOI] [PubMed] [Google Scholar]
- 52.Rinke A, Muller H-H, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study of the effect of Octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID study group. J Clin Oncol. 2009;27:4656–63. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 53.Arnold R, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study of the effect of Octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID study group. ASCO GI Cancers Symposium. 2009 doi: 10.1200/JCO.2009.22.8510. abst 121. Abstract. [DOI] [PubMed] [Google Scholar]
- 54.Arnold R, Muller H, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study of the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID study group. J Clin Oncol, ASCO Annual Meeting Proceedings. 2009;27 doi: 10.1200/JCO.2009.22.8510. Abst 4508. [DOI] [PubMed] [Google Scholar]
- 55.NCCN Practice Guidelines in Oncology, v.1.2009. Neuroendocrine tumors. 2009 Available at: http://www.nccn.org.
- 56.Ruszniewski P, Ish-Shalom S, Wymenga M, et al. Rapid and sustained relief from the symptoms of carcinoid syndrome: results from an open 6-month study of the 28-day prolonged-release formulation of lanreotide. Neuroendocrinology. 2004;80:244–51. doi: 10.1159/000082875. [DOI] [PubMed] [Google Scholar]
- 57.Eriksson B, Oberg K. Summing up 15 years of somatostatin analog therapy in neuroendocrine tumors: future outlook. Ann Oncol. 1999;10(Suppl 2):S31–8. doi: 10.1093/annonc/10.suppl_2.s31. [DOI] [PubMed] [Google Scholar]
- 58.Oberg K. Established clinical use of octreotide and lanreotide in oncology. Chemotherapy. 2001;47(Suppl 2):40–53. doi: 10.1159/000049160. [DOI] [PubMed] [Google Scholar]
- 59.Corleto VD, Angeletti S, Schillaci O, et al. Long-term octreotide treatment of metastatic carcinoid tumor. Ann Oncol. 2000;11:491–3. doi: 10.1023/a:1008398431246. [DOI] [PubMed] [Google Scholar]
- 60.Janson ET, Oberg K. Long-term management of the carcinoid syndrome. Treatment with octreotide alone and in combination with alpha-interferon. Acta Oncol. 1993;32:225–9. doi: 10.3109/02841869309083916. [DOI] [PubMed] [Google Scholar]
- 61.Koelz A, Kraenzlin M, Gyr K, et al. Escape of the response to a long-acting somatostatin analogue (SMS 201–995) in patients with VIPoma. Gastroenterology. 1987;92:527–31. doi: 10.1016/0016-5085(87)90153-3. [DOI] [PubMed] [Google Scholar]
- 62.Lamberts SW, Pieters GF, Metselaar HJ, et al. Development of resistance to a long-acting somatostatin analogue during treatment of two patients with metastatic endocrine pancreatic tumours. Acta Endocrinol (Copenh) 1988;119:561–6. doi: 10.1530/acta.0.1190561. [DOI] [PubMed] [Google Scholar]
- 63.Ruszniewski P, Ducreux M, Chayvialle JA, et al. Treatment of the carcinoid syndrome with the long-acting somatostatin analogue lanreotide: a prospective study in 39 patients. Gut. 1996;39:279–83. doi: 10.1136/gut.39.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ricci S, Antonuzzo A, Galli L, et al. Long-acting depot lanreotide in the treatment of patients with advanced neuroendocrine tumors. Am J Clin Oncol. 2000;23:412–5. doi: 10.1097/00000421-200008000-00020. [DOI] [PubMed] [Google Scholar]
- 65.Janmohamed S, Bloom SR. Carcinoid tumours. Postgrad Med J. 1997;73:207–14. doi: 10.1136/pgmj.73.858.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garland J, Buscombe JR, Bouvier C, et al. Sandostatin LAR (long-acting octreotide acetate) for malignant carcinoid syndrome: a 3-year experience. Aliment Pharmacol Ther. 2003;17:437–44. doi: 10.1046/j.1365-2036.2003.01420.x. [DOI] [PubMed] [Google Scholar]
- 67.O'Toole D, Ducreux M, Bommelaer G, et al. Treatment of carcinoid syndrome: a prospective crossover evaluation of lanreotide versus octreotide in terms of efficacy, patient acceptability, and tolerance. Cancer. 2000;88:770–6. doi: 10.1002/(sici)1097-0142(20000215)88:4<770::aid-cncr6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 68.Welin SV, Janson ET, Sundin A, et al. High-dose treatment with a long-acting somatostatin analogue in patients with advanced midgut carcinoid tumours. Eur J Endocrinol. 2004;151:107–12. doi: 10.1530/eje.0.1510107. [DOI] [PubMed] [Google Scholar]
