Abstract
Exposure to air pollution is associated with increased morbidity and mortality from cardiovascular disease. We hypothesized that increases in exposure to ambient air pollution are associated with platelet activation and formation of circulating tissue-factor-expressing microparticles. We studied 19 subjects with type 2 diabetes, without clinical evidence of cardiovascular disease, who had previously participated in a human clinical study of exposure to ultrafine particles (UFP). Blood was obtained for measurements of platelet activation following an overnight stay in the Clinical Research Center, prior to each of their two pre-exposure visits. Air pollution and meteorological data, including UFP counts, were analyzed for the 5 days prior to the subjects’ arrival at the Clinical Research Center. Contrary to expectations, increases in UFP were associated with decreases in surface expression of platelet activation markers. The number of platelet-leukocyte conjugates decreased by −80 (95% confidence interval (CI) −123 to −37, p=0.001) on the first lag day (20–44 hours prior to the blood draw) and by −85 (CI −139 to −31, p=0.005) on combined lag days 1 to 5, per interquartile range (IQR) increase in UFP particle number (2482). However, levels of soluble CD40L increased 104 (CI 3 to 205, p=0.04) pg/ml per IQR increase in UFP on lag day 1, a finding consistent with prior platelet activation. We speculate that, in people with diabetes, exposure to UFP activates circulating platelets within hours of exposure, followed by an increase in soluble CD40L and a rebound reduction in circulating platelet surface markers.
INTRODUCTION
Exposure to air pollution is associated with increases in morbidity and mortality related to cardiovascular disease, including myocardial infarction, cardiac arrhythmias, congestive heart failure, and stroke (Frampton et al., 2000, Peters et al., 2000, Peters et al., 2004, Pope III et al., 2004, Utell et al., 2002). One important mechanism may involve increased susceptibility to the formation of thrombi in diseased arteries. The initiating events in thrombus formation involve platelet activation, aggregation, and adherence. Circulating microparticles (MP), cell fragments smaller than 1 μm released from cells in response to injury, activation, or apoptosis (Simak and Gelderman, 2006), are thrombogenic and can express tissue factor, an important initiator of the coagulation cascade. People with type 2 diabetes may be particularly susceptible to the cardiovascular effects of air pollution because they have accelerated development of atherosclerotic vascular disease and its complications, mediated in part by enhanced oxidative stress. Diabetes is associated with baseline platelet activation and increased platelet responsiveness to agonists (Natarajan et al., 2008). Some air pollution epidemiology studies indeed suggest that diabetes confers an increased risk for health effects from air pollution (Goldberg et al., 2006, Liao et al., 2005, Zanobetti and Schwartz, 2002).
The wealth of evidence linking ambient pollutants, especially particulate matter, to adverse health effects has resulted in more stringent air quality standards and major efforts to reduce emissions around the world. For example, in 2006 the US Environmental Protection Agency promulgated a reduction in the National Ambient Air Quality Standards (NAAQS) for fine particles, less than 2.5 μm in diameter (PM2.5), to 35 μg/m3 as a 24-hour average because of evidence for health effects at ambient concentrations below the previous Standard of 65 μg/m3.
However, the current NAAQS for particulate matter, which are based on particle mass measurements, do not directly address the issue of particles less than 100 nm in diameter, often referred to as ultrafine particles (UFP). The vast majority of particles in ambient air are UFP, when considered by particle number. These particles have very little mass, and mass-based regulation of fine particle concentrations may not reduce UFP number concentrations. The physical properties of UFP enable them to deposit efficiently in the alveolar compartment of the lung when inhaled (Chalupa et al., 2004, Daigle et al., 2003), and even to enter epithelial cells and the pulmonary vasculature, where they could theoretically perturb vascular function by further enhancing vascular oxidative stress (Frampton et al., 2006, Shah et al., 2008). The potential role of UFP in contributing to the cardiovascular effects of air pollution exposure remains an important and open question.
We recently reported the results of a human clinical study of people with type 2 diabetes who inhaled clean air and laboratory-generated carbon UFP (50 μg/m3, count median diameter 32 nm) for two hours at rest, with the exposures separated by 3 weeks (Stewart et al., 2010). We found evidence for platelet and possibly vascular activation 3.5 hours after exposure, with UFP-related increases in von Willebrand factor, platelet expression of CD40 ligand (CD40L), and platelet-leukocyte conjugates. For the current study we explored the relationship between the level of platelet activation and numbers of circulating tissue-factor-expressing MP in these diabetic subjects prior to their experimental exposures, and their exposure to ambient outdoor air pollutants over the preceding 5 days. We hypothesized that increases in exposure to ambient UFP, PM2.5, carbon monoxide (CO), and/or ozone, would be associated with increases in platelet activation. We further hypothesized that a genetically determined reduction in antioxidant defense, characterized by a polymorphism involving deletion of the glutathione-S-transferase-M1 gene (GSTM1 null), would increase ambient pollutant effects.
