Abstract
We previously reported the identification of TUSC1 (Tumor Suppressor Candidate 1), as a novel intronless gene isolated from a region of homozygous deletion at D9S126 on chromosome 9p in human lung cancer. In this study, we examine the differential expression of TUSC1 in human lung cancer cell lines by western blot and in a primary human lung cancer tissue microarray by immunohistochemical analysis. We also tested the functional activities and mechanisms of TUSC1 as a tumor suppressor gene through growth suppression in vitro and in vivo. The results showed no expression of TUSC1 in TUSC1 homozygously deleted cells and diminished expression in some tumor cell lines without TUSC1 deletion. Interestingly, the results from a primary human lung cancer tissue microarray suggested that higher expression of TUSC1 was correlated with increased survival times for lung cancer patients. Our data demonstrated that growth curves of tumor cell lines transfected with TUSC1 grew slower in vitro than those transfected with the empty vector. More importantly, xenograph tumors in nude mice grew significantly slower in vivo in cells stably transfected with TUSC1 than those transfected with empty vector. In addition, results from confocal microscopy and immunohistochemical analyses show distribution of TUSC1 in the cytoplasm and nucleus in tumor cell lines and in normal and tumor cells in the lung cancer tissue microarray. Taken together, our results support TUSC1 has tumor suppressor activity as a candidate tumor suppressor gene located on chromosome 9p.
Introduction
Lung cancer is the most common form of cancer mortality in men and women in the world with an estimated 226,160 new cases and 160,340 deaths occurring in the United States in 2012 [1]. Lung cancer develops through a multistage process involving a variety of genetic and epigenetic changes in dominant oncogenes and tumor suppressor genes (TSGs) [2]. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer subtypes with small cell lung cancer accounting for the remaining 15% [3], NSCLC is further subdivided into adenocarcinoma (39%), squamous cell carcinoma (21%), large cell carcinoma adenocarcinoma (3%), and uncommon types and combined types comprising the remaining 22% [3].
Alteration of chromosome 9p is implicated in a variety of tumor types including melanoma, non small cell lung carcinoma, breast cancer, leukemia, and hepatocellular carcinoma, clear cell renal cell carcinomas and gastrointestinal stromal tumors through chromosomal inversions, translocations, loss of heterozygosity (LOH) and homozygous deletion (HD). Genetic alterations of chromosome 9p occur early and frequently in lung cancer. These data suggest chromosome 9p contains a tumor suppressor locus (loci) critical in the development of several tumor types including lung [4]–[17]. Two candidate tumor suppressor loci were identified in the chromosome 9p21 region. One locus is p16/CDKN2A, which encodes the p16 and p14ARF proteins. The other locus is p15/CDKN2B encoding the p15 protein [7], [18], [19]. Since p16/CDKN2A is frequently inactivated genetically or epigenetically in cancer cells, the p16/CDKN2A locus is suspected to be a major tumor suppressor gene [20]–[25]. However, our previous studies in primary NSCLC, a large number of human lung cancer cell lines, and primary tumor samples identified a region of homozygous deletion (HD) at the microsatellite marker D9S126 which is distinct from the p16/CDKN2A locus and lies approximately 3.7 Mb proximal to p16/CDKN2A, [10], [14]. We had reported the identification of TUSC1 (Tumor Suppressor Candidate 1) from this region and showed that expression of TUSC1 was absent or diminished in cell lines with or without homozygous deletion of TUSC1. These findings prompted us to suggest that TUSC1 may function as a tumor suppressor gene in lung tumorigenesis [14].
In this study, we have developed a C-terminal peptide antibody specific to TUSC1 and stably transfected lung cancer cell lines (Nu6-1 and H290) in order to study TUSC1’s effect on cell growth of tumor cell lines with homozygous deletion of TUSC1 in vitro and tumor growth in vivo to characterize TUSC1’s potential tumor suppressing activity. We also examined the correlation between expression of TUSC1 and survival times of lung cancer patients by immunohistochemical analysis of a human lung cancer tissue microarray. Our results demonstrated that cell growth curves of cells transfected with TUSC1 grow slower in vitro than cells transfected with the empty vector. Moreover, subcutaneous injection of stably transfected cells containing TUSC1 significantly suppresses growth of xenografts. The data also showed a trend towards increased survival times for lung cancer patients with higher levels of TUSC1 expression. We were able to demonstrate localization of TUSC1 protein in both the cytoplasm and nucleus in transfected cells, untransfected cells and primary tumor tissue. Taken together, we provide evidence that TUSC1 functions as a tumor suppressor gene in tumor development and TUSC1 may be a potential biomarker for diagnosis, prognosis and treatment. Future studies using TUSC1 knock-out mice and identification of its interacting partner(s) and functional pathway(s) will address TUSC1’s physiological roles in tumor development.
