Abstract
Different individuals have different degrees of neuroplasticity due to their different experiences. Neuroplasticity may play a role in individual differences among neuropsychiatric disease treatment efficacy. Since the nervous system monitors and coordinates internal organ function, neuroplasticity may be associated with other diseases. Cardiovascular disease (CVD) is associated with depression, which is a disorder of disrupted neuroplasticity. MicroRNA-132 (miR-132) has a roles in neuroplasticity and cardiovascular function. Thus, we hypothesize that miR-132 may play a role in coexistence of depression and CVD.
Keywords: microRNA-132, neuroplasticity, depression, cardiovascular disease
Background
Neuroplasticity is the ability of the nervous system to respond to intrinsic or extrinsic stimuli by reorganizing its structure, function, and connections [1]. Individuals show different degrees of neuroplasticity due to their different courses of growth [2]. It has been documented that even monozygotic twins may develop differing neural structure and function despite having an identical genetic background [2]. For instance, some monozygotic twins are discordant for many diseases, such as bulimia nervosa, schizophrenia, and bipolar disorders, and even in sexual orientation [3–6]. Experiences prior to a cerebral injury may influence not only spontaneous recovery but also the efficacy of post-injury treatment [7]. Thus, we propose that neuroplasticity may play a role in individual differences in the treatment response of neuropsychiatric diseases [2]. Since the nervous system monitors and coordinates internal organ function, neuroplasticity may be associated with other diseases. Cardiovascular disease (CVD) is associated with depression, and depression is closely related to neuroplasticity. MicroRNA (miR) is receiving intense research interest at present and microRNA-132 (miR-132) has roles in neuroplasticity and cardiovascular function. Here, we focus on miR-132 as a common component to discuss the mechanism of coexistence of depression and CVD.
CVD is Associated with Depression
Depression significantly increases the risk of incident CVD. Many prospective and retrospective studies have investigated the association of depression and incident CVD. These studies showed that depression is independently associated with CVD and mortality due to causes such as coronary heart disease, heart failure, myocardial infarction, ischemic heart disease, and stroke [8–13]. Lifetime association between major depression (MD) and coronary artery disease (CAD) was modest (odds ratio, approximately 1.3). The effect of MD on CAD is largely acute, and the longer-term effects are apparently mediated via recurrence of depression [14]. Some studies have demonstrated a significant positive correlation with a moderate effect size of 1.5–2.7 between depression and CVD [15]. Similarly, several studies have investigated the role of depression status as a prognostic factor in patients with CVD. Meta-analysis of these studies suggests that depressed patients have a 1.6–2.7-fold increased risk for further cardiovascular events within 24 months [16,17].
Depression is a Disorder of Disrupted Neuroplasticity
It is broadly accepted that stress triggers the activation of the HPA axis and causes the brain to be exposed to corticosteroids, affecting neurobehavioral functions with a strong down-regulation of hippocampal neurogenesis, and is a major risk factor for depression [18–20]. Chronic or severe stress and high-dose treatment with glucocorticoids decrease hippocampal synaptic plasticity and morphological neuroplasticity. Prolonged stress can negatively affect hippocampal function and its capacity for neuroplasticity. Additionally, chronic restraint stress leads to significant regression of the apical dendrites of pyramidal cells in the medial prefrontal cortex (mPFC) and negatively affects mPFC function. Glial loss and neuronal abnormalities have been observed in the prefrontal cortex in MD. Noradrenergic axons have been found with decreased axonal arborization and density after exposure to stress. Increasing evidence demonstrates that depression is a disorder of disrupted neuroplasticity [21]. Accumulating evidence shows that antidepressant treatment may reverse the atrophy of hippocampal neurons, increase cell survival, and increase monoamine axonal sprouting [22].