METHODS
Subjects
Written, informed consent was obtained from all subjects, and the studies were approved by the University of Rochester Research Subjects Review Board. Volunteers were 19 never-smokers 30–60 years of age with type 2 diabetes who participated in a clinical study of exposure to ultrafine carbon particles. Details on subject recruitment and findings from that study have been published (Stewart, et al., 2010). Subjects were required to be on a stable medication regimen for at least 3 months prior to entry into the study. Exclusion criteria included clinical cardiovascular disease, major organ dysfunction, uncontrolled hypertension, frequent hypoglycemia, statin-type lipid-lowering medications (because of the anti-inflammatory effects of these medications), platelet-active drugs including aspirin, and occupational exposure to particles (e.g., welding, foundry work). Subjects were asked to avoid nonsteroidal anti-inflammatory drugs and phosphodiesterase-5 enzyme inhibitors during the study. We attempted to balance subject recruitment by sex and by age group (30 to 45 and 46 to 60 years of age). Table 1 provides further characterization of the subjects.
Table 1.
Subject Characteristicsa
| Parameter | Men | Women | Age groups | |
|---|---|---|---|---|
| 30–45 y | 46–60 y | |||
| Number of Subjects | 9 | 10 | 10 | 9 |
| Age (years) | 48.3 ± 8.8a | 43.7 ± 10.0 | 38.0 ± 1.4 | 54.7 ± 1.3 |
| Body Mass Index (BMI) | 34.7 ± 5.2 | 31.1 ± 5.5 | 32.8 ± 2.1 | 32.8 ± 1.5 |
| Cholesterol (mg/dl) | 191.0 ± 36.7 | 168.5 ± 31.9 | 181.7 ± 11.9 | 176.3 ± 11.4 |
| Triglycerides (mg/dl) | 185.9 ± 55.1 | 102.9 ± 80.5* | 142.6 ± 31.0 | 141.8 ± 20.1 |
| High Density Lipoprotein (mg/dl) | 40.6 ± 7.0 | 47.5 ± 8.1 | 44.7 ± 2.5 | 43.7 ± 2.9 |
| Low Density Lipoprotein (mg/dl) | 113.2 ± 38.0 | 100.5 ± 27.8 | 108.4 ± 12.1 | 104.4 ± 9.3 |
| Cholesterol/High Density Lipoprotein Ratio | 4.8 ± 0.8 | 3.7 ± 0.9* | 4.2 ± 0.3 | 4.2 ± 0.4 |
| Hemoglobin A1c (%) | 7.6 ± 1.1 | 7.7 ± 1.8 | 7.6 ± 0.6 | 7.7 ± 0.4 |
Data are Mean ± SD.
p < 0.05 between sexes.
For the current study, subjects were contacted and invited to provide a blood sample for genotyping of the GSTM1 polymorphism, using polymerase chain reaction (PCR) amplification of exons 4 and 5 of the GSTM1 allele (Schwartz et al., 2005). Fourteen of the 19 subjects participating in the original study consented and provided blood samples. Eleven of the 14 subjects (79%) were GSTM1 null, a larger proportion than the approximately 40% expected prevalence.
Study Design
Prior to each of the two exposure sessions of the clinical study, subjects were admitted to the University of Rochester General Clinical Research Center on Monday evening and remained overnight. Blood measurements were performed the following Tuesday morning, at approximately 0900 hours, prior to the experimental exposures. Only these pre-exposure measurements were used in the current study. Thus, each of the 19 subjects had 2 blood draws, separated by at least 3 weeks, on the same day of the week and the same time of day, which were used in the current analysis.