Materials and Methods
Cell Culture, RNA Extraction and RT-PCR
The CHO cells were obtained from the American Type Culture Collection (ATCC). A squamous carcinoma cell line (SKMES-1) and three previously reported TUSC1 homozygously deleted human lung cancer cell lines H290, Nu6-1 and NE18 were a gift of Dr. Steve Belinsky at Lovelace Respiratory Research Institute and were used for in vitro and in vivo experiments [26]–[28]. The genomic status of the TUSC1, CDKN2A and CDKN2B loci have been determined previously [14]. The cell lines were maintained at 37°C in RPMI 1640 medium (Gibco, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS), 1% penicillin and streptomycin, and 5% L-glutamine (Gibco, Carlsbad, CA). Total RNA was isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA) following the supplier’s protocol. RT-PCR for TUSC1 was performed as described previously [14].
Mutation Analysis
Mutational analysis was performed using primer sets covering the entire open reading frame of the TUSC1 gene (GenBank: AY168647). Briefly, 50 ng of DNA from primary tumors and cell lines was used for amplification. The reaction conditions were optimized using the GC-RICH PCR System following the provided protocol (Roche, Indianapolis, IN). PCR products were purified by using a PCR purification kit (Qiagen, Valencia, CA) and then directly sequenced using a Bigdye terminator 1.1 and Applied Biosystems model 3130XL analyzer DNA (Applied Biosystems Inc, Foster City, CA). Sequencer DNA sequencing software was used to analyze and assemble sequences to determine nucleotide alterations.
Antibody Production, Cell Lysates and Immunoblotting
Polyclonal specific antisera to TUSC1 were raised in rabbits by injecting a synthetic peptide corresponding to the carboxy-terminal sequence of the deduced TUSC1 protein (GenBank: AY168647) (Animal Pharmacy, Healdsburg, CA). The rabbit antibodies were affinity purified using the synthetic peptide coupled to Affigel-15 (Bio-Rad, Hercules, CA) [29]. Specificity of the TUSC1 antibody was determined by western blots with proteins from TUSC1 homozygously deleted cells and proteins purified through ProBond™ Purification System (Invitrogen, Carlsbad, CA) from CHO cells stably transfected with TUSC1 in pcDNA3.1/V5-His vectors (Invitrogen, Carlsbad, CA).
For Western blot analysis, protein lysates were prepared following the provided protocol (Thermo Fisher Scientific Inc, Rockford, IL). Proteins (25–30 µg) were loaded on a 10% SDS-polyacrylamide gel, followed by blotting on a nitrocellulose membrane (Invitrogen, Carlsbad, CA/Bio-Rad, Hercules, CA). Membranes were blocked for two hours with 5% nonfat milk in TBST buffer (0.1% Tween 20 in TBS) at room temperature, and the membrane was probed with the polyclonal antibody to TUSC1. After three washings with TBST, membranes were incubated with anti-rabbit secondary antibody (Cell Signaling, Danvers, MA) and washed five times with TBST at room temperature. The membranes were developed by SuperSignal@ West Pico Chemiluminescent Substrate Western blotting detection reagents for Kodak Biomax MR film exposure (Thermo Fisher Scientific Inc, Rockford, IL).
Immunofluorescence and Immunohistochemistry Analyses
For immunofluorescence analysis, cells were seeded on coverslips in six-well plates one day before the experiment. Cells were fixed in 3% paraformaldehyde for 15 minutes at room temperature and washed two times (five minutes each) in PBS. Cells were then permeabilized with 0.1% Triton X-100 for five minutes, incubated with anti-TUSC1 or anti-V5 antibody (Invitrogen, Carlsbad, CA) over night at 4°C followed by detection with FITC/or Texas Red-conjugated anti-rabbit IgG (Vector Laboratories, Burlingame, CA) for anti-TUSC1 antibody and Texas Red-conjugated anti-mouse IgG for anti-V5 antibody. Cells were analyzed with an Axiophot microscope equipped for immunofluorescence or an LSM S10 UV System for confocal image analysis (Zeiss, Thorword, NY). Immunohistochemical staining of normal human lung tissue and a lung cancer tissue microarray were conducted following the previously described protocol [30]. Detailed information about the clinical samples selection, tissue microarray, image analysis and immunohistochemistry scoring were reported previously [30]. Briefly, 10% horse serum was used for blocking. For antibody incubation, 1 ug/ml of TUSC1 antibody was applied. After washing three times with PBS, the slide was incubated with biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA) and then incubated for 45 minutes with avidin-biotin complex method reagent (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA) and washed three times with PBS. The slide was then developed in liquid 3,3′-diaminobenzidine (DAKO, Carpinteria, CA) and lightly counterstained with Mayer’s hematoxylin, dehydrated, cleared, and mounted with resinous mounting medium.
