Abstract
Objective
To evaluate whether HAART is associated with subsequent sexual and drug-related risk behavior compensation among injection drug users (IDUs).
Design
A community-based cohort study of 362 HIV-infected IDUs initiating HAART in Baltimore, Maryland.
Methods
HAART use and risk behavior was assessed at 8316 biannual study visits (median 23). Using logistic regression with generalized estimating equations (GEE), we examined the effect of HAART initiation on changes in risk behavior while adjusting for sociodemographics, alcohol use, CD4+ cell count, year of initiation and consistency of HAART use.
Results
At HAART initiation, participants were a median of 44.4 years old, 71.3% men and 95.3% African–American. In multivariable analysis, HAART initiation was associated with a 75% reduction in the likelihood of unprotected sex [adjusted odds ratio (aOR) 0.25; 95% confidence interval (CI), 0.19–0.32] despite no change in overall sexual activity (aOR 0.95; 0.80–1.12). Odds of any injecting decreased by 38% (aOR 0.62; 0.51–0.75) after HAART initiation. Among the subset of persistent injectors, needle-sharing increased nearly two-fold (aOR 1.99; 1.57–2.52). Behavioral changes were sustained for more than 5 years after HAART initiation and did not differ by consistency of HAART use. Reporting specific high-risk behaviors in the year prior to initiation was a robust predictor of engaging in those behaviors subsequent to HAART.
Conclusion
Overall, substantial declines in sexual risk-taking and active injecting argue against significant behavioral compensation among IDUs following HAART initiation. These data also provide evidence to support identifying persons with risky pre-HAART behavior for targeted behavioral intervention.
Keywords: antiretroviral therapy, HIV prevention, injecting, injection drug users, risk compensation, sexual behavior
Introduction
For more than a decade, strong evidence has supported the benefit of HAART for reducing the morbidity and mortality associated with HIV infection [1–3]. Accumulated data have now also proven that HAART significantly reduces sexual transmission of HIV among serodiscordant couples [4,5], leading to recognition of HAART as an important tool for HIV prevention [6,7]. Informed by these data, numerous researchers have estimated how reductions in transmissibility with HAART may translate to population-level reductions in HIV incidence [8–10].
An important yet poorly characterized parameter in models of HIV incidence is the degree to which HAART may actually lead to an increase in transmission-risk behaviors. In early modeling studies, the presence or absence of compensatory increases in risk behavior following HAART initiation largely determined the net effect of treatment on further HIV transmission [11]. Although behavioral risk compensation has been cited as a concern in numerous trials of HIV prevention strategies [12–14], data describing behavior change in these settings show mixed results. Two primary hypotheses favoring an increase in high-risk behavior following HAART initiation have been postulated. First, improvement in overall clinical status may increase interest or ability to engage in risky behavior [15,16]. Second, attitudes or knowledge regarding the lower risk for transmission while virologically suppressed on HAART may lead to more risky practices [17,18]. The latter effect may grow in importance with increasing public recognition of reduced HIV transmission associated with HIV treatment through scientific and lay press reports [19,20].
Early after HAART availability, several studies of HIV-infected men suggested increases in sexual risk-taking after treatment initiation [21–24]. The causative factors proposed to explain increases in high-risk behaviors included immunological improvements [15], diminished concerns about transmission, safe-sex fatigue [17], and HIV optimism [25]. More recent studies on this topic have not shown HAART initiation to be associated with greater risk-taking [26], and some studies in low-income settings showed reductions in sexual risk behaviors [27,28]. Among injection drug using populations, in addition to sexual risk behaviors, risky drug use practices (e.g. injecting, needle-sharing, having sex partner who injects drugs, attending shooting galleries) are of substantial concern for HIV transmission. Therefore, injection drug users (IDUs) who may engage in both sexual and drug-related high-risk behavior represent an important population in which behavioral compensation after HAART should be addressed. Previous studies of behavior change among IDUs receiving HAART have yielded mixed results and were limited by relatively small numbers of participants with brief follow-up [29–33]. We evaluated longer-term changes in sexual and drug-related risk behaviors following HAART initiation among HIV-infected IDUs in the AIDS Linked to the IntraVenous Experience (ALIVE) study.
Methods
Study participants
ALIVE is a longitudinal study of HIV among IDUs in Baltimore, Maryland [34]. The initial recruitment in 1988–1989 included individuals aged 18 years or more with a history of injecting drugs that were AIDS-free at the time of enrollment [35]; additional recruitments used similar inclusion criteria except that the prior AIDS exclusion criterion was dropped. Participants were followed on a biannual basis, with study visits comprising of an interview, physical examination, and biospecimen collection.
Behavioral outcome variables
A spectrum of sexual and drug-related behaviors was evaluated at each visit. In the current analysis, we focus on four self-reported behavioral variables: any sexual activity, unprotected sexual activity, any injection drug use and any needle-sharing. Any sexual behavior includes vaginal, anal, and oral sex; any unprotected sex includes these acts without using a condom. Needle-sharing included sharing, borrowing or buying needles previously used by someone else. Any sexual activity is not considered an HIV-transmission risk factor per se, but is included for comparison to the prevalence of high-risk sex. The other outcomes have substantial prevalence, near complete reporting, and are associated with HIV-transmission risk in our cohort. Outcomes were assessed by participants’ self-report of specified behaviors during the 6 months prior to the study visit. Behavioral assessments were conducted by audio computer-assisted self-interview (ACASI), demonstrated to elicit higher levels of reporting sensitive risk behaviors compared with interviewer-administered questionnaires [36].