Air Pollution and Meteorology Measurements
All atmospheric measurements were taken at the New York State Department of Environmental Conservation (DEC) site in Rochester, located approximately 7 km from Strong Memorial Hospital, where all subjects underwent testing. We measured counts of particles 10–100 nm in diameter (UFP) using a Scanning Mobility Particle Sizer (SMPS), version 3071 from TSI Inc. (St. Paul, MN). Concentrations of particulate matter with an aerodynamic diameter less than 2.5 μm (PM2.5) were measured using a TEOM (ThermoFisher, Franklin, MA). Ozone and CO were also measured at the DEC site, along with hourly temperature, relative humidity, and barometric pressure. Hourly data on UFP and PM2.5 were averaged over 24 hours for days 1 through 5 prior to the subject’s admission to the Clinical Research Center. Ozone data were processed in a similar manner, except we used the maximum continuous 8-hour average concentration for each 24-hour period. A 5-day average was used as well. We reasoned that, because the subjects were housed within an enclosed, air-conditioned building during the 20 hours prior to the blood draws, changes in outdoor air pollution during that time would be irrelevant, and were not included. Therefore, we initiated the above pollutant averaging intervals prior to the subjects’ admission to the Clinical Research Center.
Markers of Platelet Activation
We measured the following markers related to platelet activation: platelet expression of CD62P and CD40L, platelet-leukocyte conjugates, circulating MP expressing tissue factor (all using immunofluorescence and flow cytometry), and plasma soluble CD40L (using a customized enzyme-linked immunoassay). Platelets contain CD40L, which is expressed on the cell surface and released into plasma with activation (Rückerl et al., 2007). Blood was drawn from an antecubital vein with minimal trauma, with the subject supine. Immunofluorescence measurements were performed within 1 hr of phlebotomy. More detailed methods have been published previously (Stewart, et al., 2010).
Statistical Analysis
We first examined the relationship between the outcome measures and meteorology indicators: temperature, relative humidity, and barometric pressure. Only temperature showed significant associations, so temperature was included in the analytical model. The clinical study design included randomized order of exposure and attempted balancing of age groups and gender, so these were included in the model. Day of the week was not included because all samples were obtained on the same day of the week.
Statistical analysis therefore used mixed models with the subject as a random effect and pollutant, temperature, order of assignment, gender and age group (≤45 vs >45) included as fixed effects.
Separate analyses were conducted for each pollutant and each lag (1–5 days), and for lag days 1–5 combined. The same lag was used for temperature as for pollutant. Adjusted effect estimates are based on a change in pollutant concentration equal to the interquartile range of concentrations for that pollutant, so that the calculated coefficients compare the values of the platelet activation marker at the 75th percentile of the relevant exposure distribution with the values at the 25th percentile.
Analyses were repeated after excluding the 3 subjects that were GSTM1+ (wild-type).
RESULTS
Air Pollution and Meteorology
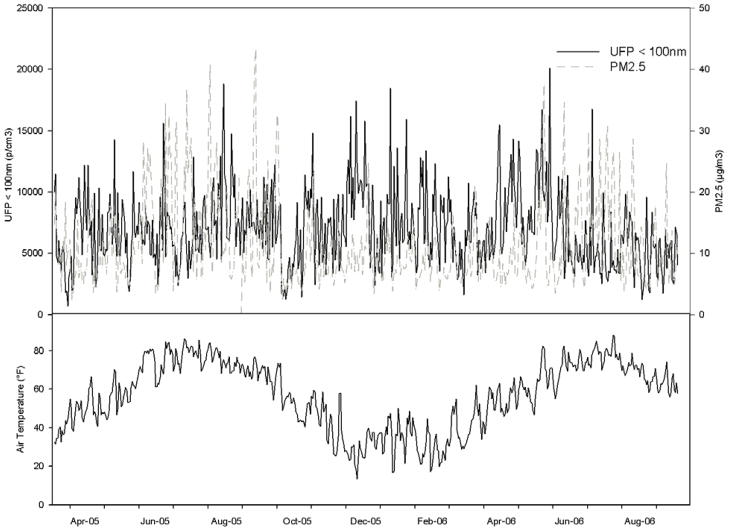
Descriptive statistics for each daily average of pollutant concentrations and weather conditions during this study are shown in Table 2, and the time trends for UFP, PM2.5, and temperature are shown in Figure 1. The 24-hour averages for PM2.5 and ozone rarely exceeded the U.S. National Ambient Air Quality Standards (NAAQS) of 35 μg/m3 and 0.075 ppm, respectively.
Table 2.
Descriptive statistics of daily air pollution concentrations and meteorology during the study period.
| Pollutant/Meteorology | Minimum | Median | Maximum | Inter-quartile range |
|---|---|---|---|---|
| UFP (#/cm3) | 1435 | 6514 | 16930 | 2482 |
| PM2.5 (μg/m3) | 2.4 | 9.8 | 38.9 | 4.3 |
| Ozone (ppm) | 0.01 | 0.03 | 0.08 | 0.01 |
| CO (ppm) | 0.12 | 0.42 | 0.80 | 0.14 |
| Temperature (°F) | 14.59 | 53.49 | 84.86 | 30.42 |
| Relative Humidity (%) | 36.59 | 66.68 | 95.34 | 12.62 |
| Barometric Pressure (inches Hg) | 28.87 | 29.57 | 30.07 | 0.18 |
Figure 1.