Construction of TUSC1 Expression Vectors and Generation of Stably Transfected Cell Lines
The open reading frame of the TUSC1 was amplified using TUSC1 specific primers (data not shown). Fragments were cloned into pcDNA3.1 or pcDNA3.1/V5-His vectors (Invitrogen, Carlsbad, CA) for transfection experiments. The inserted sequences and orientations were sequence verified.
For transfection, CHO, 9HTE, Nu6-1, and H290 cell lines were grown to 80% to 90% confluence for cell transfection. The transfections were performed with Lipofectamine™ 2000 following the provided protocol (Invitrogen, Carlsbad, CA). To establish stably transfected cell lines, cells were selected using geneticin treatment (G418, 500–800 µg/ml, Invitrogen, Carlsbad, CA) 48 hours after transfection for two to three weeks. A pool of selected cells was re-cultured under continuous selection with geneticin. The protein expression levels were determined by Western blot analyses to verify the expression of TUSC1 in the transfectants.
Cell Proliferation In Vitro and Tumor Growth In Vivo
For in vitro analysis, the cells were trypsinized and cell numbers were measured in a Coulter Counter (ZM; Scientific Instruments, Hialeah, FL). Cell proliferation was measured at 24-hour intervals for five days. The results represent the mean of three independent experiments.
All animal research was conducted in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care Institutional (AAALAC) at NIH. Animal studies were performed in accordance with U.S. National Institutes of Health guidelines and were conducted under a protocol approved by NIH Animal Care Use Committee. For xenograft experiments, ten mice for each cell line were used to study tumor development in vivo. In each animal, 1×106 stably TUSC1 transfected tumor cell lines, either Nu6-1 or H290, were injected on the left side with the corresponding tumor cell line transfected with empty vector injected on the right side of nude mice as controls. Prior to injection, the mice were anesthetized using isoflurane. Tumor size was measured weekly and approximate tumor volumes were determined by multiplying tumor height×length×width. If the tumor measurements were at or larger than 2 cm, the mice were euthanized by carbon dioxide inhalation in a chamber attached to house CO2. Humane endpoints were observed if other health issues caused the mice to experience symptoms such as rapid weight loss, debilitating diarrhea, labored breathing, bleeding from any orifice, self-induced trauma, impaired mobility, etc.
Statistical Analysis
Results of the experiments done in vitro and in vivo are reported as mean ± s.d. Statistical comparisons between test and control samples were evaluated by Student's t-test and the significance was set as p<0.05. The log-rank test was used for comparing survival distributions between positive and negative groups of staining on tumor tissue microarrays and Kaplan-Meier curves were plotted for the two groups.
Results
Analysis of TUSC1 Somatic Mutation
Our previous results located the TUSC1 gene in a homozygous deletion region on chromosome 9p and demonstrated reduced expression in lung cancer cell lines [14]. These results indicated TUSC1 may be a candidate TSG and genetically altered. To test this hypothesis, we performed mutation analysis of the open reading frame of TUSC1 (GenBank: AY168647) on 97 genomic DNA samples including 45 cancer cell lines (22 lung, 17 melanoma and six colon), 14 matched NSCLC cell line pairs, six matched Small Cell Lung Cancer (SCLC) cell line pairs and six matched SCLC primary tumors. Five previously identified single-nucleotide polymorphisms (SNPs) within the open reading frame (ORF) of TUSC1 were identified in these samples. Results from a database search identified numerous SNPs located in the 3′ and 5′ untranslated regions (UTRs) as well as seven SNPs (three nonsynonymous coding and four synonymous coding variants) located in the ORF of TUSC1 (The Wellcome Trust Sanger Institute, http://www.sanger.ac.uk/resources/databases). Three nonsynonymous SNPS (rs72631813, rs34498078 and rs72631815) and two synonymous SNPs (rs72631814 and rs35110225) were found in our samples. We observed the SNPs in multiple samples and some samples had multiple SNPs (data not shown). Furthermore, the SNPs at nucleotides 189, 358, 378 and 613 occurred at a high frequently in the samples tested for this report. However, we and others did not detect nonsense mutations in TUSC1 as mutational analysis reported for biliary tract, breast, central nervous system, large intestine and pancreas also did not identify any mutations [31]–[34]. These results indicate somatic mutation of the TUSC1 was not a major event.
