Abstract
Relapse remains a leading cause of death after allogeneic hematopoietic cell transplantation (HCT) for patients with high-risk leukemias. The potentially beneficial donor T-cell-mediated graft-versus-leukemia (GVL) effect is often mitigated by concurrent graft versus host disease (GVHD). Providing T-cells that can selectively target Wilms’ Tumor Antigen 1 (WT1), a transcription factor over-expressed in leukemias that contributes to the malignant phenotype, represents a potential opportunity to promote anti-leukemic activity without inducing GVHD. HLA A*0201-restricted WT1-specific donor-derived CD8+ cytotoxic T-cell (CTL) clones were administered post-HCT to 11 relapsed or high-risk leukemia patients without any evidence of on-target toxicity. The last four treated patients received CTL clones generated with exposure to IL-21 as a means to prolong in vivo CTL survival, as IL-21 can limit terminal differentiation of antigen-specific T-cells generated in vitro. Transferred cells exhibited direct evidence of anti-leukemic activity in 2 patients: a transient response in one patient with advanced progressive disease and the induction of a prolonged remission in a patient with minimal residual disease (MRD). Additionally, three treated patients at high risk for relapse post-HCT survive without leukemia relapse, GVHD or additional anti-leukemic treatment. CTL generated in the presence of IL-21, which were transferred in these latter three patients and the patient with MRD, all remained detectable long-term and maintained/acquired in vivo phenotypic and functional characteristics associated with long-lived memory CD8+ T-cells. This study supports expanding efforts to immunologically target WT1, and provides insights into the requirements necessary to establish potent persistent T-cell responses in patients.
Introduction
Leukemic relapse after HCT remains a major cause of treatment failure in high-risk patients who enter HCT with poor prognostic characteristics. Patients who develop GVHD have reduced relapse rates, suggesting that lymphocytes present in engrafted cells can mediate a concurrent therapeutic GVL effect (1, 2). However, as graft T-cells have not been selected for specificity for leukemia antigens, and commonly recognize proteins expressed by many other host tissues, substantial morbidity and mortality from GVHD can occur.
One strategy to enhance the GVL effect without promoting GVHD in post-HCT patients is to target leukemia-associated antigens with purified antigen-specific CD8+ CTL. In this approach, CD8+ CTL are isolated and cloned from donor peripheral blood mononuclear cells (PBMCs) based on antigen-specific T-cell-mediated lysis of target cells, and the highest avidity clone selected from each patient-donor pair and expanded for infusion. Limiting adoptively transferred CD8+ T-cells to a homogenous well-characterized product allows for tracking the provided response, facilitating analyses to help define parameters for immune-mediated eradication and long-term control of leukemic relapse. The most ideal target antigens are unique mutated proteins that are also obligate for the leukemic phenotype. However, T-cell responses to common mutations such as epitopes created by TEL-AML1 or BCR-abl fusions have been hampered, in part due to limited processing and/or few unique epitopes that bind to HLA alleles (3, 4). Alternatively, non-polymorphic proteins over-expressed by leukemic cells that contain many potential epitopes can be attractive candidate targets for CTL (5). The zinc finger transcription factor WT1 is expressed at 10–1000x fold higher levels in leukemic cells compared to normal CD34+ cells, and the magnitude of expression correlates with clinical aggressiveness of acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), and acute lymphoid leukemia (ALL) (6–8). As WT1 promotes proliferation and oncogenicity, loss of expression is disadvantageous for the tumor, making outgrowth of antigen-loss variants less likely (9). Although essential during embryogenesis, WT1 expression after birth is limited to low levels predominantly in kidney podocytes and CD34+ hematopoietic stem cells (HSC) (10–12). WT1-specific CD8+ T lymphocytes can distinguish over-expressing targets from normal cells and have been demonstrated to inhibit the growth of and to lyse leukemic but not normal CD34+ cells (13). Although vaccines targeting WT1 have resulted in clear anti-tumor responses in some patients, most patients have failed to benefit clinically, potentially reflecting the induction of weak responses due to the limited immunogenicity of vaccine regimens, the presence/generation of WT1-specific CD4 regulatory T-cells, and/or compromised patient immune systems or T-cell repertoires (14).
Adoptive transfer of donor-derived ex vivo-expanded WT1-specific CD8+ CTLs in post-transplant patients can potentially bypass the limitations encountered during vaccination by increasing the number and quality of T-cells targeting tumor-associated antigens. An analogous strategy has proven effective in reducing tumor burdens in melanoma patients, with clinical responses tightly correlated with the duration of in vivo persistence of transferred T-cells (15–18). Re-infusion of CD8+ CTLs derived from less terminally differentiated populations such as central memory T-cells (Tcm), which possess the ability to self-renew and maintain robust responses over time, has been shown to establish prolonged responses (19–21). Increased persistence has also been observed with murine CD8+ CTLs derived from the naïve pool when these cells were primed in the presence of the γc-chain cytokine Interleukin-21 (IL-21) (22), which promotes expansion of responding T-cells in vitro that phenotypically appear less terminally differentiated (23). Because CTL clones for this study were generated from the repertoire of healthy donors and likely derived from the naïve cell population, we utilized IL-21 after it became available for clinical use in a subset of patients on this trial, hypothesizing that generating WT1-specific CTLs clones in the presence of IL-21 might confer an increased ability for these cells to survive and persist in vivo after transfer.
Our results show that adoptive transfer to post-HCT patients of donor-derived WT1-specific CTLs followed by low-dose s.c IL-2 is safe and results in direct evidence of anti-leukemic activity. Furthermore, all patients who received WT1-specific CTL generated in the presence of IL-21 (three patients at high risk of relapse post-HCT and one patient treated with MRD) have survived in the absence of leukemia relapse for >30 months, with no other anti-leukemic treatment or GVHD. In these patients only, transferred T-cells remained detectable long-term and maintained/acquired phenotypic and functional characteristics associated with long-lived memory CD8+ T-cells.
Results
WT1-specific CD8+ CTL clonal populations primed in the presence of IL-21 have higher fractions of cells expressing CD27, CD28 and CD127
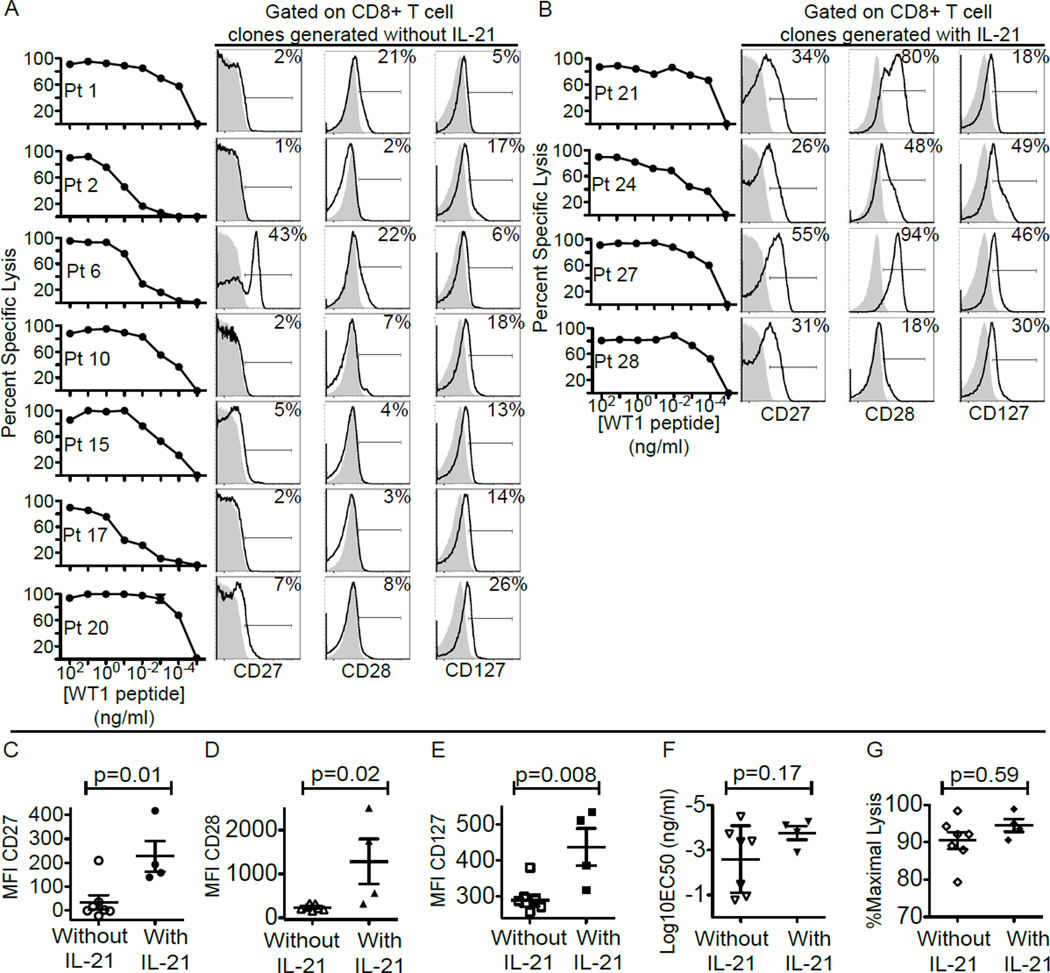
WT1-specific CD8+ T-cells were generated from each donor after stimulation with WT1 peptide, and expanded and cloned as described in the Methods. The clones for each patient/donor pair were tested for specific recognition and lysis of WT1+ targets in a 51Cr-release assay with HLA-A*0201, transporter for antigen presentation (TAP)-deficient, B-lymphoblastoid cells (T2 B-LCL) pulsed with titrating doses of the HLA-A*0201-restricted WT1126–134 (RMFPNAPYL) epitope, and the clones that recognized targets pulsed with the lowest concentration of the peptide were selected for infusions (Fig. 1A and B). Immediately before infusion, all clones, whether generated in the presence or absence of IL-21 supplementation, expressed CD3, CD8 and CD45RO but not CD4, CD16, CD19 or CD45RA, consistent with an antigen-experienced but not terminally-differentiated phenotype. However, as we reported previously (15, 24), the expanded cell populations, despite being clonal and derived from a single cell, do contain cells expressing different amounts of costimulatory and differentiation markers. In the WT1-specific clonal populations generated with exposure to IL-21, the median frequencies of cells expressing CD27, CD28, or CD127 were 32.5, 64 and 38% respectively, compared to 2, 7 and 14% within clonal populations generated in the absence of IL-21. Median fluorescence intensities of CD27, CD28 and CD127 were also significantly higher (p values = 0.01, 0.02 and 0.008 respectively) on clones generated in the presence of IL-21 (Fig. 1C, D and E). However, previous exposure to IL-21 did not appear to impact the avidity or cytolytic function of the CTL clones generated, with no significant difference detected in the mean effective peptide concentrations required to induce 50% lysis (EC50) of WT1-pulsed T2 B-LCL (mean EC50 of CTL clones generated without IL-21 was 10−2.59 ng/ml, compared to mean EC50 with IL-21 of 10−3.77 ng/ml) or the percent maximal lysis at an effector to target ratio of 10:1 (the mean for CTL clones generated without IL-21 was 90.46% compared to the mean with IL-21 of 94.55%) (Fig.1F and G).