Ultrafine particle counts (UFP, <100 nm), PM2.5, and temperature during the study period.
The UFP particle counts and CO were not well-correlated with each other, the other pollutants, or any weather condition (Table 3). The only correlation coefficients >0.40 were for PM2.5 and ozone (r=0.60), PM2.5 and temperature (r=0.62), ozone and temperature (r=0.67), and ozone and relative humidity (r=−0.50).
Table 3.
Correlation (Pearson coefficients) between each hourly pollutant concentration and meteorology.
| Pollutant/Meteorology | UFP | PM2.5 | Ozone | CO | Temperature | Relative Humidity |
|---|---|---|---|---|---|---|
| PM2.5 | 0.19 | --- | ||||
| Ozone | −0.04 | 0.60 | --- | |||
| CO | 0.12 | 0.19 | 0.35 | --- | ||
| Temperature | −0.20 | 0.62 | 0.67 | 0.29 | --- | |
| Relative Humidity | −0.25 | 0.21 | −0.50 | −0.21 | −0.23 | --- |
| Barometric Pressure | 0.38 | 0.28 | 0.04 | −0.07 | 0.02 | −0.30 |
Platelet Activation
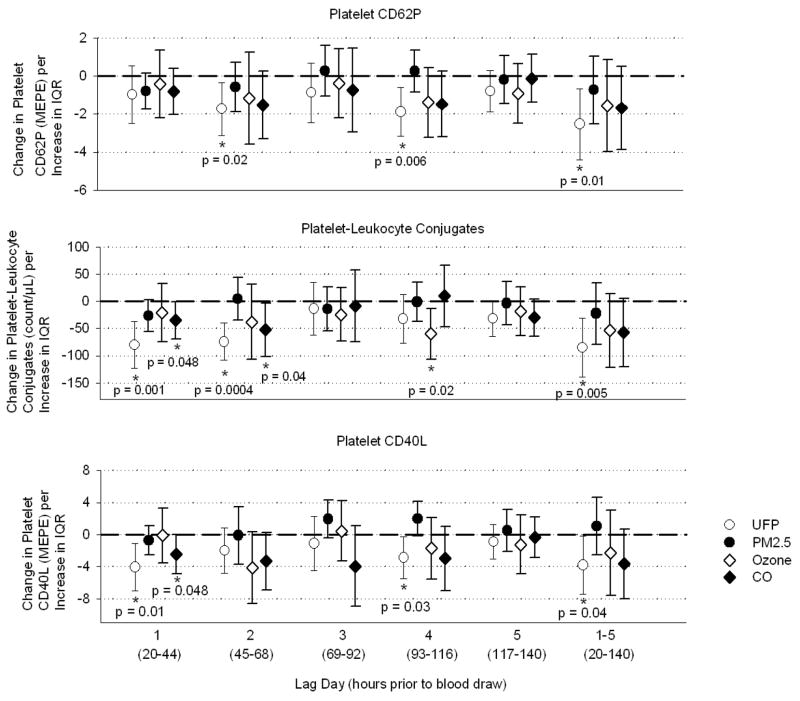
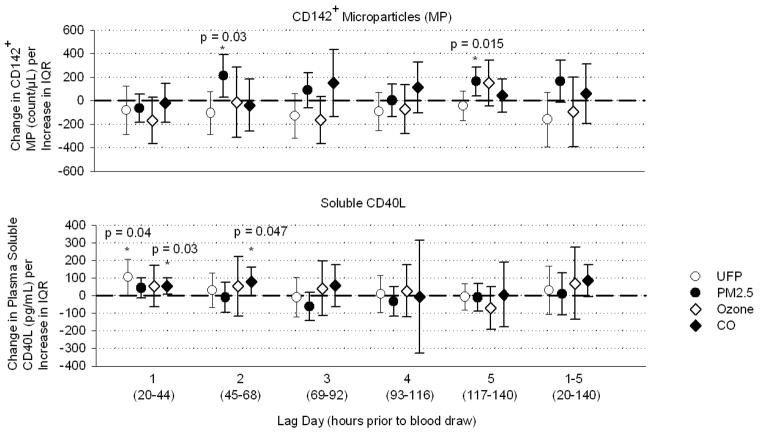
Figures 2 and 3 show pollutant effects after adjustment for temperature, order of measurement, age-group, and sex. Platelet expression of CD62P decreased on lag day 2, day 4, and days 1–5 combined following changes in UFP, but was not significantly associated with the other pollutants (Figure 2). Platelet-leukocyte conjugates decreased day 1, day 2, and days 1–5, and marginally on day 5, following UFP. Conjugates also decreased day 4 after ozone and days 1 and 2 after CO. Platelet expression of CD40L decreased days 1, 4, and 1–5 following UFP, and day 1 after CO. Tissue-factor-expressing MP increased day 2 and 5 following PM2.5. Plasma soluble CD40L increased on day 1 following UFP, and day 1 and 2 following CO. Table 4 provides a summary of the significant associations and direction of change.