Exposure variables
Self-reported antiretroviral therapy during the prior 6-month period was assessed by trained interviewers using pill books, which included the actual medicines to prompt participant recall [37,38]. We used a standardized definition of HAART as previously reported and used in collaborating cohorts [38–40]. The visit at which each participant first reported HAART use was considered the HAART initiation visit. Exposure to HAART is considered a binary variable, separating visits before initiation from those following initiation. Participants were included in the analysis if they did not report ever using HAART at study enrollment, initiated HAART during follow-up, and had at least one visit prior to and one visit after HAART initiation. Of 464 HIV-infected participants initiating HAART between July 1996 and October 2007, 102 (22%) were excluded because risk-behavior data were unavailable for visits prior to initiation. The 102 excluded patients were similar to those included with respect to sex, race, and age but had lower median CD4 count, higher median HIV RNA level, and lower prevalence of unprotected sex and needle-sharing reported at the initiation visit.
Statistical analysis
We separately evaluated the proportion of IDUs reporting each of the four behaviors at post-HAART compared with pre-HAART visits. We used logistic regression with generalized estimating equations (GEE) to account for intra-subject correlation, assuming an exchangeable correlation structure. Multivariable models controlled for potential confounders including age, sex, race, alcohol use, employment, and CD4+ cell count. To control for secular trends in drug use and unmeasured contextual factors, models also adjusted for calendar year of initiation. For unprotected sex and needle-sharing, models included only the subset of visits when participants reported any sex or any injection drug use, respectively.
We next assessed changes in risk behaviors during extended follow-up after HAART initiation. Time relative to HAART initiation was categorized into five groups: 1 year pre-HAART, 1 year post-HAART, 2nd year post-HAART, 3–5 years post-HAART, and more than 5 years post-HAART, with indicator variables added to models to compare risk behaviors in the four post-HAART time-periods relative to the pre-HAART period. To provide a uniform period for comparison, which reflects behavior occurring just prior to initiation, visits occurring more than 1 year prior to HAART initiation were excluded. We conducted stratified analyses according to treatment adherence (consistent HAART use vs. one or more treatment interruptions) and according to whether a participant reported each risk behavior during the year prior to HAART in order to evaluate whether other baseline characteristics modified the effect of HAART initiation on risk behavior.
Finally, we constructed logistic regression models to identify predictors of engaging in high-risk behavior after HAART initiation. Time-varying predictor variables included increase in CD4+ cell count from baseline and current HIV viral load. Fixed covariates assessed at the HAART initiation visit included age, sex, calendar year, risk behavior in the year prior to initiation, baseline CD4+ cell count and baseline HIV viral load. Analyses were conducted using STATA software (Stata Corp., College Station, Texas, USA).
Results
Characteristics of study participants
A total of 362 participants contributed 8316 person-visits. The median number of semiannual follow-up visits after HAART initiation was seven (IQR: 3–12) with a maximum of 22. Participants were predominantly men (258, 71.3%), African–American (345, 95.3%), had a median age at HAART initiation of 44.4 years (IQR: 40.2–48.3) and initiated in 1999 (IQR: 1998–2001) with a median CD4+ cell count of 218 cells/μl (IQR: 100–333) (Table 1). During the year prior to initiation, 244 (67.4%) participants reported any sexual activity and half engaged in unprotected sex (175, 48.3%). A majority (222, 61.3%) reported injecting drugs during the year prior to initiating HAART, but fewer reported needle-sharing (97, 26.8%). After HAART initiation, the majority of participants demonstrated inconsistent HAART use, with 261 (73.3%) experiencing at least one treatment interruption.
Table 1.
Demographic, clinical and behavioral characteristics of 362 HIV-infected AIDS Linked to the IntraVenous Experience study participants that initiated HAART from 1996 to 2007.
| n (%) | |
|---|---|
| Sex | |
| Men | 258 (71.3) |
| Women | 104 (28.7) |
| Race | |
| African–American | 345 (95.3) |
| Other | 17 (4.7) |
| Median age at HAART initiation, years (IQR) | 44.4 (40.2–48.3) |
| Median CD4 cell count at visit before HAART initiation (cells/μl) (IQR) | 218.5 (100–333) |
| Median HIV RNA level at visit before HAART initiation (copies/ml) (IQR) | 23 494 (2263–102 870) |
| Median year of HAART initiation (IQR) | 1999 (1998–2001) |
| Consistency of HAART use | |
| Consistent | 95 (26.7) |
| Inconsistent | 261 (73.3) |
| Risky behaviors in year prior to HAART | |
| Engaged in any sex | 244 (67.4) |
| Engaged in any unprotected sex | 175 (48.3) |
| Injected drugs | 222 (61.3) |
| Shared needles | 97 (26.8) |
IQR, interquartile range.