Figure 1. Phenotypic and functional characteristics of WT1-specific CD8+T-cell clones isolated and expanded for infusions.
(A and B) From the left: Lysis by WT1-specific CD8+T-cell clones of TAP-deficient HLA-A*0201+ B-LCL (T2 B-LCL) pulsed with decreasing concentrations of WT1 peptide in a 51Cr-release assay, and expression of CD27, CD28 and CD127 by WT1-specific CD8+T-cell clones (bold line) compared to isotype control (grey area) for clones generated without IL-21 (A) and with exposure to IL-21 (B). Inset values represent percentages of CD27+, CD28+ and CD127+CD8+T-cells respectively. Median fluorescent intensity (MFI) of staining for CD27 (C), CD28 (D), and CD127 (E). Mean effective concentrations of peptide required to achieve 50% lysis (EC50) (F), and percent maximal lysis at an effector to target ratio (E:T) of 10:1 (G) of clones generated without (left) or with IL-21 (right). An unpaired two-tailed equal variance test was used for statistical analysis.
Adoptive transfer of escalating doses of donor-derived WT1-specific CTLs does not injure normal tissues expressing low physiologic levels of WT1
Eleven patients with AML, MDS or ALL received a total of 36 escalating doses of CTL clones post-HCT specific for the peptide RMFNAPYL, an HLA-A*0201-restricted epitope of WT1 (Table 1 and Fig. S1). Overall, 13/36 doses administered represented the maximum target dose of 1 × 1010 WT1-specific CTL/m2 and 11/36 doses (3.3 × 109 or 1010/m2) were followed by low-dose s.c. IL-2, which was administered to enhance the survival of transferred T-cells (25). With the exception of expected transient side effects associated with activation of large numbers of antigen-specific CTL transferred into patients with targets expressing the antigen and/or low-dose s.c. IL-2, the infusions were well tolerated. Specifically, fevers (≥38.3°C) +/− chills resolving without specific treatment within 24 hours, a temporary drop in total lymphocyte counts that returned to pre-infusion levels within 7–11 days in patients with no evidence of relapsed disease, and transient mild injection site reactions accompanying low-dose s.c. IL-2 occurred in 25%, 77% and 82% of cases respectively (Table S1). No toxicities to the hematopoietic or renal systems, reflecting potential WT1+ targets, were detected during the monitoring period, nor was there any evidence of new-onset GVHD. This absence of toxic effects or GVHD was also observed for the 4 patients in whom the T cell clones remained detectable in the blood for ≥7–14 months after infusions (Fig. 2). Thus, infusion of the most avid WT1-specific CTL clone derived from each patient’s donor, with or without low-dose s.c. IL-2, was well-tolerated and safe at doses up to 1010 cells/m2.
Table 1.
Patient characteristics
| Pt | M/F | Age | Disease | Disease characteristics at HCT |
Conditionning Regimen | Days Between HSCT and 1st CTL infusion |
Disease Characteristics Between HSCT and 1st CTL Infusion |
Disease Characteristics at 1st CTL infusion |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 29 | ALL; normal cytogenetics. |
Chemorefractory after 2 inductions (Ara-C/Idarubicin), 12% BM blasts entering HCT. |
MA: CY/12Gy TBI. | 193 | 1.7% BM blasts D28 after HCT. Received 2 re- inductions (Mylotarg then Mitoxantrone, Ara- C) resulting in persistent disease. |
20% BM blasts 21 days prior. |
| 2 | F | 59 | AML with multilineage dysplasia; normal cytogenetics. |
Hematopoietic aplasia after 2 inductions (Ara-C/Idarubicin). |
NMA: Fludarabine/2Gy TBI | 302 | Relapse D156 post HCT. Received 2 courses of Mylotarg resulting in progression (30% BM blasts). Received Mitoxantrone, VP-16 44 days prior to CTL infusion. |
Hypocellular BM no blasts, persistent peripheral pancytopenia 1 day prior. |
| 6 | F | 42 | ALL; BCR/ABL+ | Hematologic remission after induction (Ara-C/Idarubicin). Molecular relapse BCR-ABL+ by PCR. |
MA: 12 Gy TBI/CY. | 455 | Persistent BCR-ABL+D35 post HCT PCR for BCR-ABL resulted negative with increased imatinib and taper of immunosuppression. |
Molecular CR (BCR-ABL neg) 260 days prior On imatinib 600mg daily. |
| 10 | F | 51 | AML secondary to breast cancer treatment; t(8;21), del (19q). |
CR after induction (Ara- C/Idarubicin). |
MA: Busulfan/CY. | 127 | Relapse D86 after HCT (43% BM blasts). Received Mitoxantrone, VP-1 6, Ara-C resulting in CR. |
Re-induction 44 days prior to CTL. CR 5 days prior. |
| 15 | F | 51 | AML secondary to MDS; Complex cytogenetics including monosomy 7. |
Chemorefractory after 2 inductions (Ara-C/Idarubicin, Clofarabine/Ara-C), 3.4% BM blasts entering HCT. |
RI: 131I–BC8 (anti-CD45 antbody) 24Gy targetted/ Fludarabine/2 Gy TBI. |
192 | Persistent monosomy 7 post HCT (1.8% D28, 4.5% D45). GVHD/infectious complications delaying CTL start. |
Relapse by flow (6.3% BM blasts, 11.8% PB blasts), morphology and cytogenetics, 2 days prior. |
| 17 | M | 65 | AML; normal cytogenetics. |
2nd CR (Ara-C/Idarubicin, Mitoxantrone/VP-16/Ara-C). |
RI:31I–BC8 (anti-CD45 antbody) 24Gy targetted/ Fludarabine/2 Gy TBI. |
876 (2.4 years) |
Relapse 2 years post HCT (85% BM blasts). Received Mitoxantrone/Ara-C resulting in morphological CR. |
Morphologic CR 62 days pnorto CTL. CR (morphology, flow, cytogenetics) 1 day prior. |
| 20 | F | 52 | AML; normal cytogenetics. |
3rd relapse (Ara-C/Idarubicin, Cytosine/Arabinoside/Idarubi cin/Pravastatin) entering HCT (4.6%BM blasts, 0.9% PB blasts). |
RI:Treosulfan/Fludarabine. | 419 | Relapse D383 post HCT with (0.01% PB and BM blasts). Patient elected to receive WT1- specific CTL. |
Relapse (1.6% BM blasts, 0.3% PB blasts) by flow 1 day prior. |
| 21 | F | 59 | AML secondary to MDS; normal cytogenetics. |
Chemorefractory after 4 inductions (Ara-C/Idarubicin, Clofarabine/Ara-C ×2 Vidaza/Mylotarg), 47% BM blasts entering HCT. |
MA: 12Gy TB/CY. | 168 | CR. | CR. |
| 24 | F | 42 | AML; t(8;21). | Chemorefractory after 2 inductions (Fludarabine/Mylotarg/Ara-C, Clofarabine/Idarubicin/Ara- C), 4.4% BM blasts, 3.5% PB blasts entering HSCT. |
RI:Treosulfan/Fludarabine. | 159 | Persistent MRD post HCT. Recevied Azacitidine/Mylotarg D66 post HCT resulting in CR D83 post HCT |
CR since 76 days prior. |
| 27 | F | 32 | B-ALL; complex cytogenetics, BCR/ABL- |
Relapse after 1st HCT (12Gy TB, CY). Achieved CR with Hyper-CVAD. |
MA: CY/Busulfan. | 64 (after 2nd HCT) |
Persistent MRD (clonal B-cell population) | Morpholgic and flow cytometric CR Clonal B- cell population detected by PCR 8 days prior. |
| 28 | M | 26 | AML, normal cytogenetics. |
CR after 2 indudions (Ara- C/Idarubicin/Mylotarg, Ara- C/Idarubicin). |
RI:Treosulfan/Fludarabine/ 2Gy TBI. |
594 (1.6 years) |
MRD (2% BM blasts) 1-year post HCT responding to taper of immunosuppression. |
CR since 182 days prior. |
MA: myelo-ablative, NMA: non-myelo-ablative, RI: reduced intensity, CR: complete remission, MRD: minimal residual disease, CY: cyclophosphamide.