Generation of Cell Lines Expressing TUSC1 and a Polyclonal Antibody for TUSC1
To test the functional activity of TUSC1, we generated stably transfected cells by transfecting expression vectors containing the TUSC1 open reading frame into cells harboring a homozygous deletion of the TUSC1 gene (Nu6-1 and H290). The expression of TUSC1 mRNA and protein in the pooled transfected cell cultures was verified by RT-PCR and Western blot (Figure 1A, B). As expected, TUSC1 expression was only detected in the cells transfected with TUSC1 expression vectors, and not in the parental cell lines transfected with either empty vector or not transfected. The results demonstrated stable expression of exogenous TSUC1 and provided an important reagent to test the specificity of the TUSC1 antibody.
Figure 1. Expression of TUSC1 mRNA and protein in cell lines.
(A–B) The tumor cell lines (H290 and Nu6-1) were stably transfected with the TUSC1 gene in pcDNA3.1 vector and CHO cells were transfected with TUSC1 in pcDNA3.1/V5-His vector. TUSC1 mRNA was amplified (RT-PCR) and exogenous protein (Western blot) was detected in all stably transfected cell lines containing TUSC1 expression clones but not the parental cell lines transfected with empty vector. (C) Western blot analysis of endogenous TUSC1 proteins in tumor cell lines. Red box: Cell lines with homozygous deletion of TUSC1; Green box: cell lines with reduced TUSC1 expression but without TUSC1 deletion. As an internal control for the amount of protein loaded, the same membrane was incubated with Anti-GAPDH antibody.
A polyclonal antibody was generated for TUSC1 using four peptides based on the predicted TUSC1 amino acid sequence (Gene Bank access no: AY168647). The peptides were synthesized and used to immunize rabbits. Polyclonal antibodies were affinity purified and the specificity and sensitivity were verified by a series of western blots, using protein extracts from CHO cells stably transfected with TUSC1 in pcDNA3.1/V5-His vectors and proteins from cells with or without the TUSC1 homozygous deletion (data not shown). We demonstrated that a C-terminal peptide antibody was specific as it detected a single band with the expected molecular weight of TUSC1 in the protein lysates isolated from CHO cells stably transfected with the TUSC1 expressing vector. The corresponding protein band was detected in cell lysates from TUSC1 homozygously deleted cells (Nu6-1, H290) containing the TUSC1 expression vector. However, no signals were detected in the protein lysates from the parental Nu6-1 and H290 cell lines transfected with empty vector (Figure 1B).
Reduced Expression of TUSC1 Correlates with Poor Survival Rates in Patients with NSCLC
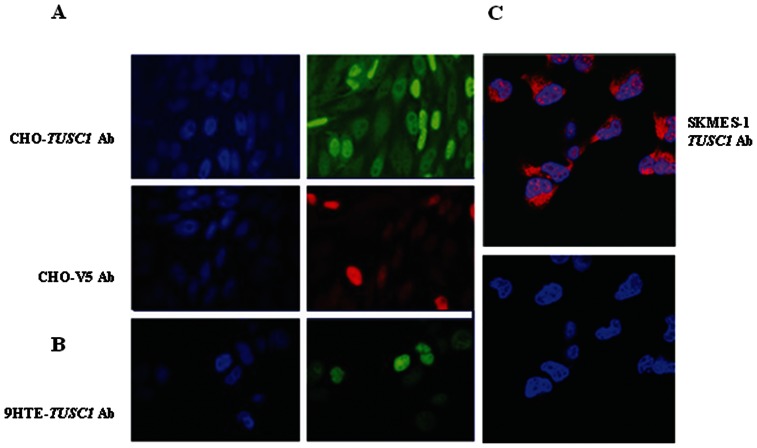
We previously reported the expression of TUSC1 mRNA in normal adult tissues tested by northern blot, and a lack of expression in cells bearing homozygous deletion of TUSC1, as well as reduced expression of TUSC1 in other cell lines without TUSC1 deletion [14]. In western blot, expression of TUSC1 was not detected in cell lines (Nu6-1 and NE18) bearing homozygous deletion of the TUSC1 gene and had diminished expression of TUSC1 in cell lines without the deletion of TUSC1 gene (Figure 1C). These findings led us to test expression levels in primary tumors and to correlate these levels with patient outcome in order to further test TUSC1’s potential tumor suppressor activity. We evaluated the expression patterns of TUSC1 in a previously described primary human lung cancer tissue microarray by immunohistochemistry (IHC) [30]. The tissue microarray contains 150 adenocarcinomas and 150 squamous cell carcinomas. In normal bronchial epithelial cells of the lung, TUSC1 expression was localized in the differentiated cells of the upper cell layers but expression was reduced or absent in the basal cell layers. We also observed three TUSC1 staining patterns including cytoplasmic, nuclear and nuclear/cytoplasmic patterns (Figure 2A). However, the proportion of positive staining of TUSC1 in both cytoplasm and nucleus was not equal in all the samples, and there were various levels of staining intensity (Figure 2A–C). We scored the tissue microarray samples based on the expression level and staining intensity of TUSC1 using a method described previously [30]. Following scoring of the tumors, the results suggested a correlation between increased expression of TUSC1 and longer survival times in patients having the higher score [30]. The correlation was somewhat stronger in patients with squamous carcinomas (Figure 2F) and the results showed a trend towards statistical significance (p = 0.064). We also determined the cellular location of TUSC1 by immunofluorescence with confocal microscopy on both untransfected and stably transfected cell lines. The results showed exogenous and endogenous TUSC1 proteins are located in both the cytoplasm and nucleus of CHO transfected cells as well as in untransfected 9HTE cells and a lung cancer cell line (SKMES-1) endogenously expressing TUSC1 (Figure 3A–C). The results suggest TUSC1 may function in multiple pathways depending on subcellular localization.