Figure 2.
Effect estimates for changes in markers of platelet activation, adjusted for temperature, relative humidity, order of measurement, age-group, and sex. Bars represent 95% confidence intervals. MEPE: molecules of equivalent phycoerythrin (measure of fluorescence intensity). IQR: interquartile range of pollutant concentration.
Figure 3.
Effect estimates for changes in MP expressing tissue factor (CD142) and soluble CD40L, adjusted for temperature, relative humidity, order of measurement, age-group, and sex. Bars represent 95% confidence intervals. IQR: interquartile range of pollutant concentration.
Table 4.
Summary of findings.
| Marker | UFP | PM2.5 | Ozone | CO |
|---|---|---|---|---|
| Platelet CD62P | ↓ D2,4, 1–5 | --- | --- | --- |
| Platelet-Leukocyte Conjugates | ↓ D1,2, 1–5 | --- | ↓ D4 | ↓ D1,2, |
| Platelet CD40L | ↓ D1,4, 1–5 | --- | --- | ↓ D1 |
| CD142+ MP | --- | ↑ D2,5 | --- | --- |
| Soluble CD40L | ↑ D1 | --- | --- | ↑ D1,2 |
Repeating the analyses with exclusion of the 3 subjects that were GSTM1+ did not substantially change the effect estimates for any of the outcomes.
DISCUSSION
In this study, we used data from a human clinical study of the effects of inhaling laboratory-generated ultrafine particles on platelet activation, to test the hypothesis that daily changes in ambient outdoor pollutants, as measured from a central monitor, can affect circulating platelets, and therefore affect pre-exposure testing in study participants.
Table 4 summarizes the findings of this study. Among the three pollutants, after adjustment for temperature effects, UFP had the most consistent and statistically robust relationship with platelet activation markers, but in the opposite of the direction hypothesized. Increases in UFP were associated with decreases in all three surface markers of platelet activation: platelet CD62P, platelet-leukocyte conjugates, and platelet CD40L. Soluble CD40L levels, on the other hand, were increased, which is consistent with increased platelet activation. Tissue-factor-expressing-MP increased significantly on days 2 and 5, and non-significantly on days 1–5 following increases in PM2.5, which is the hypothesized direction of change, suggesting pro-thrombotic vascular effects. Ozone showed largely nonsignificant relationships except for reduced platelet-leukocyte conjugates on day 4. Results with CO paralleled those with UFP, consistent with traffic as the primary source for both pollutants.
There is growing evidence that exposure to air pollution has adverse cardiovascular effects, and that thrombogenic effects are likely to play a role. Franchini et al. (Franchini and Mannucci, 2011) recently reviewed the thrombogenic and cardiovascular effects of air pollution. The proposed mechanisms include release in the lung of pro-inflammatory mediators in response to particle deposition, leading to endothelial activation, with binding and activation of platelets, formation of platelet-leukocyte conjugates, and generation of tissue factor-bearing microparticles. However, there are relatively few studies of UFP health effects, in part because of a relative lack of ambient UFP measurements.
UFP are directly emitted from combustion engines, especially diesel engines, or formed secondarily in the atmosphere through photochemical nucleation. Relative to fine particles, their small size, high surface area, high alveolar deposition, and redox capabilities suggest they may enter the circulation and cause systemic inflammation and related cardiovascular consequences. A recent study in patients undergoing cardiac rehabilitation (Rich et al., 2012) found strong associations between ambient UFP concentrations and adverse changes in a variety of cardiovascular parameters, including systolic blood pressure, heart rate variability, and markers of systemic inflammation. Platelet function was not examined in that study. UFP and nanoparticles of varying composition have been shown to activate platelets in vitro (Radomski et al., 2005), and to promote vascular thrombus formation in animal models (Nemmar et al., 2006). Relationships have been observed between UFP exposure and blood markers of platelet activation (Delfino et al., 2008), including increases in soluble CD40L and decreased platelet counts (Rückerl, et al., 2007).