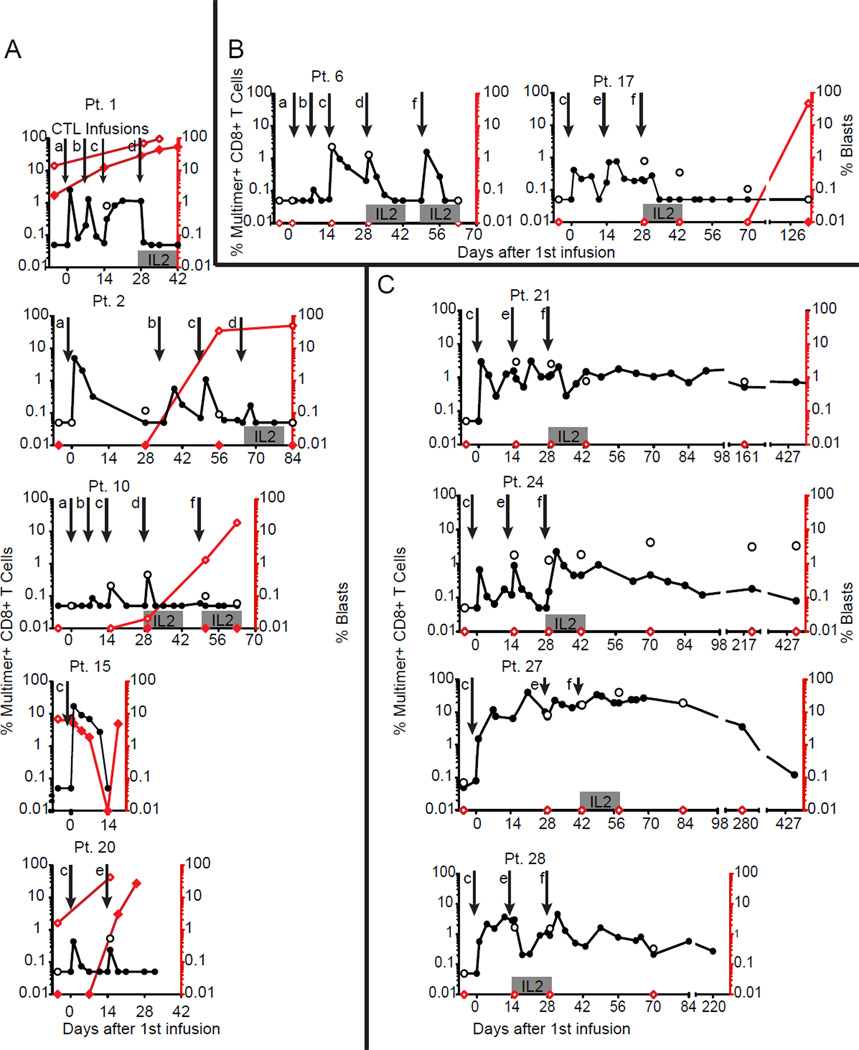
Figure 2. Kinetics of in vivo persistence of WT1-specific CTL clones and leukemia disease burden.
Percent multimer+CD8+ T-cells (left y-axis) in PBMCs (solid circles) and BM (open circles), and percent leukemic blasts (right y-axis) in PBMCs (solid red diamonds) and BM (open red diamonds) collected 7 days (+/−2 days) before and at defined timepoints after infusions are shown. (A) The 5 Pts who received clones generated without IL-21 with detectable leukemia at the time of treatment, (B) The 2 Pts who received clones generated without IL-21 without detectable leukemia at the time of treatment, and (C) the 4 patients who received clones generated in the presence of IL-21, including 1 with MRD (Pt 27) and 3 without detectable leukemia at the time of treatment. Infusion schedule indicated by downward arrows: (a) 3.3 × 108 WT1-specific CTL/m2; (b) 1 × 109 CTL/m2; (c) 3.3 × 109 CTL/m2; (d) 3.3 × 109 CTL/m2 followed by low dose s.c IL-2 × 14 days; (e) 1 × 1010 CTL/m2; (f) 1 × 1010 CTL/m2 followed by low dose s.c IL-2 × 14 days.
Transferred WT1-specific CTL clones can persist after infusion into patients with MRD or undetectable leukemic burden
Analysis of patients for the presence of pre-existing WT1-specific multimer+ T-cells in PBMC (range 0-0.06%) and bone marrow (BM) (range 0%-0.13%) revealed median frequencies of 0% at both sites, attesting to the essentially undetectable frequency of endogenous WT1-specific CD8+ T-cells in most patients. The first 7 infused patients received escalating doses of WT1-specific CTL clones generated in the absence of IL-21. Five had detectable leukemia during all or some infusions and 2 were in CR after salvage therapy for relapse after HCT at least 60 days prior to the first infusion and remained in CR throughout the infusions (patients [Pts] 6 and 17) (Fig. 2A and B). In this subset of patients, none of the clones persisted beyond 14 days in blood or BM (range 0–14, median 1 day). In contrast, the last 4 patients received WT1-specific CTL clones generated in the presence of IL-21. Three were in CR at least 60 days prior to the first infusion and one (Pt 27) had MRD (B-cell clonal population detected in the BM). For this subset of patients, the infused CTL clones persisted in all recipients at levels >0.05% well beyond 14 days, at ≥430 days in Pts 21, 24 and 27, and at ≥230 days in Pt 28 (Fig. 2C).
For patients who had received the maximal dose of 1010 CTL/m2 (irrespective of exposure to IL-21), direct intra-patient comparisons revealed that the median peak CTL frequency, achieved 24–72 hours after infusions, appeared to be higher if followed by IL-2 than without IL-2 (3.1% multimer+ CD8+ T-cells compared to 1.5%), as did the frequencies sustained at day 14 (1.5% with IL-2 compared to 0.6% without IL-2) (Fig. S2 A and B). Although the difference, with the limited numbers of infusions being analyzed, did not achieve statistical significance, the benefits of IL-2 administration after T-cell transfer have been previously reported (25). Regulatory T-cells (Tregs) can be sensitive to exogenous IL-2, and could negatively impact the persistence and function of transferred CTL. Therefore, we assessed Treg numbers after all infusions based on expression of the surrogate markers CD4 and CD25 and the absence of CD127. Treg frequencies increased after infusions followed by low-dose IL-2 from baseline levels at 7 (p=0.04) and 14 days (p=0.01), and returned to near baseline levels by day 28 (p=0.26) (Fig. S3). Changes in Treg frequencies never achieved statistical significance after infusion of T-cells without IL-2, and at day 28 the Treg frequencies after infusions with or without IL-2 were nearly identical.