- 69.Redfern JS, Fortuner WJ. Octreotide-associated biliary tract dysfunction and gallstone formation: pathophysiology and management. Am J Gastroenterol. 1995;90:1042–52. [PubMed] [Google Scholar]
- 70.Catnach SM, Anderson JV, Fairclough PD, et al. Effect of octreotide on gall stone prevalence and gall bladder motility in acromegaly. Gut. 1993;34:270–3. doi: 10.1136/gut.34.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paisley AN, Roberts ME, Trainer PJ. Withdrawal of somatostatin analogue therapy in patients with acromegaly is associated with an increased risk of acute biliary problems. Clin Endocrinol (Oxf) 2007;66:723–6. doi: 10.1111/j.1365-2265.2007.02811.x. [DOI] [PubMed] [Google Scholar]
- 72.Weckbecker G, Raulf F, Stolz B, et al. Somatostatin analogs for diagnosis and treatment of cancer. Pharmacol Ther. 1993;60:245–64. doi: 10.1016/0163-7258(93)90009-3. [DOI] [PubMed] [Google Scholar]
- 73.Froidevaux S, Eberle AN. Somatostatin analogs and radiopeptides in cancer therapy. Biopolymers. 2002;66:161–83. doi: 10.1002/bip.10256. [DOI] [PubMed] [Google Scholar]
- 74.Susini C, Buscail L. Rationale for the use of somatostatin analogs as antitumor agents. Ann Oncol. 2006;17:1733–42. doi: 10.1093/annonc/mdl105. [DOI] [PubMed] [Google Scholar]
- 75.Schally AV, Szepeshazi K, Nagy A, et al. New approaches to therapy of cancers of the stomach, colon and pancreas based on peptide analogs. Cell Mol Life Sci. 2004;61:1042–68. doi: 10.1007/s00018-004-3434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slijkhuis WA, Stadheim L, Hassoun ZM, et al. Octreotide therapy for advanced hepatocellular carcinoma. J Clin Gastroenterol. 2005;39:333–8. doi: 10.1097/01.mcg.0000155136.35315.de. [DOI] [PubMed] [Google Scholar]
- 77.Dimitroulopoulos D, Xinopoulos D, Tsamakidis K, et al. The role of sandostatin LAR in treating patients with advanced hepatocellular cancer. Hepatogastroenterology. 2002;49:1245–50. [PubMed] [Google Scholar]
- 78.Kwekkeboom DJ, Teunissen JJ, Kam BL, et al. Treatment of patients who have endocrine gastroenteropancreatic tumors with radiolabeled somatostatin analogues. Hematol Oncol Clin North Am. 2007;21:561–73. doi: 10.1016/j.hoc.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 79.De Jong M, Valkema R, Jamar F, et al. Somatostatin receptor-targeted radionuclide therapy of tumors: preclinical and clinical findings. Semin Nucl Med. 2002;32:133–40. doi: 10.1053/snuc.2002.31027. [DOI] [PubMed] [Google Scholar]
- 80.Rosenoff SH, Gabrail NY, Conklin R, et al. A multicenter, randomized trial of long-acting octreotide for the optimum prevention of chemotherapy-induced diarrhea: results of the STOP trial. J Support Oncol. 2006;4:289–94. [PubMed] [Google Scholar]
- 81.Zidan J, Haim N, Beny A, et al. Octreotide in the treatment of severe chemotherapy-induced diarrhea. Ann Oncol. 2001;12:227–9. doi: 10.1023/a:1008372228462. [DOI] [PubMed] [Google Scholar]
- 82.Baillie-Johnson HR. Octreotide in the management of treatment-related diarrhoea. Anticancer Drugs. 1996;7(Suppl 1):11–15. doi: 10.1097/00001813-199601001-00003. [DOI] [PubMed] [Google Scholar]
- 83.Nikou GC, Toumpanakis C, Katsiari C, et al. Treatment of small intestinal disease in systemic sclerosis with octreotide: a prospective study in seven patients. J Clin Rheumatol. 2007;13:119–23. doi: 10.1097/RHU.0b013e3180645d2a. [DOI] [PubMed] [Google Scholar]
- 84.Gambineri A, Patton L, De Iasio R, et al. Efficacy of octreotide-LAR in dieting women with abdominal obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:3854–62. doi: 10.1210/jc.2004-2490. [DOI] [PubMed] [Google Scholar]
- 85.Lustig RH, Greenway F, Velasquez-Mieyer P, et al. A multicenter, randomized, double-blind, placebo-controlled, dose-finding trial of a long-acting formulation of octreotide in promoting weight loss in obese adults with insulin hypersecretion. Int J Obes (Lond) 2006;30:331–41. doi: 10.1038/sj.ijo.0803074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramage JK, Davies AH, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut. 2005;54(Suppl 4):iv1–16. doi: 10.1136/gut.2004.053314. [DOI] [PMC free article] [PubMed] [Google Scholar]