Risk behaviors before and after HAART initiation
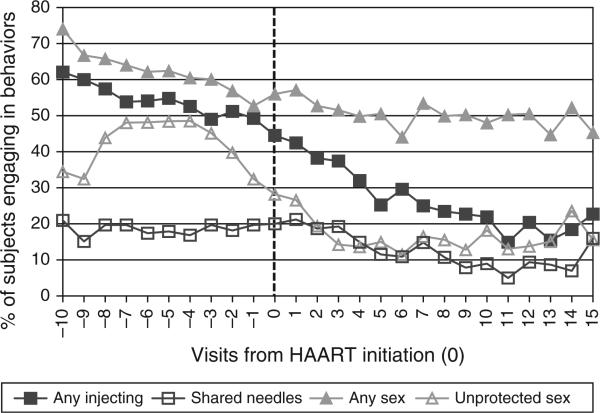
Figure 1 illustrates longitudinal changes in the proportion of IDUs engaging in sexual and drug-related behaviors before and after HAART initiation. Declines were generally observable for all behaviors with reductions notably occurring prior to HAART initiation. Marked declines both prior to and following HAART initiation were seen for unprotected sex and for any injecting. Comparing each individual's proportion of visits reporting each behavior before vs. after HAART initiation showed declines in the proportion reporting any sex (68 vs. 48%), unprotected sex (34 vs. 17%), any IDU (65 vs. 34%) and needle-sharing (26 vs. 16%).
Fig. 1.
Proportion of ALIVE participants reporting sexual and drug-related behavioral outcomes at 6-month study visits before and after HAART initiation (Time 0).
To compare post-HAART behavior to behavior just prior to initiation, only visits in the year prior to initiation were included as the referent group. Logistic regression models estimated the odds of engaging in the four behaviors post-HAART relative to pre-HAART periods (Table 2). In unadjusted analysis, HAART initiation was associated with decreases in both measures of sexual behavior and in overall drug injecting. In multivariable analyses adjusting for socio-demographic characteristics and other potential confounders, we observed a 75% reduction in the odds of reporting unprotected sex [adjusted odds ratio (aOR) 0.25; 95% confidence interval (CI), 0.19–0.32] and a 38% reduction in the odds of reporting injection drug use (aOR 0.62, 0.51–0.75) after HAART initiation. When restricted to the participants reporting active injection, needle-sharing appeared to increase after HAART initiation. That is, although overall needle-sharing declined concurrent with the declining frequency of drug injecting in the cohort, the likelihood of sharing needles among those participants who continued to inject was 40% higher after HAART initiation (OR 1.40; 95% CI, 1.14–1.71). This association appeared even stronger after adjustment for other demographic, clinical and behavioral covariates (aOR 1.99, 95% CI, 1.57–2.52).
Table 2.
Likelihood and durability of sexual and drug-related behavior change after HAART initiation.
| Outcome | Overall ORa (95% CI) | Overall aORa,c (95% CI) | aORb,c (95% CI) |
|---|---|---|---|
| Any sexual behavior | |||
| pre-HAART | 1.00 | 1.00 | 1.00 |
| post-HAART | 0.42 (0.36–0.49)* | 0.95 (0.80–1.14) | |
| 1 year | 1.15 (0.98–1.36) | ||
| 1–2 years | 0.95 (0.76–1.18) | ||
| 2–5 years | 0.88 (0.69–1.13) | ||
| > 5 years | 0.73 (0.51–1.04) | ||
| Unprotected sexd | |||
| pre-HAART | 1.00 | 1.00 | 1.00 |
| post-HAART | 0.54 (0.44–0.67)* | 0.25 (0.19–0.32)* | |
| 1 year | 0.35 (0.26–0.46)* | ||
| 1–2 years | 0.15 (0.10–0.24)* | ||
| 2–5 years | 0.18 (0.12–0.27)* | ||
| > 5 years | 0.17 (0.10–0.29)* | ||
| Any injection of drugs | |||
| pre-HAART | 1.00 | 1.00 | 1.00 |
| post-HAART | 0.26 (0.22–0.31)* | 0.62 (0.51–0.75)* | |
| 1 year | 0.69 (0.58–0.83)* | ||
| 1–2 years | 0.63 (0.50–0.79)* | ||
| 2–5 years | 0.46 (0.36–0.60)* | ||
| > 5 years | 0.28 (0.18–0.43)* | ||
| Needle-sharinge | |||
| pre-HAART | 1.00 | 1.00 | 1.00 |
| post-HAART | 1.40 (1.14–1.71)* | 1.99 (1.57–2.52)* | |
| 1 year | 1.73 (1.31–2.30)* | ||
| 1–2 years | 2.12 (1.48–3.04)* | ||
| 2–5 years | 2.24 (1.51–3.33)* | ||
| > 5 years | 2.53 (1.37–4.68)* |
aOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
Statistically significant association (α = 0.05).
Time was modeled as a binary variable: pre-HAART visits vs. all visits post-HAART.
Time was modeled with only the visits 1 year prior to HAART initiation vs. visits categorized into time-periods after initiation up to more than 5 years of follow-up.
Adjusted for age, sex, race, alcohol usage, employment status, CD4 cell count, and year of HAART initiation (1996–1997, 1998–2000, 2001–2007).
Unprotected sex includes the subgroup of participants that reported any sexual behavior.
Needle-sharing includes the subgroup of participants that reported any injection of drugs.
To evaluate whether behavior changes following HAART initiation were stable over time, we next compared risk behaviors reported in the year prior to initiation to behavior reported during post-HAART follow-up intervals of 1 year, 1–2 years, 2–5 years, and more than 5 years (Table 2). No significant changes in the report of any sex were observed during follow-up. Unprotected sex and any injection drug use were reported significantly less in all post-HAART time periods. The odds of reporting unprotected sex decreased by almost two-thirds during the first year after initiation and decreased further in subsequent time intervals. The odds of reporting any injection drug use decreased by around one-third during the first 2 years after HAART initiation with further decline afterwards. Within the subgroup of active injectors, needle-sharing increased with time after initiation, from a 73% increase after 1 year to more than 2.5-fold increase after more than 5 years of follow-up.