Transferred WT1-specific CD8 CTL preferentially localize to the BM compartment
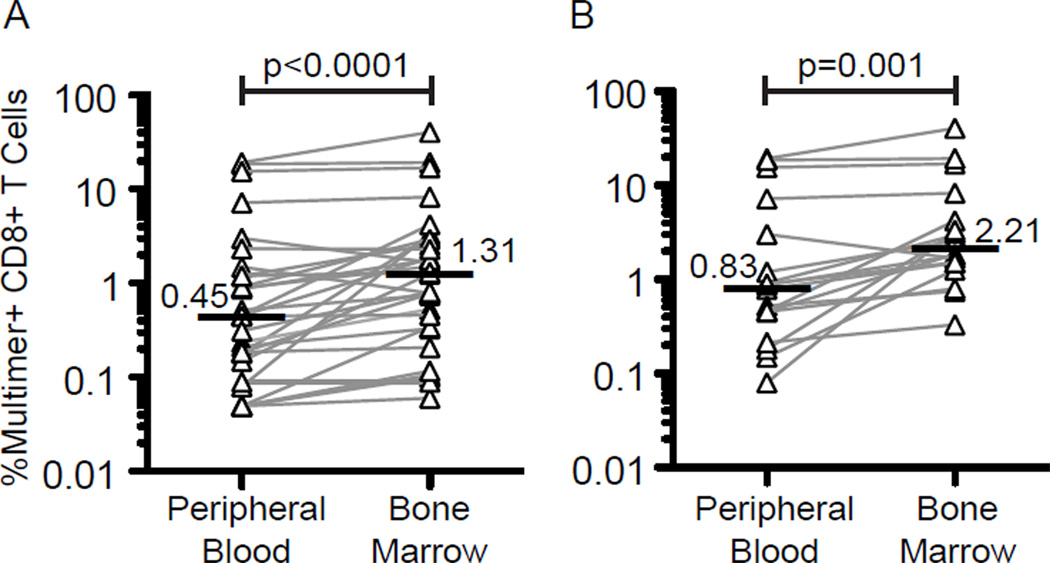
We next assessed whether transferred CTL clones reached the BM, the main site of accumulation of leukemic cells and the most common site of relapse. All patients consented to BM assessments prior to CTL infusions, 1 day after the second infusion, and 1 day after the last dose of s.c IL-2. The median frequencies of WT1-specific CTL in PBMC and BM from all patients, and at all time-points in which transferred cells could be detected at either site were 0.45% and 1.31% respectively (p<0.001), suggesting preferential accumulation of the transferred cells in the BM (Fig. 3A). For the subset of patients who received WT1-specific CTL generated with exposure to IL-21, which coincided with patients who demonstrated T-cell persistence beyond 14 days, the median WT1-specific CTL frequencies in PBMC and BM over multiple time points were 0.83% and 2.21% respectively (p=0.001) (Fig. 3B).
Figure 3. Localization of adoptively transferred WT1-specific CTL to the BM.
(A) Percent multimer+ CD8+ cells in blood (left) and BM (right) at all time points from the 11 patients in whom blood and BM were analyzed at the same time. (B) Same analysis as above performed at all time points on the 4 patients who received CTL clones that were generated with exposure to IL-21 and persisted long-term in vivo. Only samples in which at least one site showed detectable transferred cells are shown. Horizontal bars indicate medians. A two-tailed paired signed-rank test was used for statistical analysis.
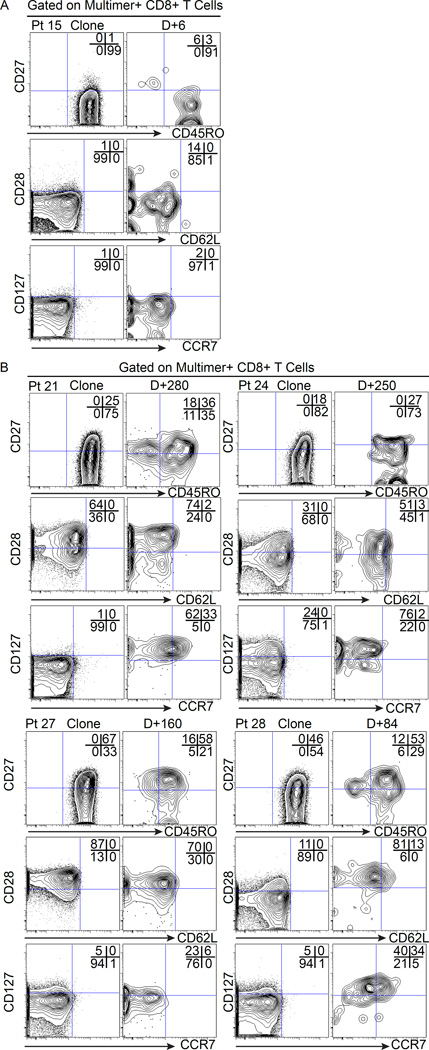
CTL clones generated with exposure to IL-21 maintain/acquire in vivo characteristics associated with long-lived memory
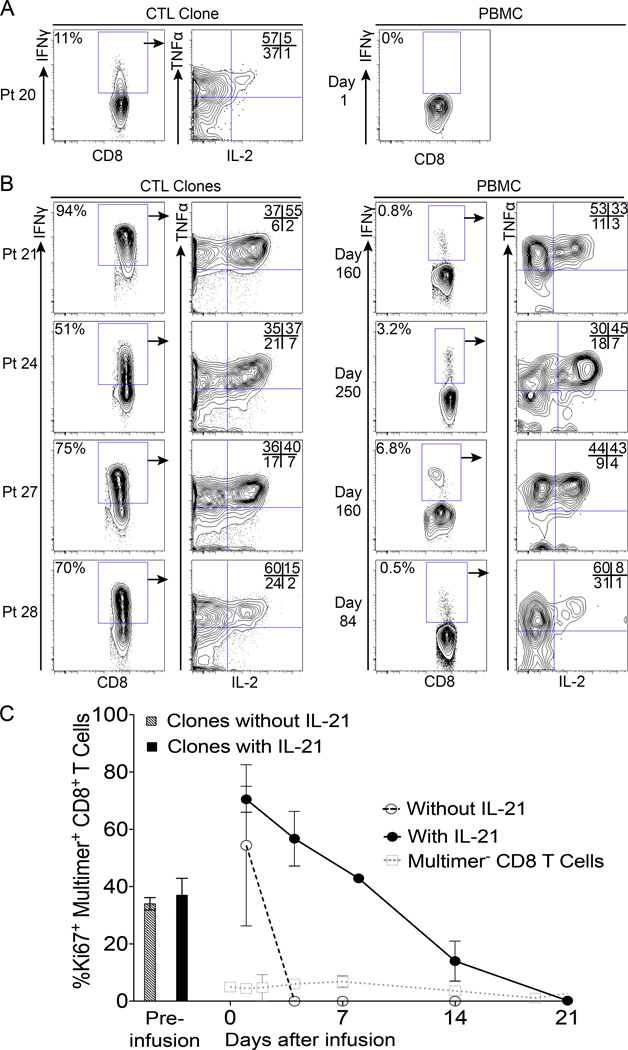
Patients who received CTL clones generated without IL-21 had absent or low expression of CD27, CD28 and CD127 (Fig. 1A), and all these clones persisted ≤14 days in vivo. Consequently, the in vivo phenotype could only be assessed for the brief period that the T-cells persisted (≤14 days), but no change in expression of CD45RO, CD27, CD28, CD127, CD62L or CCR7 was observed (Fig. 4A and Fig. S4). In contrast, as previously described, CTL clones generated with exposure to IL-21 expressed higher levels of CD27, CD28 and CD127 before being infused (Fig. 1B) and demonstrated long-term in vivo survival after infusion. Based on gating multimer+CD8+T-cells at days 280, 250, 160 and 84 for Pts 21, 24, 27 and 28 respectively (Fig. 4B), subpopulations of the infused cells maintained or up-regulated phenotypic markers associated with long-lived memory CD8 T-cells (CD27, CD28, CD127, CD62L and CCR7) (Fig. S4).
Figure 4. Phenotypic characteristics of transferred WT1-specific CD8+ T-cells persisting in vivo.
(A) Expression of CD27 (y-axis) and CD45RO (x-axis) (upper plots), CD28 (y-axis) and CD62L (x-axis) (middle plots), and CD127 (y-axis) and CCR7 (x-axis) (lower plots) on gated multimer+ cells for CD8+ CTL clones generated without IL-21 for Pt 15 (representative) immediately before infusion and after 6 days in vivo; and (B) CTL clones generated with exposure to IL-21 for Pts 21, 24, 27 and 28 immediately before infusions, and 280, 250, 160 and 84 days respectively in vivo after the first infusion.
The functional profile of persistent infused cloned T-cells was determined by gating on IFNγ-producing cells. Prior to infusions, all CTL clones secreted IFNγ and TNFα in response to WT1-pulsed T2 B-LCL. Clones expressing CD28 secreted the highest levels of IL-2, consistent with the known contribution of a costimulatory signal via CD28 (26, 27). Consistent with the results obtained by multimer staining, which revealed a low to undetectable frequency of endogenous WT1-reactive cells, no IFNγ-producing cells could be detected in any patient prior to infusions. In patients who received CTL clones generated without IL-21, no IFNγ-producing cells could be detected in vivo after infusions, despite the transient persistence of transferred T-cells revealed by multimer staining (Fig. 5A). In contrast, clones generated with exposure to IL-21 secreted IFNγ, TNFα and IL-2 both prior to infusion (consistent with the expression of CD28) and in vivo after adoptive transfer for the entire period the cells could be detected by multimer staining (Fig. 5B).