Some clinical studies of exposure to diesel exhaust, which is rich in UFP, have shown evidence for platelet activation (Lucking et al., 2008). However, other diesel studies at lower concentrations have not found prothrombotic effects (Carlsten et al., 2007). A few human clinical studies have examined the effect of concentrated ambient ultrafine particles, and have not found evidence for systemic inflammatory effects (Samet et al., 2009). However, none have systematically evaluated platelet activation.
We expected to find that exposure to ambient air pollution would be associated with either no change, or an increase, in indicators of platelet activation. To our surprise, our findings were the opposite of the effects observed in the clinical study (Stewart, et al., 2010), in which inhalation of laboratory generated carbon UFP, relative to clean air exposure, increased surface markers of platelet activation 3-1/2 hours after exposure, while decreasing plasma levels of soluble CD40L. The timing of sampling in relation to exposure is one possible reason for the decreases in surface markers. Our subjects were admitted to the Clinical Research Center about 20 hours before the baseline blood draw, so we were not able to examine the effects of increases in outdoor ambient pollution during that interval. Thus, any early (less than 20 hours), transient platelet activation would have been missed. It is possible that UFP exposure triggers an early, transient activation of platelets in the hours following exposure, with a compensatory reduction in platelet activation in the following 1 to 2 days.
Thus, in the current study, the surface marker measurements may have missed transient activation, and only detected subsequent compensatory changes in response to that activation. The increase observed in soluble CD40L provides evidence that an earlier activation occurred, with subsequent release of CD40L from activated platelets. It is known that platelet activation is reversible (Michelson et al., 1994, Schmitz et al., 1998). Circulating platelets that have degranulated and increased their expression of P-selectin in response to a stimulus rapidly lose P-selectin from the surface while continuing to circulate, in the absence of a continuing stimulus (Linden et al., 2004). Compensatory reduction in platelet activation could occur in response to increases in nitric oxide or prostaglandin E2, both of which are inhibitors of platelet activation.
This explanation for our findings remains speculative. However, some epidemiology and panel studies support early adverse effects of PM exposure. A recent panel study in subjects with both type 1 and type 2 diabetes (Jacobs et al., 2010) found that an increase in PM10 exposure 2 hours before the blood draw was associated with increases in a functional assay of platelet activation. Longer lag times were not examined. The risk of MI may increase in as little as 1 hour following elevations in PM (Peters, et al., 2004). A recent study (Bhaskaran et al., 2011) confirmed an increased risk of MI 1–6 hours after elevations in PM10 and NO2 exposure, but found a compensatory decrease in risk at longer lag times. Such findings would be consistent with early (a few hours) pollutant-induced increases in platelet reactivity and activation, followed later (a few days) by compensatory mechanisms to counteract the thrombophilic effects of PM exposure. Our findings are consistent with this.
It is also possible that the differing findings relate to the lower concentrations of ambient UFP (maximum 16930 particles/cm3) compared with the larger concentrations of carbon UFP used in our experimental exposures (approximately 107 particles/cm3), or differences in particle size, surface area, and composition.
Among the strengths of this study are a well-characterized group of subjects with type 2 diabetes studied on the same day of the week and the same time of day, with ambient air quality measurements that included UFP counts. There are a number of limitations to this study. The original clinical study was not designed to assess the effects of ambient pollution exposure. Ambient pollution levels were measured at a single monitoring site, with the possibility for exposure misclassification, especially for UFP, which can have considerable spatial variability. We only measured outdoor air pollutant levels, while people on average spend most of their time indoors (Klepeis et al., 2001). The overall sample size was small, with 2 blood draws for each of the 19 subjects. These limitations would tend to reduce our ability to detect pollutant effects, biasing the study toward the null. The subjects all had type 2 diabetes and were not on statin-type medications, so the findings may not be applicable to subjects without diabetes or taking statins. The number of subjects with the GSTM1 wild-type gene was too small to allow a meaningful analysis of genotype-related effects.
In conclusion, we found significant relationships between ambient concentrations of UFP and markers of platelet activation. However, surface markers of platelet activation decreased in association with UFP, the opposite direction of that expected, while soluble CD40L increased in association with increased UFP. We speculate that, in people with diabetes, exposure to UFP activates circulating platelets within hours of exposure, with later release of platelet CD40L into the plasma, and a rebound reduction in surface activation markers on circulating platelets. Further studies are required to confirm this hypothesis.