Brain-derived neurotrophic factor (BDNF) signaling, through its tyrosine kinase B (TrkB) receptor, plays an important role in neuroplasticity. BDNF has also been shown to be expressed at high levels in the hippocampus and to play an important role in synaptic transmission and in the plasticity of the hippocampus [23,24]. BDNF also mediates some of the injurious effects of glucocorticoids on the hippocampus [25,26]. BDNF expression is regulated by stress-responsive corticosteroids, and increased glucocorticoid exposure induces a reduction in BDNF level [27]. Chronic stress has been shown to result in alterations in BDNF/TrkB signaling and changes in neuronal functions [28]. Serotonergic axon sprouting appears to be dependent on BDNF, which appears to be decreased after stress exposure. Thus, stress and depression may increase neuronal atrophy degeneration. Furthermore, hippocampal neurons continue to proliferate well into adulthood. This continued neurogenesis depends on the presence of BDNF and serotonin, both of which are altered in depression, and are inhibited by glucocorticoids [29,30]. Most circulating BDNF is produced in the brain and passes through the blood–brain barrier [31], so serum BDNF level can be a biomarker for depression [32].
MiR-132 is an Activity-Regulated MiR Controlling Neuroplasticity
MiRs are short, non-coding, single-stranded RNA molecules approximately 19–23 nucleotides in length that regulate gene expression by binding to complementary elements in the untranslated regions of target mRNAs and inhibiting protein synthesis [33–35]. Based on sequence homology, each miR has the potential to regulate the translation of hundreds of different genes [36], and greater than 30% of all mammalian genes may be regulated by miRs [37].
MiR-132, a highly conserved miR transcribed from an intergenic region on human chromosome 17 by the transcription factor cAMP response element-binding protein (CREB), is a key coordinator of the intracellular pathways that mediate experience-dependent changes in the brain [38–40]. Using an unbiased genome-wide screen for CREB-bound transcripts in vitro, Impey et al. [41] identified 16 non-coding miR that are induced by CREB-mediated transcription. Further characterization of 1 of these, miR-132, has recently revealed that it is induced by BDNF and neuronal activity, being demonstrated to affect neuronal characteristics such as neurite outgrowth and cell excitability [40,42,43]. miR-132 is able to modulate dendritic morphology via suppression of a specific target, p250 GTPase-activating protein, and regulate cellular excitability via regulation of ion channels [40,42,43].
Interestingly, the CREB-dependent miR-132 has been shown to control the development of dendrites and spines, and synaptic integration in cultured hippocampal neurons and newborn hippocampal neurons [40,42,44–48]. For example, it was reported that knockout of the miR-212/132 locus using conditional transgenic mice or knockdown of miR-132 using viral vectors induced reduced dendritic complexity and spine density, respectively, in newborn neurons of the adult hippocampal neurogenic zone [47,48]. The dendritic effect was shown to be preferentially due to miR-132 loss. A recent study has demonstrated that miR-132 is rapidly transcribed in the hippocampus in vivo following enhanced neuronal activity and contextual fear conditioning [39]. Based on the documented ability of miR-132 to regulate cellular characteristics in an activity-dependent manner, Lambert et al. [49] has provided evidence that overexpression of miR-132 in cultured hippocampal neurons leads to selective changes in short-term synaptic plasticity.
BDNF is essential for a variety of neuronal aspects, including cell differentiation, survival, and synaptic plasticity in the central nervous system (CNS). Intriguingly, a recent study suggests that BDNF exerts its beneficial effects on CNS neurons via up-regulation of miR-132 [50]. BDNF increases CREB activation; the CREB pathways are among the most critical and are the pathways on which BDNF exerts its effects [51]. Therefore, it is concluded that BDNF affects CNS by CREB-miR-132 pathway. Additionally, increased blood levels of glucocorticoids cause suppression in BDNF-dependent neuronal function via reducing miR-132 expression [52].
The dysfunction of adult hippocampal neurogenesis is proposed to be an essential mechanism explaining the etiology of depression. BDNF, CREB, and glucocorticoids are the key components for hippocampal neurogenesis, all of which are directly related to miR-132. Thus, it is suggested that miR-132 plays an important role in the etiology of depression.
MiR-132 has Functions in the Cardiovascular System
There is scant literature on the function of miR-132 in the cardiovascular system. However, the existing literature suggests that miR-132 has functions in the cardiovascular system.