Figure 5. Functional characteristics of persisting transferred WT1-specific CTL.
(A) left-most plot: Percent cells producing IFNγ by the CTL clone generated without IL-21 for Pt 20 (representative) in response to WT1-pulsed T2 B-LCL at an E:T ratio of 10:1; next plot to the right: TNFα (y axis) and IL-2 (x-axis) production of IFNγ+ cells. Right-most plot: the same analysis performed on PBMC 1 day after transfer in vivo. (B) Plots to the left: Percent IFNγ production for CTL clones generated with exposure to IL-21 (Pts 21, 24, 27 and 28), and the respective TNFα and IL-2 production. Plots to the right: The same analysis performed on PBMC obtained after 160, 250, 160 and 84 days respectively in vivo. (C) Intranuclear Ki-67 expression on pre-infusion CTL clones harvested on day 14 of the ex vivo expansion cycle generated without (striped/grey column) or in the presence of IL-21 (solid column). Intranuclear Ki-67 expression on post-infusion CD8+multimer+ cells averaged from PBMCs of patients who received CTL clones generated without (open circles, dashed lines) or in the presence of IL-21 (solid circles, solid lines). Open squares and grey dotted lines represent average Ki-67 expression on patient endogenous CD8+multimer- cells for all patients combined.
As cells possessing the potential to divide might also persist better, we investigated whether infused CTL clones expressed Ki-67 in vivo, a marker of recent proliferation (28). For clones generated without/with exposure to IL-21 and harvested for infusion on day 14 of the stimulation cycle, a median of 37% and 37.5% of cells expressed Ki-67 respectively (Fig. 5C left columns). Early after transfer (day 1), most infused multimer+ clonal CTL populations entered the cell cycle, with >55% of cells expressing Ki-67. Cloned CTL populations generated without exposure to IL-21 lost Ki-67 expression by day 4 (Fig. 5C dashed black line), but CTL clones generated with exposure to IL-21 expressed Ki-67 on a fraction of cells until day 21 after transfer (Fig. 5C solid black line). Infusions of WT1-specific CTL clones had no impact on the proliferation of host multimer– CD8 T cells (Fig. 5C dotted grey line). The in vivo proliferation of CTL clones after infusions followed by low-dose s.c. IL-2 (irrespective of the use of IL-21), as reflected by Ki-67 expression, tended to increase 4 days after transfer (mean with IL-2 65.8% compared to 30.7% without IL-2), but this difference did not reach statistical significance (Fig. S5). Furthermore, administration of low-dose s.c. IL-2 did not affect the proliferation of host multimer– CD8+ populations (Fig. S5). Thus, the CTL clones generated with exposure to IL-21 and infused into patients with MRD or undetectable leukemia exhibited phenotypic and functional properties associated with long-lived CD8+ T-cells capable of proliferating independent of CD4 Thelper cells, and a fraction of these CD8+ T-cells sustained a proliferative state until day 21 after transfer.
Evidence of antileukemic activity mediated by WT1-specific CD8 CTL
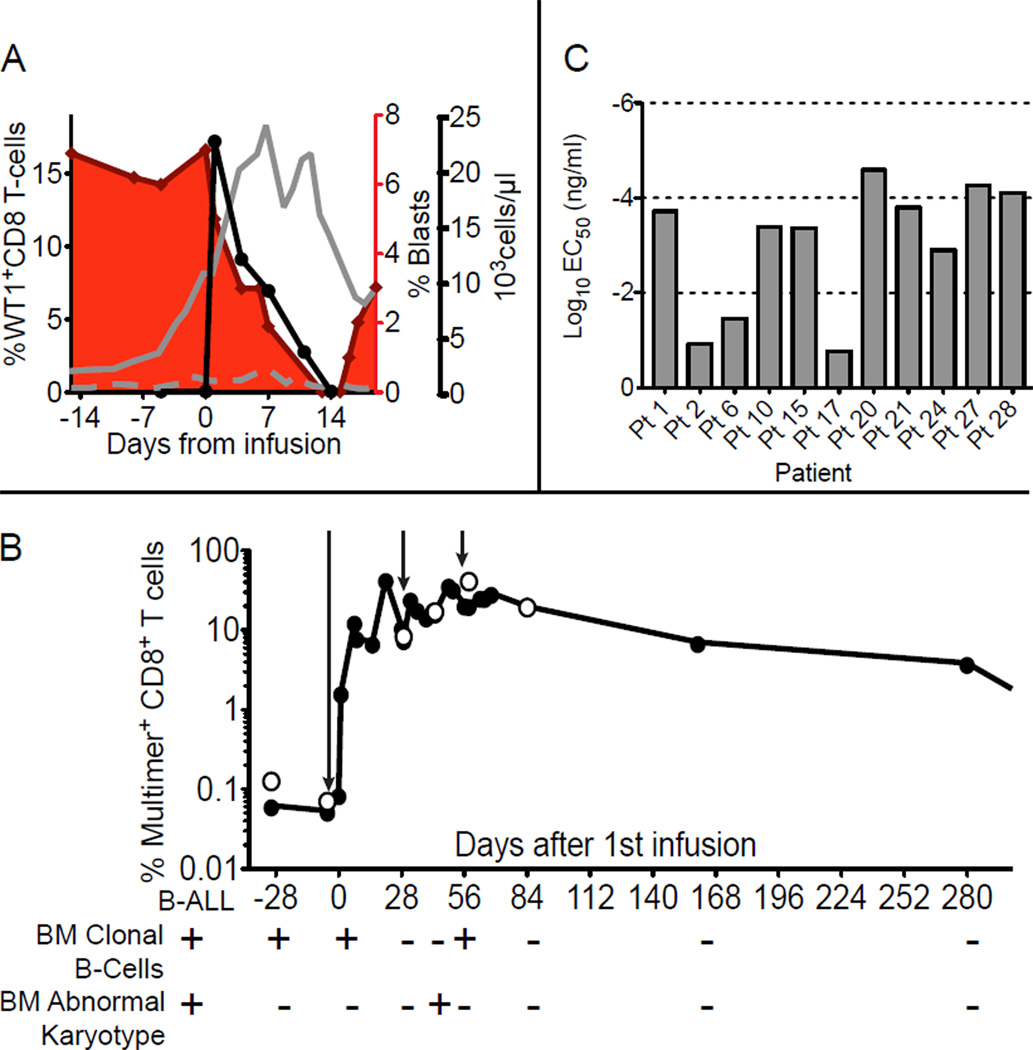
Eleven patients with high-risk disease were treated on this study (Table 1). High-risk features included poor prognostic indicators conferred by the cytogenetics of the primary leukemia (29), AML secondary to MDS or prior chemotherapy treatments (30), refractoriness to induction therapy prior to HCT (31), entering HCT with detectable blasts (32) (Pt 21 entered HCT with refractory disease and had a BM containing 40% blasts), or relapse after HCT (32, 33). Due to the heterogeneity of the patients treated, a comparative ranking of the adverse prognosis of patients at study entry who received clones generated with or without IL-21 is not reliable (34), but all had at least 2 high-risk features (Table S2). Of patients who received clones generated without IL-21, all had relapsed after HCT (associated with >95% mortality at 2 years (33)), and 5/7 had detectable leukemia at the time they received WT1-specific T-cells. Of the patients who received clones generated with IL-21, 3/4 had relapsed after HCT and 1 patient entered HCT with >40% blasts, which is associated with a <5% disease free survival 1 year after HCT (32). However, only 1 patient (Pt 27) had detectable disease at the time of WT1-specific T-cell infusions. Thus, although all patients had high-risk disease, patients who received clones generated with IL-21 had MRD or no detectable leukemia at the time of infusions, and therefore better disease characteristics compared to patients who received WT1-specific clones generated without IL-21. In 10/11 patients, leukemia cells were available for analysis, and expression of WT1 in the leukemic cells was confirmed. Table 2 summarizes the clinical outcomes of each patient. One patient (Pt 15) with detectable leukemic blasts in the blood (7% of total white blood cells [WBC]) exhibited a rapid reduction in the percentage of blasts after the infusion of 3.3 × 109 WT1-specific CTL/m2, with blasts declining to 0% of total WBCs after 14 days (Fig. 6A). The decrease in detectable leukemic blasts was associated with increasing absolute numbers of circulating normal WBCs and coincided with the presence of infused WT1-specific CTL in the blood. The patient developed toxicity from prior anti-leukemic therapy that precluded qualifying for subsequent T-cell infusions, and died with progressive disease after disappearance of the infused T-cells. Another patient (Pt 27) had no detectable leukemic blasts but presented with MRD prior to infusions as revealed by detection of a clonal B cell population and an abnormal karyotype in the BM. After the infusions of WT1-specific CTL were completed, which resulted in percentages of WT1-specific multimer+ CD8+ T-cells maintained at 6.6% and 3.6% 168 and 280 days respectively from the time infusions began, the patient exhibited clearing of the clonal B cell population and cytogenetic analysis no longer revealed any abnormalities (Fig 6B). Based on the ability to lyse T2 B-LCL pulsed with titrated peptide doses, the avidities of the clones infused for Pts 15 and 27, who had detectable disease, and Pts 21, 24, and 28, who were treated with undetectable disease, were similar to the highest avidities obtained in this study (Fig. 6C). All four patients who received CTL clones generated in the presence of IL-21 demonstrated long-term CTL persistence and were in CR without additional chemotherapy or the development of GVHD at 22, 33, 35 and 38 months after HCT despite historical probabilities of relapse estimated at 90–95% after 1 year and 95–97% after 2 years (Table S3). Among the 7 patients who received CTL clones generated in the absence of IL-21, all of whom demonstrated only short-term CTL persistence, 5 had detectable disease and 2 (Pts 6 and 17) had no detectable disease during infusions. However, Pt 6 was receiving concurrent immunosuppressive therapy for pre-existing GVHD, which included mycophenolate mofetil (MMF), which may have contributed to poor T-cell persistence, especially in the presence of concurrent administration of exogenous IL-2, which likely promoted entry into the cell cycle. Pt 17 relapsed after clearance of the infused CTL. WT1 expression in both blood and BM was assessed longitudinally by PCR in all patients. Except for Pt 17, whose leukemic cells expressed WT1 prior to therapy and who relapsed with 40% blasts in the BM 138 days after the first infusion with no increase in detectable levels of WT1 expression, the presence of leukemic blasts by morphology and/or flow cytometry correlated with levels of WT1. These results are consistent with continued expression of the pro-oncogenic WT1 protein in leukemia blasts independent of targeting with T-cells in most patients (Fig. S6).