Footnotes
DECLARATION OF INTEREST
This research was funded wholly or in part by the American Petroleum Institute, the United States Environmental Protection Agency through the Science to Achieve Results (STAR) program (RD832415) to the University of Rochester, and by grants from the National Institutes of Health (RC1 ES018519, R01 ES017428, P30 ES01247, UL1 RR024160).
Dr. Frampton has provided consulting services for the American Petroleum Institute, the Health Effects Institute, and the Environmental Protection Agency. The other authors report no declarations of interest.
References
- Bhaskaran K, Hajat S, Armstrong B, Haines A, Herrett E, Wilkinson P, Smeeth L. The effects of hourly differences in air pollution on the risk of myocardial infarction: Case crossover analysis of the minap database. BMJ. 2011;343:d5531. doi: 10.1136/bmj.d5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsten C, Kaufman JD, Peretz A, Trenga CA, Sheppard L, Sullivan JH. Coagulation markers in healthy human subjects exposed to diesel exhaust. Thromb Res. 2007;120(6):849–855. doi: 10.1016/j.thromres.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa DC, Morrow PE, Oberdörster G, Utell MJ, Frampton MW. Ultrafine particle deposition in subjects with asthma. Environ Health Perspect. 2004;112:879–882. doi: 10.1289/ehp.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle CC, Chalupa DC, Gibb FR, Morrow PE, Oberdörster G, Utell MJ, Frampton MW. Ultrafine particle deposition in humans during rest and exercise. Inhal Toxicol. 2003;15:539–552. doi: 10.1080/08958370304468. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Polidori A, Arhami M, Gillen DL, Kleinman MT, Vaziri ND, Longhurst J, Zaldivar F, Sioutas C. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ Health Perspect. 2008;116(7):898–906. doi: 10.1289/ehp.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton MW, Samet JM, Utell MJ. Cardiopulmonary consequences of particle inhalation. In: Heyder Gehr PJ., editor. Particle-lung interactions. New York: Marcel Dekker, Inc; 2000. pp. 653–670. [Google Scholar]
- Frampton MW, Stewart JC, Oberdörster G, Morrow PE, Chalupa D, Pietropaoli AP, Frasier LM, Speers DM, Cox C, Huang L-S, Utell MJ. Inhalation of carbon ultrafine particles alters blood leukocyte expression of adhesion molecules in humans. Environ Health Perspect. 2006;114:51–58. doi: 10.1289/ehp.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini M, Mannucci PM. Thrombogenicity and cardiovascular effects of ambient air pollution. Blood. 2011;118(9):2405–2412. doi: 10.1182/blood-2011-04-343111. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Burnett RT, Yale JF, Valois MF, Brook JR. Associations between ambient air pollution and daily mortality among persons with diabetes and cardiovascular disease. Environ Res. 2006;100(2):255–267. doi: 10.1016/j.envres.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Jacobs L, Emmerechts J, Mathieu C, Hoylaerts MF, Fierens F, Hoet PH, Nemery B, Nawrot TS. Air pollution related prothrombotic changes in persons with diabetes. Environ Health Perspect. 2010;118(2):191–196. doi: 10.1289/ehp.0900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, Behar JV, Hern SC, Engelmann WH. The national human activity pattern survey (nhaps): A resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11(3):231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- Liao D, Heiss G, Chinchilli VM, Duan Y, Folsom AR, Lin HM, Salomaa V. Association of criteria pollutants with plasma hemostatic/inflammatory markers: A population-based study. J Expo Anal Environ Epidemiol. 2005;15(4):319–328. doi: 10.1038/sj.jea.7500408. [DOI] [PubMed] [Google Scholar]
- Linden MD, Frelinger AL, 3rd, Barnard MR, Przyklenk K, Furman MI, Michelson AD. Application of flow cytometry to platelet disorders. Semin Thromb Hemost. 2004;30(5):501–511. doi: 10.1055/s-2004-835671. [DOI] [PubMed] [Google Scholar]
- Lucking AJ, Lundback M, Mills NL, Faratian D, Barath SL, Pourazar J, Cassee FR, Donaldson K, Boon NA, Badimon JJ, Sandstrom T, Blomberg A, Newby DE. Diesel exhaust inhalation increases thrombus formation in man. Eur Heart J. 2008;29(24):3043–3051. doi: 10.1093/eurheartj/ehn464. [DOI] [PubMed] [Google Scholar]