Sensitivity analyses
We investigated whether the observed decreases in risk behavior were influenced by factors other than HAART through sensitivity analyses. To explore whether behavior change reflected differential loss-to-follow-up among higher-risk IDUs, we repeated our analyses while limiting inclusion to participants with at least three post-HAART visits. In the restricted models, similar changes in unprotected sex and any drug use were observed; the magnitude of the association between needle-sharing and HAARTwas slightly attenuated but remained statistically significant (aOR = 1.83, 95% CI: 1.41–2.37). The associations described above did not significantly differ between participants who reported consistent HAART use and those who had one or more treatment interruptions. Stratifying the sample by pre-HAART risk behavior, we found significant decreases in unprotected sex (aOR = 0.26, 95% CI: 0.17–0.41 and 0.19, 95% CI: 0.14–0.27) and drug injecting (aOR = 0.59, 95% CI: 0.39–0.92 and 0.38, 95% CI: 0.29–0.49) after HAART initiation among both high-risk and lower-risk IDUs, respectively, supporting that the observed differences represent actual behavior changes associated with HAART rather than effects of reduced risk only among high-risk persons.
Predictors of sexual and drug-related behaviors
We examined selected demographic, behavioral and clinical factors as predictors of sexual or drug-related risk behaviors following HAART initiation (Table 3). After adjusting for potential confounders, pre-HAART behavior was strongly and consistently predictive of reporting all post-HAART behaviors examined. Participants who engaged in unprotected sex in the year prior to HAART initiation had 3.34-fold greater odds of reporting unprotected sex (95% CI, 1.87–5.95) after HAART initiation. In a subset analysis which included as the referent group those who resumed sexual activity after initiating HAART, those who reported any sex before and after HAART initiation had nearly twice the odds of reporting unprotected sex after HAART (OR 1.89; 95% CI, 1.13–3.37). Participants reporting any injection drug use or needle-sharing in the year prior to HAART had 10.8-fold (95% CI, 6.58–17.8) and 2.55-fold (95% CI, 1.70–3.83) greater odds, respectively, of reporting these behaviors after HAART initiation.
Table 3.
Predictors of sexual and drug-related risk behavior following HAART initiationa.
| Engaged in sex-related behaviors after HAART initiation |
Engaged in drug-related behaviors after HAART initiation |
|||
|---|---|---|---|---|
| Variables | Any sexual behavior aORc (95% CI) | Unprotected sex aORc (95% CI) | Injecting drugs aORc (95% CI) | Needle-sharing aORc (95% CI) |
| Age at HAART initiation | ||||
| <40 years | 1.00 | 1.00 | 1.00 | 1.00 |
| 40–43.9 years | 0.71 (0.40–1.27) | 1.67 (0.89–3.13) | 0.68 (0.38–1.23) | 0.76 (0.41–1.39) |
| 44–47.9 years | 1.00 (0.60–1.66) | 1.23 (0.68–2.22) | 0.94 (0.54–1.61) | 0.50 (0.29–0.86)* |
| ≥ 48 years | 0.71 (0.41–1.22) | 0.72 (0.40–1.32) | 0.61 (0.35–1.06) | 0.67 (0.37–1.22) |
| Sex | ||||
| Men | 1.00 | 1.00 | 1.00 | 1.00 |
| Women | 0.73 (0.49–1.08) | 2.20 (1.34–3.63)* | 0.62 (0.40–0.95)* | 1.28 (0.83–1.99) |
| Engaged in behavior in year prior to HAART initiation | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 5.65 (3.72–8.60)* | 3.34 (1.87–5.95)* | 10.8 (6.58–17.8)* | 2.55 (1.70–3.83)* |
| Year of HAART initiation | ||||
| 1996–1997 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1998–2000 | 0.91 (0.50–1.66) | 0.41 (0.22–0.75)* | 1.22 (0.54–2.76) | 2.92 (1.39–6.15)* |
| 2001 and after | 0.82 (0.44–1.53) | 0.72 (0.35–1.47) | 1.05 (0.47–2.37) | 1.93 (0.91–4.09) |
| Baseline CD4 count (cells/μl) | ||||
| < 100 | 1.00 | 1.00 | 1.00 | 1.00 |
| 100–199 | 0.94 (0.55–1.60) | 1.25 (0.60–2.60) | 1.27 (0.72–2.23) | 0.66 (0.36–1.23) |
| 200–349 | 0.84 (0.51–1.37) | 1.40 (0.73–2.67) | 0.84 (0.50–1.41) | 0.79 (0.46–1.34) |
| 350–499 | 1.63 (0.91–2.91) | 1.15 (0.50–2.65) | 0.79 (0.44–1.44) | 0.90 (0.44–1.82) |
| ≥ 500 | 1.46 (0.62–3.44) | 1.53 (0.57–4.15) | 1.78 (0.73–4.32) | 0.95 (0.43–2.08) |
| Increase in CD4 count of 50 cells from baselineb | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.37 (1.09–1.72)* | 0.86 (0.62–1.21) | 0.77 (0.59–1.02) | 1.18 (0.80–1.75) |
| Baseline HIV viral load, copies/ml | ||||
| <1000 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1000- 74 999 | 1.54 (0.94–2.53) | 0.91 (0.51–1.63) | 1.65 (1.00–2.74)* | 2.13 (1.24–3.67)* |
| ≥75 000 | 2.05 (1.17–3.58)* | 1.29 (0.65–2.58) | 1.36 (0.78–2.38) | 1.27 (0.68–2.36) |
| Current HIV viral load <1000 copies/mlb | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.09 (0.92–1.29) | 0.98 (0.73–1.32) | 0.63 (0.48–0.82)* | 0.84 (0.57–1.24) |
aOR, adjusted odds ratio; CI, confidence interval.