Table 2.
Assessment of clinical outcomes
| Pt | IL21 | Leukemia WT1 expression |
Leukemia burden at time of CTL infusions |
CTL persistence (days) |
Outcome* | GVHD after CTL? |
Additional treatment after CTL? |
Alive/ Dead |
|---|---|---|---|---|---|---|---|---|
| 1 | No | Yes** | Present | 14 | Progressed while receiving CTL |
NO | YES | dead |
| 2 | No | Yes** | Present | 14 | Relapsed while receiving CTL |
NO | YES | dead |
| 6 | No | Unavailable | Absent | 14 | CR/chronic GVHD 4.9 years after CTL, 6 years post HCT |
YES (pre- existing) |
NO | alive |
| 10 | No | Yes** | Present | 14 | Relapse while receiving CTL |
NO | YES | dead |
| 15 | No | Yes** | Present | 14 | Progressed after responding to 1st CTL infusion |
NO | NO | dead |
| 17 | No | Yes** | Absent | 7 | Relapsed D+132 post CTL |
NO | NO | dead |
| 20 | No | Yes** | Present | 5 | Progressed while receiving CTL |
NO | YES | dead |
| 21 | Yes | Yes*** | Absent | 430 | CR 30 months post 1st CTL, 35 months post HCT |
NO | NO | alive |
| 24 | Yes | Yes*** | Absent | 430+ | CR 28 months post 1st CTL, 33 months post HCT |
NO | NO | alive |
| 27 | Yes | Yes** | Present | 430+ | CR 19 months post 1st CTL, 22 months post HCT |
NO | NO | alive |
| 28 | Yes | Yes*** | Absent | 230+ | CR 18 months post 1st CTL, 38 months post HCT |
NO | NO | alive |
As of 08/15/2012
Assessed by PCR
Assessed by immunohistochemistry
Figure 6. Evidence of anti-leukemic activity.
(A) Percent WT1-specific multimer+ cells detected in PBMC (solid black circles, black line) of Pt 15 after infusion of 3.3 × 109 cells/m2 (left y-axis), concurrent percent leukemic blasts in the blood (red area) (inner right y-axis). Total white blood cells (solid grey line) and lymphocytes (dashed grey line) (outer right y-axis) in samples collected before and after the infusion (x-axis). (B) Percent multimer+CD8+ T-cells (y-axis) among PBMCs (solid circles) and in BM (open circles) collected before and after infusions for Pt 27. Arrows indicate time of infusions. Below the graph are characteristics of the patient’s primary B-ALL at diagnosis (left, below B-ALL), and at timepoints at which clonal B cells or the abnormal karyotype were analyzed and detected (+) in BM. (C) Mean effective concentrations of peptide required to achieve 50% lysis (EC50) of pulsed T2 BLCL at an effector to target ratio (E:T) of 10:1 by the CTL clones infused for each patient.
Discussion
Establishing robust T-cell mediated anti-leukemic activity after HCT without inducing accompanying GVHD remains a major challenge. WT1 is an antigen that is overexpressed in leukemic cells, which contributes to the leukemic phenotype and has been shown to be capable of eliciting T-cell responses (9). Therefore, we have examined the potential to achieve a selective anti-leukemic effect by the adoptive transfer of homogenous WT1-specific CD8+ CTL clones. Clones were generated from the repertoire of each patient’s HLA-matched donor that was not compromised by prior chemotherapy, and the clone exhibiting the highest avidity for targets expressing WT-1 was selected for infusion. Infusions were well tolerated, non-toxic, and not associated with new-onset GVHD after doses of ≤1010cells/m2, including when cell infusions were followed by administration of low-dose s.c IL-2. This contrasts with previous reports of adoptive transfer of unselected T-cells containing potentially CML-reactive CTL, in which the incidence of GVHD doubled compared to control patients transplanted with CD34+-selected cells who did not receive T-cell infusions (30% vs 14%) (35, 36).
The establishment of a persistent functional population of antigen-specific CTL capable of eliminating cells responsible for late leukemic recurrences will likely be necessary to consistently derive long-term benefit from infused CTL. Studies of transferred CD8+ T-cells for murine LCMV infection revealed that transferred Tcm provided enhanced protective immunity from in vivo challenge compared to effector memory (Tem) (21). Furthermore, studies in non-human primates have shown that expanded CD8+ T cell clones derived from Tcm exhibit greater replicative potential in response to antigen and prolonged in vivo persistence compared to CD8+ T cell clones derived from Tem. Despite all cells displaying a differentiated Tem phenotype after in vitro expansion, ultimately, a fraction of the transferred CD8+ T cell clones derived from Tcm revert back to a Tcm phenotype in vivo (20). However, as spontaneous memory responses to WT1 are rare/non-existent in healthy individuals, and were absent in the HLA-matched donors in our study, the CTL clones for therapy were most likely derived from the naïve repertoire rather than from a memory population (37). When used with IL-15 during the priming of antigen-specific CD8+ T-cells, the γc cytokine IL-21 has been shown to drive further expansion and prevent apoptosis of the cells responding to antigen stimulation, and leads to in vitro generation of CD8+ T-cells with a CD28hi, less terminally differentiated phenotype in both murine and human studies (23, 38). In this study, the WT1-reactive CTL clones that had been primed in the presence of IL-21 expressed significantly higher levels of CD27, CD28 and CD127 prior to infusions, consistent with a less differentiated phenotype, compared to CTL clones generated without IL-21. Similar to previous observations in a murine model (22), the less differentiated phenotype induced by IL-21 conferred the CTL clones enhanced capacities for in vivo persistence and proliferation compared to CTL clones generated in the absence of IL-21. The latter expressed a fully differentiated effector phenotype prior to infusion, stopped proliferating shortly after transfer, and exhibited only brief in vivo survival (≤14 days). Furthermore, as previously shown in the murine model of LCMV infection, the persisting cells generated with exposure to IL-21 displayed phenotypic and functional characteristics of Tcm (39). Although the number of patients treated is insufficient to allow definitive conclusions, the data suggest that CTL clones derived from healthy donors and primed in vitro in the presence of IL-21 may provide a method to establish long-lived memory responses, bypassing the alternative strategy of generating CTL populations for transfer from Tcm reactive with tumor antigens that must already exist in vivo, which will often not likely be feasible. The high antigenic burdens of persistent/recurrent leukemia in most of the patients who received CTL clones generated in the absence of IL-21 may have contributed to their short survival (40). However, leukemic blasts were not detected in 2 patients for whom the CTL clones were generated without exposure to IL-21, and even in this setting long-term T-cell survival was not observed in either patient.