- Michelson AD, Benoit SE, Kroll MH, Li JM, Rohrer MJ, Kestin AS, Barnard MR. The activation-induced decrease in the platelet surface expression of the glycoprotein ib-ix complex is reversible. Blood. 1994;83(12):3562–3573. [PubMed] [Google Scholar]
- Natarajan A, Zaman AG, Marshall SM. Platelet hyperactivity in type 2 diabetes: Role of antiplatelet agents. Diab Vasc Dis Res. 2008;5(2):138–144. doi: 10.3132/dvdr.2008.023. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoylaerts MF, Nemery B. Effects of particulate air pollution on hemostasis. Clin Occup Environ Med. 2006;5(4):865–881. doi: 10.1016/j.coem.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Peters A, Liu E, Verrier RL, Schwartz J, Gold DR, Mittleman M, Baliff J, Oh JA, Allen G, Monahan K, Dockery DW. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11:11–17. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Peters A, Von Klot S, Heier M, Trentinaglia I, Hörmann A, Wichmann HE, Löwel H. Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- Pope CA, III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution. Epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, Radomski MW. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol. 2005;146(6):882–893. doi: 10.1038/sj.bjp.0706386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Zareba W, Beckett W, Hopke PK, Oakes D, Frampton MW, Bisognano J, Chalupa D, Bausch J, O’shea K, Wang Y, Utell MJ. Are ambient ultrafine, accumulation mode, and fine particles associated with adverse cardiac responses in patients undergoing cardiac rehabilitation? Environ Health Perspect. 2012 doi: 10.1289/ehp.1104262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rückerl R, Phipps R, Schneider A, Frampton M, Cyrys J, Oberdörster G, Wichmann HE, Peters A. Ultrafine particles and platelet activation in patients with coronary heart disease - results from a prospective panel study. Part Fibre Toxicol. 2007;4:1–14. doi: 10.1186/1743-8977-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM, Rappold A, Graff D, Cascio WE, Berntsen JH, Huang YC, Herbst M, Bassett M, Montilla T, Hazucha MJ, Bromberg PA, Devlin RB. Concentrated ambient ultrafine particle exposure induces cardiac changes in young healthy volunteers. Am J Respir Crit Care Med. 2009;179(11):1034–1042. doi: 10.1164/rccm.200807-1043OC. [DOI] [PubMed] [Google Scholar]
- Schmitz G, Rothe G, Ruf A, Barlage S, Tschope D, Clemetson KJ, Goodall AH, Michelson AD, Nurden AT, Shankey TV. European working group on clinical cell analysis: Consensus protocol for the flow cytometric characterisation of platelet function. Thromb Haemost. 1998;79(5):885–896. [PubMed] [Google Scholar]
- Schwartz J, Park SK, O’neill MS, Vokonas PS, Sparrow D, Weiss S, Kelsey K. Glutathione-s-transferase m1, obesity, statins, and autonomic effects of particles: Gene-by-drug-by-environment interaction. Am J Respir Crit Care Med. 2005;172(12):1529–1533. doi: 10.1164/rccm.200412-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AP, Pietropaoli AP, Frasier LM, Speers DM, Chalupa DC, Delehanty JM, Huang L-S, Utell MJ, Frampton MW. Effect of inhaled carbon ultrafine particles on reactive hyperemia in healthy human subjects. Environ Health Perspect. 2008;116:375–380. doi: 10.1289/ehp.10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simak J, Gelderman MP. Cell membrane microparticles in blood and blood products: Potentially pathogenic agents and diagnostic markers. Transfus Med Rev. 2006;20(1):1–26. doi: 10.1016/j.tmrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Chalupa DC, Devlin RB, Frasier LM, Huang L-S, Little EL, Lee SM, Phipps RP, Pietropaoli AP, Taubman MB, Utell MJ, Frampton MW. Vascular effects of ultrafine particles in persons with type 2 diabetes. Environ Health Perspect. 2010;118:1692–1698. doi: 10.1289/ehp.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utell MJ, Frampton MW, Zareba W, Devlin RB, Cascio WE. Cardiovascular effects associated with air pollution: Potential mechanisms and methods of testing. Inhal Toxicol. 2002;14:1231–1247. doi: 10.1080/08958370290084881. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. Cardiovascular damage by airborne particles: Are diabetics more susceptible? Epidemiology. 2002;13(5):588–592. doi: 10.1097/00001648-200209000-00016. [DOI] [PubMed] [Google Scholar]