The cardiovascular system is controlled by the nervous system, mainly by the autonomic nervous system; therefore, BDNF can influence the cardiovascular system via the autonomic nervous system. BDNF is important for autonomic nervous system function. BDNF is known to play an important role in regulating the survival of neurons in the autonomic nervous system and the formation of their synaptic connectivity with their peripheral targets in the cardiovascular, digestive, and other organ systems. Emerging evidence suggests that BDNF may also affect the function of the autonomic nervous system during adult life and may, in part, mediate the effects of environmental factors, such as exercise and dietary energy intake, on autonomic nervous system neurons and target cells [53]. BDNF has also been shown to be a modulator of visceral sensory transmission, suggesting that BDNF is involved in maturation and/or plasticity in the arterial baroreceptor pathway [54]. As noted above, BDNF influences CNS via the CREB-miR-132 pathway, and most of circulating BDNF is produced in the brain and passes through the blood-brain barrier. Thus, it is suggested that miR-132 may play an important role in cardiovascular function via the autonomic nervous system. Additionally, BDNF may also influence energy homeostasis through its role in neurogenesis and in the neuroplasticity of the HPA axis [55–57], and is involved in the maintenance of cardiometabolic homeostasis [58]. Therefore, it is suggested that miR-132 may also influence cardiovascular function via the HPA axis.
Endothelial dysfunction is a critical step in development of CVD pathology, such as hypertension, atherosclerosis, and thrombosis [59–61]. The action of vascular endothelial growth factor (VEGF) is essential to maintain proper endothelial and vascular function [62]. The major function of VEGF is angiogenesis [63]. VEGF stimulates virtually all aspects of endothelial function: proliferation, migration, permeability, and nitric oxide production and release. In addition, the action of VEGF makes the endothelium anti-apoptotic. In turn, the inhibition of VEGF action is associated with endothelial dysfunction [62].
The effect of VEGF on the endothelium is related to miR. Research on effects of miR on the endothelium has been conducted, showing that miR-132 is an angiogenic growth factor inducible miR in the endothelium [64,65]. VEGF triggers phosphorylation of CREB and subsequent transcription of miR-132. MiR-132 downregulates p120 Ras GTPase-activating protein, thereby removing the endogenous brake on Ras activity and activating quiescent endothelium [65].
MiR-132 mediates the deleterious effect of angiotensin II in vascular smooth muscle cells [66]. However, endothelial dysfunction is the first step to CVD and plays a central role in its pathogenesis [67]. Additionally, miR-132 may have an important role in cardiovascular function via the autonomic nervous system and the HPA axis. BDNF also maintains vessel stability in the heart through direct angiogenic actions on endothelial cells [68]. Although at present there is no literature on the role of miR-132 in BDNF-induced angiogenesis, it is likely that CREB and miR-132, which are the common components in BDNF-induced neuroplasticity and VEGF-induced angiogenesis, are involved in the mechanism. Thus, the positive effect of miR-132 on the cardiovascular system may be greater than the negative one. For example, Katare et al. [69] investigated the therapeutic activity and mechanistic targets of saphenous vein-derived pericyte progenitor cells (SVPs) in a mouse myocardial infarction model and concluded that SVP transplantation produces long-term improvement of cardiac function by a novel paracrine mechanism involving the secretion of miR-132 and inhibition of its target genes. Furthermore, a study of long-term β-adrenergic administration on the expression levels of the cardiac L-type Ca channel β2 subunit, which regulates channel trafficking and function, showed that cardiac L-type Ca channel β2 subunit protein expression may be down-regulated by miRs, including miR-132, in response to long-term activation of β-adrenergic signaling, possibly as an adaptive response in cardiac hypertrophy and sustained β-adrenergic states [70].