Statistically significant association (α = 0.05).
Time was modeled with visits after HAART initiation.
Time-varying variable.
Adjusted for all other variables in the table.
There were mixed findings on the associations of treatment responses and other demographic characteristics with post-HAART behavior. Baseline immunological status was not associated with risk behaviors (Table 3). Moderate or high baseline viral loads were associated with an increased likelihood for injection-related risk behavior or having any sex after HAART initiation, respectively. Immunological improvement (defined by an increase in CD4 cell count by at least 50 cells/μl from baseline) was associated with higher odds of having any sex (aOR = 1.37; 95% CI, 1.09–1.72), but was not significantly associated with risk behaviors. Virological suppression (defined as current HIV viral load < 1000 copies/ml) was associated with a significant decrease in any injecting after HAART initiation (aOR = 0.63; 95% CI, 0.48–0.82). There were no consistent patterns of risk behavior change associated with age or with calendar-period of HAART initiation, although needle-sharing appeared to increase in later periods. After HAART initiation, women were 2.20-fold more likely to report unprotected sex compared with men (95% CI, 1.34–3.63) but were less likely to inject drugs (aOR 0.62; 95% CI, 0.40–0.95).
Discussion
In longitudinal analysis of 362 HIV-infected IDUs, both sexual and drug-related risk behavior generally declined over a two-decade period of study follow-up. Notable reductions in risk behaviors were observable prior to HAART initiation, and over a substantially longer period of observation following HAART initiation than reported from most prior studies. Our data do not support the premise that HAART is associated with generally increased risky behavior among IDUs.
We found no evidence of sexual behavioral risk compensation following initiation of HAART. Among IDUs who remained sexually active, we observed a significant decline in unprotected sex after HAART initiation, and this effect remained durable for over 5 years. Our findings are consistent with a previous longitudinal study conducted among French IDUs, which reported reduced episodes of unprotected sex after initiating HAART [29] and several studies demonstrating either a reduction or no change in sexual risk behaviors [29,32,41]. In total, the body of evidence provides substantial reassurance that the benefits of HAART for IDUs are not undermined by compensatory increases in sexual risk-taking [30].
Our data suggest a more complex relationship between HAART and injection-related risk behavior. As seen for sexual behavior, we observed a significant and sustained reduction in active injecting after initiating HAART, findings in agreement with similar analyses from France [42] and the Netherlands [43]. However, for the small subset of IDUs who continued or resumed injecting after HAART, the odds of needle-sharing increased approximately two-fold, with no diminution of this excess risk over more than 5 years of follow-up. This finding indicates that for a minority of IDUs unable to abstain from injecting, starting HIV treatment could mark a transition to riskier injecting. Importantly, because of the notable decline in the proportion injecting overall following HAART initiation (65% before to 34% after), the population prevalence of needle-sharing was relatively low and declined following HAART (26% before to 16% after).
Our research group previously evaluated HIV-infected IDUs in Baltimore in the first 2 years after HAART availability (1996–1998) and identified a subgroup of IDUs that reported small increases in unprotected sex, needle-sharing and shooting gallery attendance following initiation of therapy [33]. In the present study, we build upon this early work by incorporating data from 229 additional HAART initiators with up to 11 years of follow-up after initiation. Our updated analysis does support the existence of a high-risk subset of IDUs for whom HIV treatment may coincide with increased vulnerability to intensification of risky injecting. This contrasts with a recent study evaluating 260 active IDUs initiating HAART in Vancouver between 1996 and 2008, which found no association between syringe lending behavior and HAART initiation [31]. Using baseline data from a multisite prevention trial, a lower prevalence of syringe lending was observed among IDUs currently prescribed HAART compared with those not receiving HAART, although temporal relationships of behavior and HAART initiation could not be evaluated [44]; at 6 and 12 months after the intervention, no association between HAART and syringe lending was observed [45]. Behavioral risk compensation due to decreased perceived infectiousness is a plausible hypothesis to explain increased needle-sharing post-HAART among persistent injectors [18]. Changes in risk environment may also contribute. For example, HAART initiation may signal increased social functioning and mobility, resulting in exposure to riskier drug-using networks. Further research is needed to investigate mechanisms underlying this phenomenon and the existence of context-specific factors, which may help explain why observations differ across studies.
Our analyses extended beyond assessment of behavior changes following HAART to identification of predictors of subsequent high-risk behavior. We consistently found that individuals who engaged in high-risk behaviors in the year prior to HAART initiation were substantially more likely to engage in those same behaviors following HAART, providing a strong rationale for targeting these high-risk persons with additional prevention interventions. In contrast to the paradigm of treatment optimism and riskier behavior among HAARTresponders [22–25], we found that persons with virologic suppression were less likely to inject drugs overall. In our cohort, positive behavior change leading to engagement in HIV care, adherence, and favorable treatment responses appear to often overlap with less risky sexual and drug-related behavior.