Direct evidence of anti-leukemic activity was observed in Pt 15, but the response was short lived, as leukemic blasts rapidly repopulated the peripheral blood after the transferred CTL were cleared. The patient was not eligible for subsequent T-cell infusions due to toxicity from extensive prior cytotoxic therapies that fulfilled exclusion criteria. Exposure to IL-21 during priming may have been able to endow the cells transferred into this patient with sufficient survival and proliferative ability to maintain a more prolonged anti-leukemic effect, but unfortunately could not be assessed as Pt 15 was treated before IL-21 became available for this study. However, direct evidence of anti-leukemic activity was observed in Pt 27, who had MRD at the time of T-cell therapy and received a CTL clone generated with exposure to IL-21. In this patient, the long-term persistence of the infused T-cells was associated with disappearance of the leukemic cells and a sustained complete remission. The other 3 patients who received CTL clones generated in the presence of IL-21 had no measurable disease at the time of T-cell infusions, but all had exceptionally high risks of relapse (Table S1). All are surviving in complete remission without GVHD or additional anti-leukemic therapy. The absence of leukemic relapse in these patients cannot be definitively attributed to the continued presence of WT1-specific CTL due to the lack of a comparative group, but these promising results warrant further study. Additionally, both the frequency achieved and persistence of functional transferred WT1-specific T-cells generated with exposure to IL-21 (4 out of 4) are markedly better than results obtained by vaccination to WT1 (14).
The results of our study suggest that targeting WT1 with T-cells is safe and can lead to antileukemic activity, but in its current format transferred T-cells may not be sufficient to achieve a clinical benefit in all treated patients. As WT1 over-expression is not restricted to leukemias but rather is present in many tumor types (41), the safety observed in this study also supports expanding efforts to treat other malignancies, particularly employing strategies described here for establishing persistent WT1-specific responses. Although the most avid CTL clone generated for each patient-donor pair was selected for infusion based on the ability to lyse targets pulsed with titrated doses of WT1 peptide, the avidities of the CTL clones obtained were variable. More reproducible clinical results might be achieved with T-cell therapy if the infused CD8+ T-cells exhibited consistent comparably high avidities. Fortunately, strategies to accomplish this are becoming increasingly available, such as by transducing patient T-cells with a characterized high affinity WT1-specific TCR that imparts high avidity for leukemic targets, a technology already being utilized to target other antigens (42, 43). Employing such TCRs, in concert with either expanding the transduced T-cells in the presence of IL-21 and/or directly transducing cells derived from a CD8+ Tcm pool, may predictably provide patients with potent persistent responses for the treatment of leukemia (pre- or post-HCT) as well as solid tumors.
Materials and Methods
Clinical protocol and patient characteristics
All clinical investigations were conducted according to the Declaration of Helsinki principles. Protocol #1655 was approved by the Fred Hutchinson Cancer Research Center (FHCRC) Institutional Review Board and the U.S. Food and Drug Administration. The trial was registered at clinicaltrials.org as NCT00052520. Enrolled HLA A*0201 patient-donor pairs provided written informed consent prior to receiving an HLA-matched allogeneic HCT for AML, ALL, MDS, or CML defined as high-risk, comprising MDS RAEB or RAEB-T, CML beyond chronic phase, AML beyond first remission, Philadelphia chromosome (BCR-abl)-positive ALL at any stage, any ALL beyond first remission, primary refractory AML or ALL, and secondary AML (33, 44–46). Patients with molecular, cytogenetic, or morphologic evidence of relapse post-HCT (treatment of active disease), or following recovery of hematopoiesis post-HCT if transplanted with >5% blasts in the pre-HCT BM due to the greater risk of early relapse (prophylactic therapy), were eligible to receive escalating doses of WT1-specific CTL generated from a leukapheresis obtained from the patient’s matched donor. Between 03/2006 and 08/2010, 37 patient/donor pairs were enrolled, WT1-specific CTL clones were generated for 24 patients and 11 patients received CTL infusions (Fig. S7).
Treatment plan
The first 2 treated patients (Pts 1 and 2) received escalating doses of WT1-specific CTL with an initial dose of 3.3×108 CTL/m2 on day 0, 1.0×109 CTL/m2 on day 7, 3.3×109 CTL/m2 on day 14 and again on day 28, with the last cell dose followed by low-dose s.c. IL-2 (250,000 IU/m2 twice daily) x 14 days. The next 2 treated patients (Pts 6 and 10) received the same regimen with an additional dose of 1.0×1010 CTL/m2, followed by low-dose s.c. IL-2 × 14 days on day 49 (21 days after the last infusion). As no major toxicities were observed, to increase the likelihood of delivering adequate cell doses to patients before disease progression the protocol was modified so that all remaining patients received 3.3×109 CTL/m2 on day 0, 1.0×1010 CTL/m2 on day 14 and 1.0×1010 CTL/m2 on day 28 followed by low-dose s.c. IL-2 × 14 days (Fig. S1). All the described modifications were reviewed by the FHCRC IRB and the FDA. Patients were monitored for toxicities based on Common Toxicity Criteria v4.0 (47). BM aspirates were obtained for analysis within 14 days of the first CTL infusion, 1 day after the second infusion and 1 day after the last dose of s.c. IL-2, and then as clinically indicated.
Isolation and expansion of WT1-specific CTL clones
All ex vivo manipulations involving processing of products destined for infusion were performed in the cGMP Cell Processing Facility (CPF) of the FHCRC. Donor PBMC were obtained from a leukapheresis, and CD8+ T-cells bead-selected (Miltenyi Biotec Inc.) and stimulated up to 3 times for 7–10 day cycles with autologous dendritic cells (DC) pulsed with the A*0201-restricted WT1126–134 (RMFPNAPYL) peptide (Anaspec at a DC to effector ratio of 1:2–10 to obtain sufficient frequencies (>5%) of WT1-reactive CD8+ T-cells. On Day 2 of each stimulation, the γc-chain cytokines IL-2 (12.5IU/ml), IL-7 (5ng/ml) and IL-15 (1ng/ml) were added. For Pts 21, 24, 27 and 28, IL-21 (30ng/ml) was also added once on Day 0 of each stimulation cycle before limiting dilution cloning. Clones were screened for binding to the WT1126–134 peptide-MHC multimer, and the most avid clones based on lysis of T2 B-LCL pulsed with decreasing concentrations of the WT1126–134 peptide, were further selected for expansion (25, 37). CTL clones were analyzed for surface expression of CD3, CD8, CD4, CD45RO, CD27, CD28, CD127, CD62L, CCR7 and cytotoxicity. Most selected clones were tested for monoclonality by analysis of T-cell receptor (TCR)-Vβ usage (Table S4). Briefly, DNA was isolated from WT1-specific T-cell clones and the TCRβ chains amplified by RACE PCR (Clontech). For each clone, only a single band was detected from ≥ 5 × 105 cells, which was then amplified and sequenced. The TCRβ chain and the complementarity determinant region 3 (CDR3) sequence was determined using the IMGT program (www.imgt.org).
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Cancer Institute grants P01 CA018029 and CA033084, and the Leukemia and Lymphoma Society SCOR program LLS-7008-09. AGC is supported by a Walker Fellowship Award, and GBR was supported by a Leukemia Lymphoma Society Special Fellow Award 4442-09.
Footnotes
List of Supplementary Materials:
Supplementary Materials and Methods:
Cytotoxicity assays, T-cell monitoring by MHC-peptide multimers, Flow cytometry, WT1-PCR analysis, WT1 immunohistochemistry, Evaluation of MRD for B-ALL cells, and Statistical analysis.
Figure S1: Treatment plan.
Figure S2: Effect of low-dose s.c IL-2 on post-infusion WT1-specific CTL frequencies.
Figure S3: Dynamics of CD4+ T-regulatory cells after WT1-specific CTL infusions and exogenous low-dose s.c. IL-2.
Figure S4: Phenotypic changes of WT1-specific CTL before and after infusions.
Figure S5: Effect of low-dose s.c IL-2 on post-infusion WT1-specific CTL proliferation 4 days after transfer.
Figure S6: Correlation between WT1 expression by PCR and percentages of leukemic blasts in blood and BM.
Figure S7: Flow diagram of patients enrolled in the clinical study.
Figure S8: FoxP3 expression by CD4+ CD25+ CD127– T-cells.
Table S1: List of adverse events related to treatment.
Table S2: Adverse prognostic factors of treated patients.
Table S3: Prognosis of the patients who demonstrated long-term persistence of transferred WT1-specific CTL clones.
Table S4: Monoclonality of WT1-specific CTL clones.
Author Contributions: AGC analyzed and interpreted data, designed and performed experiments, and drafted the manuscript. GBR lead the clinical trial, CNC and HNN collected patient data. GLP performed all PCR analyses. TMS, JSP, HNN, and IMR performed flow cytometry analysis. SO, MW, MB, WYH and ND performed activities that supported the clinical trial. JPR analyzed and interpreted data, CY developed the use of IL-21 as described. PDG designed and supervised the research, analyzed and interpreted data, and revised the manuscript. All authors approved and edited the paper.
Competing interests: Dr C. Yee is the inventor of the patent entitled ‘Methods of using IL-21 for adoptive immunotherapy and identification of tumor antigens’: 20100310533. All other authors declare that they do not have commercial or other associations that might cause conflict of interest.