Hypothesis
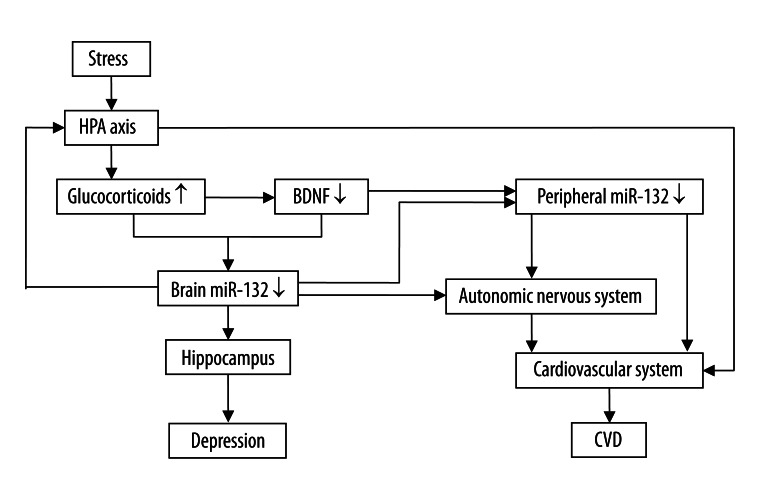
MiR-132 has functions in both the nervous and cardiovascular system. Stress decreases BDNF level. Low BDNF level reduces CREB activation, resulting in down-regulation of miR-132, and then disrupts neuroplasticity and leads to depression. Stress also increases the level of glucocorticoid, and increased glucocorticoid level also down-regulates miR-132. MiR-132 may affect cardiovascular function by the autonomic nervous system and the HPA axis. Circulating BDNF, most of which is produced in the brain and passes through the blood–brain barrier [31], may also influence cardiovascular function involving miR-132. In addition, miRs are also found in microvesicles, which are plasma membrane fragments shed from virtually all cells [71]. Microvesicles circulate in peripheral blood, where they transport mRNA and proteins between cells and play a pivotal role in cell-to-cell communication. They are also implicated in the process of angiogenesis [72]. Recent studies also raise the possibility that CNS-derived vesicles may enter the bloodstream and interact with endothelial cells in the peripheral circulation [73], suggesting that the synthesis of miR-132 in the brain may be related to miR-132 level in the cardiovascular system. Thus, we hypothesize that miR-132 may play a role in coexistence of depression and CVD. Figure 1 presents an integrative model that shows the possible role of miR-132 in coexistence of depression and CVD. This model is not intended to be complete or all-encompassing, but rather to highlight and connect certain interesting evidence pointing to this miR-132 role.
Figure 1.
An integrative model that shows the possible role of miR-132 in coexistence of depression and CVD. miR-132 may play a role in pathogenesis of coexistence between depression and CVD. Abbreviations: HPA, hypothalamus-pituitary-adrenal; BDNF, brain-derived neurotrophic factor; miR-132, microRNA-132; CVD, cardiovascular disease.
Based on this hypothesis, miR-132 may be a potential target for treating depression and CVD. Both depression and CVD may benefit from up-regulated miR-132, and more research should be conducted in this field.
Footnotes
Source of support: Self financing
Conflicts of interest statement
None declared.
Statement
The work was done in Guangdong Province Pharmaceutical Association.
References
- 1.Cramer SC, Sur M, Dobkin BH, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134:1591–609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Z, Xu F. Neuroplasticity may play a role in inter-individual difference among neuropsychiatric disease treatment efficacy. Dev Psychobiol. 2012;54:369–71. doi: 10.1002/dev.20561. [DOI] [PubMed] [Google Scholar]
- 3.Bulik CM, Wade TD, Kendler KS. Characteristics of monozygotic twins discordant for bulimia nervosa. Int J Eat Disord. 2001;29:1–10. doi: 10.1002/1098-108x(200101)29:1<1::aid-eat1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Cannon TD, Huttunen MO, Lonnqvist J, et al. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet. 2000;67:369–82. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gourovitch ML, Torrey EF, Gold JM, et al. Neuropsychological performance of monozygotic twins discordant for bipolar disorder. Biol Psychiatry. 1999;45:639–46. doi: 10.1016/s0006-3223(98)00148-6. [DOI] [PubMed] [Google Scholar]
- 6.Hall LS, Love CT. Finger-length ratios in female monozygotic twins discordant for sexual orientation. Arch Sex Behav. 2003;32:23–28. doi: 10.1023/a:1021837211630. [DOI] [PubMed] [Google Scholar]
- 7.Kolb B, Teskey GC. Age, experience, injury, and the changing brain. Dev Psychobiol. 2012;54:369–71. doi: 10.1002/dev.20515. [DOI] [PubMed] [Google Scholar]
- 8.Ariyo AA, Haan M, Tangen CM, et al. Depressive symptoms and risks of coronary heart disease and mortality in elderly Americans. Cardiovascular Health Study Collaborative Research Group. Circulation. 2000;102:1773–79. doi: 10.1161/01.cir.102.15.1773. [DOI] [PubMed] [Google Scholar]
- 9.Abramson J, Berger A, Krumholz HM, Vaccarino V. Depression and risk of heart failure among older persons with isolated systolic hypertension. Arch Intern Med. 2001;161:1725–30. doi: 10.1001/archinte.161.14.1725. [DOI] [PubMed] [Google Scholar]