Several methodological constraints and study characteristics may limit interpretation of our findings. First, the use of antiretroviral medications and engagement in risk behaviors were self-reported. We attempted to mitigate the possibility of inaccurate recall through the use of medication charts and ‘pill books’, but misclassification of HAART status may have occurred. Despite efforts to capture previous HAART use through baseline medication questionnaires, some IDUs recruited during later phases of the study may have received HAART prior to enrollment and have been misclassified as HAART initiators. Self-reported risk behavior data is subject to socially desirable responding, which could result in underestimation of the behavioral outcomes. Our approach to behavior ascertainment may also fail to reflect the significant variability in the riskiness of specific behaviors, as, for example, in our grouping together of unprotected anal, vaginal, and oral sex. Because of missing baseline risk data, only 78% of IDUs who initiated HAART were analyzed and may not be representative of all treatment initiators in the cohort. Moreover, our urban, African–American study population may not be fully representative of all HIV-infected IDUs who reside in diverse settings with different cultural norms and socio-legal contexts. The ALIVE study regularly provides risk reduction counseling and encourages engagement in HIV care for participants, but operates separately from any HIV care location. We are therefore limited in our ability to assess the degree that study participants are engaged in HIV care, and the role that clinic-based health promotion messages may influence behavior.
A limitation common to cohort studies is that higher-risk participants may be more likely lost to follow-up, introducing an attrition bias that makes the remaining cohort appear to have decreasing risk. We attempted to mitigate this possibility by conducting a sensitivity analysis limited to participants with longer duration of follow-up and our findings were unchanged, suggesting that differential loss-to-follow-up did not likely influence the behavioral associations with HAARTobserved in this population. Secular trends favoring overall reductions in risk behavior during the study period could produce spurious association between HAART and decreased behavioral risk. To control for unmeasured contextual factors influencing behavior over the course of study, we included calendar year of HAART initiation in the multivariable models.
This study underscores the relevance of behavioral research to programs implementing and evaluating the ‘Treatment as Prevention’ (TasP) paradigm [7]. Antiretroviral therapy has been considered a promising method of prevention for over a decade since the discovery that treating HIV-positive pregnant women can effectively reduce mother-to-child transmission [46]. Observational studies of serodiscordant couples [47,48] and a recent large, randomized prevention trial [5] provide powerful evidence supporting the link between viral suppression on HAART with reduced transmissibility. In theory, expansion of HAART could also yield incremental risk reduction via behavioral counseling often provided through comprehensive HIV treatment programs, or could paradoxically increase risky behavior due to diminished recognition of negative consequences associated with unsafe sexual or injection-related practices. Our results support the optimistic view that for most IDUs, risk compensation following HAART initiation is unlikely, albeit with a worrisome caveat that a small minority of active injectors may be more likely to share needles after initiating treatment. As TasP strategies are expanded, earlier treatment makes it increasingly likely that IDUs will initiate therapy in closer temporal proximity to having engaged in high-risk behavior. Based on our findings, targeting risk-reduction interventions for persons with high-risk behavior in the time-period shortly before HAART initiation should be considered.
Acknowledgements
We acknowledge the ALIVE project staff and study participants without whom this work would not have been possible.
This study was supported by Public Health Service Grants from the National Institutes of Health including from the National Institute on Drug Abuse (R01-DA12568 & R01-DA04334 to support ALIVE; and K23-DA032306 to R.W.) with additional funding from the National Institute of Allergy and Infectious Diseases (K01-AI071754 to B.L.).
Footnotes
T-C.F. and G.D.K. designed the study. T-C.F., B.L. and G.D.K. contributed to the statistical analyses. D.V., S.H.M. and G.D.K. provided data. T-C.F., R.P.W. and G.D.K. wrote the article. All authors edited and approved the final article.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Detels R, Munoz A, McFarlane G, Kingsley L, Margolick J, Giorgi J, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. JAMA. 1998;280:1497. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Walensky RP, Paltiel AD, Losina E, Mercincavage LM, Schackman BR, Sax PE, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Gay C, Kashuba AD, Blower S, Paxton L. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med. 2007;146:591–601. doi: 10.7326/0003-4819-146-8-200704170-00010. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MS, Gay CL. Treatment to prevent transmission of HIV-1. Clin Infect Dis. 2010;50(Suppl 3):S85–S95. doi: 10.1086/651478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montaner JS. Treatment as prevention–a double hat-trick. Lancet. 2011;378:208–209. doi: 10.1016/S0140-6736(11)60821-0. [DOI] [PubMed] [Google Scholar]