References
- 1.Kolb HJ, Schmid C, Barrett AJ, Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood. 2004;103:767. doi: 10.1182/blood-2003-02-0342. [DOI] [PubMed] [Google Scholar]
- 2.Weiden PL, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300:1068. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Qin H, Reese VA, Cheever MA. CTLs specific for bcr-abl joining region segment peptides fail to lyse leukemia cells expressing p210 bcr-abl protein. J Immunother. 1998;21:257. doi: 10.1097/00002371-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Popovic J, et al. The only proposed T-cell epitope derived from the TEL-AML1 translocation is not naturally processed. Blood. 2011;118:946. doi: 10.1182/blood-2010-12-325035. [DOI] [PubMed] [Google Scholar]
- 5.Cheever MA, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmann L, et al. High levels of Wilms’ tumor gene (wt1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome. Blood. 1997;90:1217. [PubMed] [Google Scholar]
- 7.Galimberti S, et al. WT1 expression levels at diagnosis could predict long-term time-to-progression in adult patients affected by acute myeloid leukaemia and myelodysplastic syndromes. Br J Haematol. 2010;149:451. doi: 10.1111/j.1365-2141.2009.08063.x. [DOI] [PubMed] [Google Scholar]
- 8.Inoue K, et al. Aberrant overexpression of the Wilms tumor gene (WT1) in human leukemia. Blood. 1997;89:1405. [PubMed] [Google Scholar]
- 9.Sugiyama H. WT1 (Wilms’ tumor gene 1): biology and cancer immunotherapy. Jpn J Clin Oncol. 2010;40:377. doi: 10.1093/jjco/hyp194. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong JF, Pritchard-Jones K, Bickmore WA, Hastie ND, Bard JB. The expression of the Wilms’ tumour gene, WT1, in the developing mammalian embryo. Mech Dev. 1993;40:85. doi: 10.1016/0925-4773(93)90090-k. [DOI] [PubMed] [Google Scholar]
- 11.Baird PN, Simmons PJ. Expression of the Wilms’ tumor gene (WT1) in normal hemopoiesis. Exp Hematol. 1997;25:312. [PubMed] [Google Scholar]
- 12.Pritchard-Jones K, et al. The candidate Wilms’ tumour gene is involved in genitourinary development. Nature. 1990;346:194. doi: 10.1038/346194a0. [DOI] [PubMed] [Google Scholar]
- 13.Gao L, et al. Selective elimination of leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood. 2000 Apr 1;95:2198. [PubMed] [Google Scholar]
- 14.Van Driessche A, Berneman ZN, Van Tendeloo VF. Active specific immunotherapy targeting the Wilms’ tumor protein 1 (WT1) for patients with hematological malignancies and solid tumors: lessons from early clinical trials. Oncologist. 2012;17:250. doi: 10.1634/theoncologist.2011-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapuis AG, et al. Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1113748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler MO, et al. Establishment of Antitumor Memory in Humans Using in Vitro-Educated CD8+ T Cells. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002207. 80ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunder NN, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 20.Berger C, et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 22.Hinrichs CS, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol. 2005;175:2261. doi: 10.4049/jimmunol.175.4.2261. [DOI] [PubMed] [Google Scholar]
- 24.Ochsenbein AF, et al. CD27 expression promotes long-term survival of functional effector-memory CD8+ cytotoxic T lymphocytes in HIV-infected patients. J Exp Med. 2004;200:1407. doi: 10.1084/jem.20040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yee C, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ragheb JA, Deen M, Schwartz RH. CD28-Mediated regulation of mRNA stability requires sequences within the coding region of the IL-2 mRNA. J Immunol. 1999;163:120. [PubMed] [Google Scholar]
- 27.Topp MS, et al. Restoration of CD28 expression in CD28− CD8+ memory effector T cells reconstitutes antigen-induced IL-2 production. J Exp Med. 2003;198:947. doi: 10.1084/jem.20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Hong WJ, Medeiros BC. Unfavorable-risk cytogenetics in acute myeloid leukemia. Expert Rev Hematol. 2011;4:173. doi: 10.1586/ehm.11.10. [DOI] [PubMed] [Google Scholar]
- 30.Larson RA. Is secondary leukemia an independent poor prognostic factor in acute myeloid leukemia? Best Pract Res Clin Haematol. 2007;20:29. doi: 10.1016/j.beha.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Kurosawa S, et al. Prognostic factors and outcomes of adult patients with acute myeloid leukemia after first relapse. Haematologica. 2010;95:1857. doi: 10.3324/haematol.2010.027516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kebriaei P, et al. Impact of disease burden at time of allogeneic stem cell transplantation in adults with acute myeloid leukemia and myelodysplastic syndromes. Bone Marrow Transplant. 2005;35:965. doi: 10.1038/sj.bmt.1704938. [DOI] [PubMed] [Google Scholar]
- 33.Mielcarek M, et al. Outcomes among patients with recurrent high-risk hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13:1160. doi: 10.1016/j.bbmt.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Chevallier P, et al. A new Leukemia Prognostic Scoring System for refractory/relapsed adult acute myelogeneous leukaemia patients: a GOELAMS study. Leukemia. 2011;25:939. doi: 10.1038/leu.2011.25. [DOI] [PubMed] [Google Scholar]
- 35.Bornhauser M, et al. Prophylactic transfer of BCR-ABL-, PR1-, and WT1-reactive donor T cells after T cell-depleted allogeneic hematopoietic cell transplantation in patients with chronic myeloid leukemia. Blood. 2011;117:7174. doi: 10.1182/blood-2010-09-308569. [DOI] [PubMed] [Google Scholar]
- 36.Elmaagacli AH, et al. Outcome of transplantation of highly purified peripheral blood CD34+ cells with T-cell add-back compared with unmanipulated bone marrow or peripheral blood stem cells from HLA-identical sibling donors in patients with first chronic phase chronic myeloid leukemia. Blood. 2003;101:446. doi: 10.1182/blood-2002-05-1615. [DOI] [PubMed] [Google Scholar]
- 37.Ho WY, Nguyen HN, Wolfl M, Kuball J, Greenberg PD. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naive repertoire. J Immunol Methods. 2006;310:40. doi: 10.1016/j.jim.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 38.Alves NL, Arosa FA, van Lier RA. IL-21 sustains CD28 expression on IL-15-activated human naive CD8+ T cells. J Immunol. 2005;175:755. doi: 10.4049/jimmunol.175.2.755. [DOI] [PubMed] [Google Scholar]
- 39.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35:792. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 41.Oji Y, et al. Expression of the Wilms’ tumor gene WT1 in solid tumors and its involvement in tumor cell growth. Jpn J Cancer Res. 1999;90:194. doi: 10.1111/j.1349-7006.1999.tb00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marr LA, Gilham DE, Campbell JD, Fraser AR. Immunology in the clinic review series; focus on cancer: double trouble for tumours: bi-functional and redirected T cells as effective cancer immunotherapies. Clin Exp Immunol. 2012;167:216. doi: 10.1111/j.1365-2249.2011.04517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006 Oct 6;314:126. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deeg HJ, et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood. 2002;100:1201. doi: 10.1182/blood-2002-02-0527. [DOI] [PubMed] [Google Scholar]
- 45.Gratwohl A, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 1998;352:1087. doi: 10.1016/s0140-6736(98)03030-x. [DOI] [PubMed] [Google Scholar]
- 46.Gyurkocza B, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28:2859. doi: 10.1200/JCO.2009.27.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Common Terminology Criteria for Adverse Events v.4.0 (CTCAE) Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- 48.Riddell SR, et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 49.Altman JD, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94. [PubMed] [Google Scholar]
- 50.Papagno L, Almeida JR, Nemes E, Autran B, Appay V. Cell permeabilization for the assessment of T lymphocyte polyfunctional capacity. J Immunol Methods. 2007;328:182. doi: 10.1016/j.jim.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Peters JH, Hilbrands LB, Koenen HJ, Joosten I. Ex vivo generation of human alloantigen-specific regulatory T cells from CD4(pos)CD25(high) T cells for immunotherapy. PLoS One. 2008;3:e2233. doi: 10.1371/journal.pone.0002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cilloni D, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009;27:5195. doi: 10.1200/JCO.2009.22.4865. [DOI] [PubMed] [Google Scholar]
- 53.van Dongen JJ, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 54.Ettinghausen SE, et al. Hematologic effects of immunotherapy with lymphokine-activated killer cells and recombinant interleukin-2 in cancer patients. Blood. 1987;69:1654. [PubMed] [Google Scholar]
- 55.Radich JP, et al. Second allogeneic marrow transplantation for patients with recurrent leukemia after initial transplant with total-body irradiation-containing regimens. J Clin Oncol. 1993;11:304. doi: 10.1200/JCO.1993.11.2.304. [DOI] [PubMed] [Google Scholar]
- 56.Folch G, Lefranc MP. The human T cell receptor beta variable (TRBV) genes. Exp Clin Immunogenet. 2000;17:42. doi: 10.1159/000019123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.