- 10.Williams SA, Kasl SV, Heiat A, et al. Depression and risk of heart failure among the elderly: a prospective community – based study. Psychosom Med. 2002;64:6–12. doi: 10.1097/00006842-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Gump BB, Matthews KA, Eberly LE, Chang YF. Depressive symptoms and mortality in men: results from the Multiple Risk Factor Intervention Trial. Stroke. 2005;36:98–102. doi: 10.1161/01.STR.0000149626.50127.d0. [DOI] [PubMed] [Google Scholar]
- 12.Ahto M, Isoaho R, Puolijoki H, Vahlberg T, Kivela SL. Stronger symptoms of depression predict high coronary heart disease mortality in older men and women. Int J Geriatr Psychiatry. 2007;22:757–63. doi: 10.1002/gps.1735. [DOI] [PubMed] [Google Scholar]
- 13.Surtees PG, Wainwright NW, Luben RN, et al. Depression and ischemic heart disease mortality: evidence from the EPIC-Norfolk United Kingdom prospective cohort study. Am J Psychiatry. 2008;165:515–23. doi: 10.1176/appi.ajp.2007.07061018. [DOI] [PubMed] [Google Scholar]
- 14.Kendler KS, Gardner CO, Fiske A, Gatz M. Major depression and coronary artery disease in the Swedish twin registry: phenotypic, genetic, and environmental sources of comorbidity. Arch Gen Psychiatry. 2009;66:857–63. doi: 10.1001/archgenpsychiatry.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baune BT, Stuart M, Gilmour A, et al. The relationship between subtypes of depression and cardiovascular disease: a systematic review of biological models. Transl Psychiatry. 2012;2:e92. doi: 10.1038/tp.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meijer A, Conradi HJ, Bos EH, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33:203–16. doi: 10.1016/j.genhosppsych.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 17.van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66:814–22. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 18.Masi G, Brovedani P. The hippocampus, neurotrophic factors and depression: possible implications for the pharmacotherapy of depression. CNS Drugs. 2011;25:913–31. doi: 10.2165/11595900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Llorens-Martin M, Trejo JL. Mifepristone Prevents Stress-Induced Apoptosis in Newborn Neurons and Increases AMPA Receptor Expression in the Dentate Gyrus of C57/BL6 Mice. PLoS One. 2011;6:e28376. doi: 10.1371/journal.pone.0028376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Sullivan E, Barrett E, Grenham S, et al. BDNF expression in the hippocampus of maternally separated rats: does Bifidobacterium breve 6330 alter BDNF levels? Benef Microbes. 2011;2:199–207. doi: 10.3920/BM2011.0015. [DOI] [PubMed] [Google Scholar]
- 21.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 22.Rush AJ, Fava M, Wisniewski SR, et al. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials. 2004;25:119–42. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 23.Berninger B, Schinder AF, Poo MM. Synaptic reliability correlates with reduced susceptibility to synaptic potentiation by brain-derived neurotrophic factor. Learn Mem. 1999;6:232–42. [PMC free article] [PubMed] [Google Scholar]
- 24.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–98. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 25.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–27. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 27.Numakawa T, Yokomaku D, Richards M, et al. Functional interactions between steroid hormones and neurotrophin BDNF. World J Biol Chem. 2010;1:133–43. doi: 10.4331/wjbc.v1.i5.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kutiyanawalla A, Terry AV, Jr, Pillai A. Cysteamine attenuates the decreases in TrkB protein levels and the anxiety/depression-like behaviors in mice induced by corticosterone treatment. PLoS One. 2011;6:e26153. doi: 10.1371/journal.pone.0026153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee TH, Kato H, Chen ST, et al. Expression disparity of brain-derived neurotrophic factor immunoreactivity and mRNA in ischemic hippocampal neurons. Neuroreport. 2002;13:2271–75. doi: 10.1097/00001756-200212030-00020. [DOI] [PubMed] [Google Scholar]