- 8.Law MG, Prestage G, Grulich A, Van de Ven P, Kippax S. Modelling the effect of combination antiretroviral treatments on HIV incidence. AIDS. 2001;15:1287–1294. doi: 10.1097/00002030-200107060-00011. [DOI] [PubMed] [Google Scholar]
- 9.Baggaley RF, Garnett GP, Ferguson NM. Modelling the impact of antiretroviral use in resource-poor settings. PLoS Med. 2006;3:e124. doi: 10.1371/journal.pmed.0030124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 11.Velasco-Hernandez JX, Gershengorn HB, Blower SM. Could widespread use of combination antiretroviral therapy eradicate HIV epidemics? Lancet Infect Dis. 2002;2:487–493. doi: 10.1016/s1473-3099(02)00346-8. [DOI] [PubMed] [Google Scholar]
- 12.Kalichman S, Eaton L, Pinkerton S. Circumcision for HIV prevention: failure to fully account for behavioral risk compensation. PLoS Med. 2007;4:e138. doi: 10.1371/journal.pmed.0040138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman PA, Roungprakhon S, Tepjan S, Yim S. Preventive HIV vaccine acceptability and behavioral risk compensation among high-risk men who have sex with men and transgenders in Thailand. Vaccine. 2010;28:958–964. doi: 10.1016/j.vaccine.2009.10.142. [DOI] [PubMed] [Google Scholar]
- 14.Guest G, Shattuck D, Johnson L, Akumatey B, Clarke EE, Chen PL, et al. Changes in sexual risk behavior among participants in a PrEP HIV prevention trial. Sex Transm Dis. 2008;35:1002–1008. [PubMed] [Google Scholar]
- 15.Dukers NHTM, Goudsmit J, de Wit JBF, Prins M, Weverling G-J, Coutinho RA. Sexual risk behaviour relates to the virological and immunological improvements during highly active antiretroviral therapy in HIV-1 infection. AIDS. 2001;15:369–378. doi: 10.1097/00002030-200102160-00010. [DOI] [PubMed] [Google Scholar]
- 16.Tun W, Gange SJ, Vlahov D, Strathdee SA, Celentano DD. Increase in sexual risk behavior associated with immunologic response to highly active antiretroviral therapy among HIV-infected injection drug users. Clin Infect Dis. 2004;38:1167–1174. doi: 10.1086/383033. [DOI] [PubMed] [Google Scholar]
- 17.Ostrow DE, Fox KJ, Chmiel JS, Silvestre A, Visscher BR, Vanable PA, et al. Attitudes towards highly active antiretroviral therapy are associated with sexual risk taking among HIV-infected and uninfected homosexual men. AIDS. 2002;16:775–780. doi: 10.1097/00002030-200203290-00013. [DOI] [PubMed] [Google Scholar]
- 18.Tun W, Celentano DD, Vlahov D, Strathdee SA. Attitudes toward HIV treatments influence unsafe sexual and injection practices among injecting drug users. AIDS. 2003;17:1953–1962. doi: 10.1097/00002030-200309050-00014. [DOI] [PubMed] [Google Scholar]
- 19.Hayden E. ‘Seek, test and treat’ slows HIV. Nature. 2010;463:1006. doi: 10.1038/4631006a. [DOI] [PubMed] [Google Scholar]
- 20.Montaner J, Wood E, Kerr T, Lima V, Barrios R, Shannon K, et al. Expanded highly active antiretroviral therapy coverage among HIV-positive drug users to improve individual and public health outcomes. J Acquir Immune Defic Syndr. 2010;55:S5–S9. doi: 10.1097/QAI.0b013e3181f9c1f0. [DOI] [PubMed] [Google Scholar]
- 21.Katz MH, Schwarcz SK, Kellogg TA, Klausner JD, Dilley JW, Gibson S, et al. Impact of highly active antiretroviral treatment on HIV seroincidence among men who have sex with men: San Francisco. Am J Public Health. 2002;92:388–394. doi: 10.2105/ajph.92.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rietmeijer CA. Resurgence of risk behaviors among men who have sex with men: the case for HAART realism. Sex Transm Dis. 2005;32:176–177. doi: 10.1097/01.olq.0000156738.09438.53. [DOI] [PubMed] [Google Scholar]
- 23.Stolte IG, Dukers NHTM, de Wit JBF, Fennema JSA, Coutinho RA. Increase in sexually transmitted infections among homosexual men in Amsterdam in relation to HAART. Sex Transm Infect. 2001;77:184–186. doi: 10.1136/sti.77.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stolte IG, Dukers NHTM, Geskus RB, Coutinho RA, de Wit JBR. Homosexual men change to risky sex when perceiving less threat of HIV/AIDS since availability of highly active anti-retroviral therapy: A longitudinal study. AIDS. 2004;18:303–309. doi: 10.1097/00002030-200401230-00021. [DOI] [PubMed] [Google Scholar]
- 25.Optimism ICoH. HIV treatments optimism among gay men: an international perspective. J Acquir Immune Defic Syndr. 2003;32:545–550. doi: 10.1097/00126334-200304150-00013. [DOI] [PubMed] [Google Scholar]
- 26.Venkatesh KK, Flanigan TP, Mayer KH. Is expanded HIV treatment preventing new infections? Impact of antiretroviral therapy on sexual risk behaviors in the developing world. AIDS. 2011;25:1939–1949. doi: 10.1097/QAD.0b013e32834b4ced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wandera B, Kamya MR, Castelnuovo B, Kiragga A, Kambugu A, Wanyama JN, et al. Sexual behaviors over a 3-year period among individuals with advanced HIV/AIDS receiving antiretroviral therapy in an urban HIV clinic in Kampala, Uganda. J Acquir Immune Defic Syndr. 2011;57:62–68. doi: 10.1097/QAI.0b013e318211b3f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venkatesh KK, de Bruyn G, Lurie MN, Mohapi L, Pronyk P, Moshabela M, et al. Decreased sexual risk behavior in the era of HAART among HIV-infected urban and rural South Africans attending primary care clinics. AIDS. 2010;24:2687–2696. doi: 10.1097/QAD.0b013e32833e78d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouhnik AD, Moatti JP, Vlahov D, Gallais H, Dellamonica P, Obadia Y. Highly active antiretroviral treatment does not increase sexual risk behaviour among French HIV infected injecting drug users. J Epidemiol Commun Health. 