Abstract
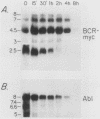
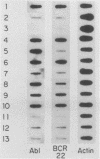
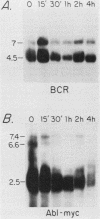
The translocation of the c-abl oncogene from chromosome 9 to the bcr gene on chromosome 22 in cases of Philadelphia chromosome-positive chronic myelogenous leukemia (CML) generates an aberrant bcr-abl fusion transcript which may be intimately related to the pathogenesis of CML. Because factors controlling normal bcr expression might also be involved in the expression of this aberrant bcr-abl transcript, we studied the patterns of expression of the normal bcr gene in different cell types. We found that the normal bcr gene was expressed in many different types of human cells. Moreover, the bcr gene was evolutionarily conserved, and homologous bcr genomic sequences and RNA transcripts were readily detected in chick tissue. The highest level of bcr expression in chick tissue was in brain tissue, the lowest level was in liver tissue, and a truncated bcr mRNA was noted in chick testes. Normal bcr transcripts, in addition to the aberrant bcr-abl hybrid transcripts, have been found in all Philadelphia chromosome-positive CML cells studied to date. Within a given CML sample, the relative amounts of normal bcr RNA and aberrant bcr-abl RNA were similar. In addition, the normal bcr and the aberrant bcr-abl hybrid transcripts demonstrated similarly prolonged half-lives compared with that of the normal abl-related transcripts in CML cells. These findings suggest that in CML cells, similar cellular mechanisms control the steady-state levels of both the normal bcr and the bcr-abl fusion RNAs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Gale R. P., Steiner-Saltz D., Berrebi A., Aghai E., Januszewicz E. Altered transcription of an oncogene in chronic myeloid leukaemia. Lancet. 1984 Mar 17;1(8377):593–595. doi: 10.1016/s0140-6736(84)90997-8. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Groudine M. T. Rearrangement and amplification of c-abl sequences in the human chronic myelogenous leukemia cell line K-562. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4813–4817. doi: 10.1073/pnas.80.15.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Kubonishi I., Miyoshi I., Groudine M. T. Altered transcription of the c-abl oncogene in K-562 and other chronic myelogenous leukemia cells. Science. 1984 Jul 6;225(4657):72–74. doi: 10.1126/science.6587568. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Martinotti S., Gallo R. C., Erikson J., Croce C. M. Translocation and rearrangements of the c-myc oncogene locus in human undifferentiated B-cell lymphomas. Science. 1983 Feb 25;219(4587):963–967. doi: 10.1126/science.6401867. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J., Jacobson R. J., Papayannopoulou T. Chronic myelocytic leukemia: clonal origin in a stem cell common to the granulocyte, erythrocyte, platelet and monocyte/macrophage. Am J Med. 1977 Jul;63(1):125–130. doi: 10.1016/0002-9343(77)90124-3. [DOI] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Stam K., Groffen J., de Klein A., Grosveld G. Structural organization of the bcr gene and its role in the Ph' translocation. 1985 Jun 27-Jul 3Nature. 315(6022):758–761. doi: 10.1038/315758a0. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Stam K., Groffen J., de Klein A., Grosveld G. Structural organization of the bcr gene and its role in the Ph' translocation. 1985 Jun 27-Jul 3Nature. 315(6022):758–761. doi: 10.1038/315758a0. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Acute myelogenous leukemia: a human cell line responsive to colony-stimulating activity. Science. 1978 Jun 9;200(4346):1153–1154. doi: 10.1126/science.306682. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Singer J. W., Collins S. J., Witte O. N. Cell lines and clinical isolates derived from Ph1-positive chronic myelogenous leukemia patients express c-abl proteins with a common structural alteration. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1810–1814. doi: 10.1073/pnas.82.6.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Kubonishi I., Miyoshi I. Establishment of a Ph1 chromosome-positive cell line from chronic myelogenous leukemia in blast crisis. Int J Cell Cloning. 1983 Jun;1(2):105–117. doi: 10.1002/stem.5530010205. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- Ponzetto C., Wolgemuth D. J. Haploid expression of a unique c-abl transcript in the mouse male germ line. Mol Cell Biol. 1985 Jul;5(7):1791–1794. doi: 10.1128/mcb.5.7.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Keath E. J., Piccoli S. P., Cole M. D. Novel myc oncogene RNA from abortive immunoglobulin-gene recombination in mouse plasmacytomas. Cell. 1982 Dec;31(2 Pt 1):443–452. doi: 10.1016/0092-8674(82)90137-4. [DOI] [PubMed] [Google Scholar]
- Stam K., Heisterkamp N., Grosveld G., de Klein A., Verma R. S., Coleman M., Dosik H., Groffen J. Evidence of a new chimeric bcr/c-abl mRNA in patients with chronic myelocytic leukemia and the Philadelphia chromosome. N Engl J Med. 1985 Dec 5;313(23):1429–1433. doi: 10.1056/NEJM198512053132301. [DOI] [PubMed] [Google Scholar]
- Taub R., Kirsch I., Morton C., Lenoir G., Swan D., Tronick S., Aaronson S., Leder P. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Baltimore D. Cellular RNA homologous to the Abelson murine leukemia virus transforming gene: expression and relationship to the viral sequence. Mol Cell Biol. 1983 May;3(5):773–779. doi: 10.1128/mcb.3.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Klein A., van Kessel A. G., Grosveld G., Bartram C. R., Hagemeijer A., Bootsma D., Spurr N. K., Heisterkamp N., Groffen J., Stephenson J. R. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982 Dec 23;300(5894):765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]