- 30.Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999;21:46S–51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- 31.Forsgren S, Grimsholm O, Dalen T, et al. Measurements in the Blood of BDNF for RA Patients and in Response to Anti-TNF Treatment Help Us to Clarify the Magnitude of Centrally Related Pain and to Explain the Relief of This Pain upon Treatment. Int J Inflam. 2011;2011:650685. doi: 10.4061/2011/650685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshimura R, Kishi T, Suzuki A, et al. The brain-derived neurotrophic factor (BDNF) polymorphism Val66Met is associated with neither serum BDNF level nor response to selective serotonin reuptake inhibitors in depressed Japanese patients. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1022–25. doi: 10.1016/j.pnpbp.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nature Methods. 2007;4:721–26. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herranz H, Cohen SM. MicroRNAs and gene regulatory networks: managing the impact of noise in biological systems. Genes Dev. 2010;24:1339–44. doi: 10.1101/gad.1937010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–20. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 36.Lim LP, Lau NC, Garrett-Engele P, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–73. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 37.Lewis BP, Shih IH, Jones-Rhoades MW, et al. Prediction of mammalian microRNA targets. Cell. 2003;115:787–98. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 38.Scott HL, Tamagnini F, Narduzzo KE, et al. MicroRNA-132 regulates recognition memory and synaptic plasticity in the perirhinal cortex. Eur J Neurosci. 2012;36:2941–48. doi: 10.1111/j.1460-9568.2012.08220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nudelman AS, DiRocco DP, Lambert TJ, et al. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus. 2010;20:492–98. doi: 10.1002/hipo.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vo N, Klein ME, Varlamova O, et al. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci USA. 2005;102:16426–31. doi: 10.1073/pnas.0508448102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Impey S, McCorkle SR, Cha-Molstad H, et al. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell. 2004;119:1041–54. doi: 10.1016/j.cell.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 42.Wayman GA, Davare M, Ando H, et al. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci USA. 2008;105:9093–98. doi: 10.1073/pnas.0803072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng HY, Papp JW, Varlamova O, et al. microRNA modulation of circadian-clock period and entrainment. Neuron. 2007;54:813–29. doi: 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luikart BW, Bensen AL, Washburn EK, et al. miR-132 mediates the integration of newborn neurons into the adult dentate gyrus. PLoS One. 2011;6:e19077. doi: 10.1371/journal.pone.0019077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edbauer D, Neilson JR, Foster KA, et al. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65:373–84. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hansen KF, Sakamoto K, Wayman GA, et al. Transgenic miR132 alters neuronal spine density and impairs novel object recognition memory. PLoS One. 2010;5:e15497. doi: 10.1371/journal.pone.0015497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Impey S, Davare M, Lesiak A, et al. An activity-induced microRNA controls dendritic spine formation by regulating Rac1-PAK signaling. Mol Cell Neurosci. 2010;43:146–56. doi: 10.1016/j.mcn.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magill ST, Cambronne XA, Luikart BW, et al. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci USA. 2010;107:20382–87. doi: 10.1073/pnas.1015691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lambert TJ, Storm DR, Sullivan JM. MicroRNA132 modulates short-term synaptic plasticity but not basal release probability in hippocampal neurons. PLoS One. 2010;5:e15182. doi: 10.1371/journal.pone.0015182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Numakawa T, Richards M, Adachi N, et al. MicroRNA function and neurotrophin BDNF. Neurochem Int. 2011;59:551–58. doi: 10.1016/j.neuint.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 51.Numakawa T, Suzuki S, Kumamaru E, et al. BDNF function and intracellular signaling in neurons. Histol Histopathol. 2010;25:237–58. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- 52.Kawashima H, Numakawa T, Kumamaru E, et al. Glucocorticoid attenuates brain-derived neurotrophic factor-dependent upregulation of glutamate receptors via the suppression of microRNA-132 expression. Neuroscience. 2010;165:1301–11. doi: 10.1016/j.neuroscience.2009.11.057. [DOI] [PubMed] [Google Scholar]
- 53.Mattson MP, Wan R. Neurotrophic factors in autonomic nervous system plasticity and dysfunction. Neuromolecular Med. 2008;10:157–68. doi: 10.1007/s12017-007-8021-y. [DOI] [PubMed] [Google Scholar]