2002;56:349–353. doi: 10.1136/jech.56.5.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall B, Milloy M, Kerr T, Zhang R, Montaner J, Wood E. No evidence of increased sexual risk behaviour after initiating antiretroviral therapy among people who inject drugs. AIDS. 2010;24:2271–2278. doi: 10.1097/QAD.0b013e32833dd101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuyper L, Milloy MJ, Marshall BD, Zhang R, Kerr T, Montaner JS, et al. Does initiation of HIV antiretroviral therapy influence patterns of syringe lending among injection drug users? Addict Behav. 2011;36:560–563. doi: 10.1016/j.addbeh.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smit C, Lindenburg K, Geskus RB, Brinkman K, Coutinho RA, Prins M. Highly active antiretroviral therapy (HAART) among HIV-infected drug users: a prospective cohort study of sexual risk and injecting behaviour. Addiction. 2006;101:433–440. doi: 10.1111/j.1360-0443.2006.01339.x. [DOI] [PubMed] [Google Scholar]
- 33.Vlahov D, Safaien M, Lai S, Strathdee SA, Johnson L, Sterling T, et al. Sexual and drug risk-related behaviours after initiating highly active antiretroviral therapy among injection drug users. AIDS. 2001;15:2311–2316. doi: 10.1097/00002030-200111230-00013. [DOI] [PubMed] [Google Scholar]
- 34.Vlahov D, Graham N, Hoover D, Flynn C, Bartlett JG, Margolick JB, et al. Prognostic indicators for AIDS and infectious disease death in HIV-infected injection drug users: Plasma viral load and CD4R cell count. JAMA. 1998;279:35–40. doi: 10.1001/jama.279.1.35. [DOI] [PubMed] [Google Scholar]
- 35.Vlahov D, Anthony JC, Munoz A, Margolick J, Nelson KE, Celentano DD, et al. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: Description of methods and characterisitics of participants. NIDA Res Monograph. 1991;109:75–100. [PubMed] [Google Scholar]
- 36.Macalino GE, Celentano DD, Latkin C, Strathde SA, Vlahov D. Risk behaviors by audio computer-assisted self-interviews among HIV-seropositive and HIV-seronegative injection drug users. AIDS Educ Prevent. 2002;14:367–1367. doi: 10.1521/aeap.14.6.367.24075. [DOI] [PubMed] [Google Scholar]
- 37.Celentano DD, Galai N, Sethi AK, Shah NGA, Strathdee SA, Vlahov D, et al. Time to initiating highly active antiretroviral therapy among HIV-infected injection drug users. AIDS. 2001;15:1707–1715. doi: 10.1097/00002030-200109070-00015. [DOI] [PubMed] [Google Scholar]
- 38.Mehta SH, Lucas G, Astemborski J, Kirk GD, Vlahov D, Galai N. Early immunologic and virologic responses to highly active antiretroviral therapy and subsequent disease progression among HIV-infected injection drug users. AIDS Care. 2007;19:637–645. doi: 10.1080/09540120701235644. [DOI] [PubMed] [Google Scholar]
- 39.Kavasery R, Galai N, Astemborski J, Lucas GM, Celentano DD, Kirk GD, et al. Nonstructured treatment interruptions among injection drug users in Baltimore, MD. J Acquir Immune Defic Syndr. 2009;50:360–366. doi: 10.1097/QAI.0b013e318198a800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris J, Golub E, Mehta S, Jacobson L, Gange S. Injection drug use and patterns of highly active antiretroviral therapy use: an analysis of ALIVE, WIHS, and MACS cohorts. AIDS Res Ther. 2007;4:12. doi: 10.1186/1742-6405-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall BD, Milloy MJ, Kerr T, Zhang R, Montaner JS, Wood E. No evidence of increased sexual risk behaviour after initiating antiretroviral therapy among people who inject drugs. AIDS. 2010;24:2271–2278. doi: 10.1097/QAD.0b013e32833dd101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouhnik AD, Carrieri MP, Rey D, Spire B, Gastaut JA, Gallais H, et al. Drug injection cessation among HIV-infected injecting drug users. Addict Behav. 2004;29:1189–1197. doi: 10.1016/j.addbeh.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Lindenburg CEA, Krol A, Smit C, Buster MCA, Coutinho RA, Prins M. Decline in HIV incidence and injecting, but not in sexual risk behaviour, seen in drug users in Amsterdam: a 19-year prospective cohort study. AIDS. 2006;20:1771–1775. doi: 10.1097/01.aids.0000242824.59377.53. [DOI] [PubMed] [Google Scholar]
- 44.Metsch LR, Pereyra M, Purcell DW, Latkin CA, Malow R, Gomez CA, et al. Correlates of lending needles/syringes among HIV-seropositive injection drug users. J Acquir Immune Defic Syndr. 2007;46(Suppl 2):S72–S79. doi: 10.1097/QAI.0b013e3181576818. [DOI] [PubMed] [Google Scholar]
- 45.Latkin CA, Buchanan AS, Metsch LR, Knight K, Latka MH, Mizuno Y, et al. Predictors of sharing injection equipment by HIV-seropositive injection drug users. J Acquir Immune Defic Syndr. 2008;49:447–450. doi: 10.1097/qai.0b013e31818a6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Cock KM, Fowler MG, Mercier E, de Vincenzi I, Saba J, Hoff E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries. JAMA. 2000;283:1175–1182. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 47.Attia S, Egger M, Mueller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 48.Donnell K, Kiarie J, Thomas K. ART and risk of heterosexual HIV-1 transmission in HIV-1 serodiscordant African couples: a multinational prospective study.. 17th Conference on Retro-viruses and Opportunistic Infections; San Francisco, CA. 2010. [Google Scholar]