- 54.Martin JL, Jenkins VK, Hsieh HY, Balkowiec A. Brain-derived neurotrophic factor in arterial baroreceptor pathways: implications for activity-dependent plasticity at baroafferent synapses. J Neurochem. 2009;108:450–64. doi: 10.1111/j.1471-4159.2008.05781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noble EE, Billington CJ, Kotz CM, Wang C. The lighter side of BDNF. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1053–69. doi: 10.1152/ajpregu.00776.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taliaz D, Loya A, Gersner R, et al. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J Neurosci. 2011;31:4475–83. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeanneteau FD, Lambert WM, Ismaili N, et al. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc Natl Acad Sci USA. 2012;109:1305–10. doi: 10.1073/pnas.1114122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaldakov G. The metabotrophic NGF and BDNF: an emerging concept. Arch Ital Biol. 2011;149:257–63. doi: 10.4449/aib.v149i2.1366. [DOI] [PubMed] [Google Scholar]
- 59.Speer T, Rohrer L, Blyszczuk P, et al. Abnormal High-Density Lipoprotein Induces Endothelial Dysfunction via Activation of Toll-Like Receptor-2. Immunity. 2013;38(4):754–68. doi: 10.1016/j.immuni.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Liu W, Peng Y, Wu B, et al. A Meta-Analysis of the Impact of EPC Capture Stent on the Clinical Outcomes in Patients with Coronary Artery Disease. J Interv Cardiol. 2013 doi: 10.1111/j.1540-8183.2013.12017.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 61.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–68. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 62.Waltenberger J. VEGF resistance as a molecular basis to explain the angiogenesis paradox in diabetes mellitus. Biochem Soc Trans. 2009;37:1167–70. doi: 10.1042/BST0371167. [DOI] [PubMed] [Google Scholar]
- 63.Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 2005;6:209. doi: 10.1186/gb-2005-6-2-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu S, Jin C, Shen X, et al. MicroRNAs as potential novel therapeutic targets and tools for regulating paracrine function of endothelial progenitor cells. Med Sci Monit. 2012;18(7):HY27–31. doi: 10.12659/MSM.883193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anand S, Cheresh DA. MicroRNA-mediated regulation of the angiogenic switch. Curr Opi Hematol. 2011;18:171–76. doi: 10.1097/MOH.0b013e328345a180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin W, Reddy MA, Chen Z, et al. Small RNA sequencing reveals microRNAs that modulate angiotensin II effects in vascular smooth muscle cells. J Biol Chem. 2012;287:15672–83. doi: 10.1074/jbc.M111.322669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanson M, Gluckman P. Endothelial dysfunction and cardiovascular disease: the role of predictive adaptive responses. Heart. 2005;91:864–66. doi: 10.1136/hrt.2004.047381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Donovan MJ, Lin MI, Wiegn P, et al. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development. 2000;127:4531–40. doi: 10.1242/dev.127.21.4531. [DOI] [PubMed] [Google Scholar]
- 69.Katare R, Riu F, Mitchell K, et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res. 2011;109:894–906. doi: 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carrillo ED, Escobar Y, Gonzalez G, et al. Posttranscriptional regulation of the beta2-subunit of cardiac L-type Ca2+ channels by MicroRNAs during long-term exposure to isoproterenol in rats. J Cardiovasc Pharmacol. 2011;58:470–78. doi: 10.1097/FJC.0b013e31822a789b. [DOI] [PubMed] [Google Scholar]
- 71.Deregibus MC, Tetta C, Camussi G. The dynamic stem cell microenvironment is orchestrated by microvesicle-mediated transfer of genetic information. Histol Histopathol. 2010;25:397–404. doi: 10.14670/HH-25.397. [DOI] [PubMed] [Google Scholar]
- 72.Ceman S, Saugstad J. MicroRNAs: Meta-controllers of gene expression in synaptic activity emerge as genetic and diagnostic markers of human disease. Pharmacol Ther. 2011;130:26–37. doi: 10.1016/j.pharmthera.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smalheiser NR. Do Neural Cells Communicate with Endothelial Cells via Secretory Exosomes and Microvesicles? Cardiovasc Psychiatry Neurol. 2009;2009:383086. doi: 10.1155/2009/383086. [DOI] [PMC free article] [PubMed] [Google Scholar]