Figure 2. Representative immunohistochemical analysis using the TUSC1 antibody in normal and primary lung tissues.
(A) Bronchioepithelium of Normal Lung. The basal layer was not positive for TUSC1 but differentiated cells in the upper layers of epithelium have high TUSC1 expression. Distribution of TUSC1 staining was cytoplasmic, nuclear and nuclear/cytoplasmic in the cells. (B, C) Lung cancer tissue microarray stained with TUSC1 antibody and representative tissue cores (1–3) for each level of staining intensity. (D–F) Kaplan-Meier survival plots for the overall patient (D), adenocarcinoma (E) and squamous cell carcinoma (F). Survival status and the associated P value are indicated. Red line represents tumors with higher expression levels of TUSC1 (score: 3), green line represents tumor with lower expression levels of TUSC1 (score: 0–2).
Figure 3. Immunofluorescent staining by confocal microscopy.
Localization of TUSC1 in stable transfectants of CHO and 9HTE cell lines. (A) Stably transfected CHO cells were incubated with V5 or TUSC1 antibodies. (B–C) Un-transfected 9HTE and SKEMS-1 lung cancer cells were incubated with TUSC1 antibody. Subcellular distributions of TUSC1 proteins are cytoplasmic and nuclear. V5 antibody was detected with Texas Red-conjugated anti-mouse IgG (Red) and TUSC1 antibody was detected with either Texas Red-conjugated anti-rabbit IgG (Red) or by FITC-conjugated anti-rabbit IgG (Green).
Restoring Expression of the TUSC1 Gene Reduces Tumor Cell Growth in vitro and Suppresses Tumor Formation in vivo
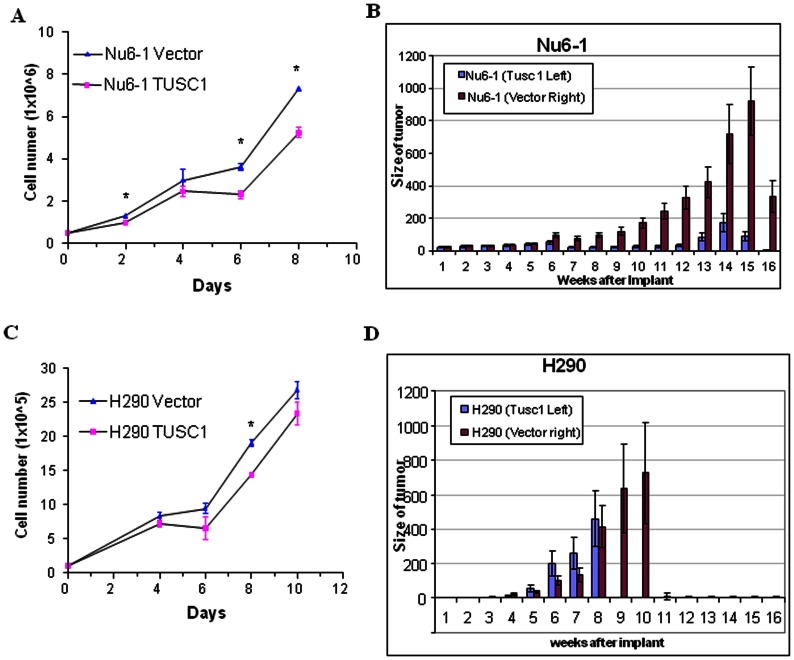
Restoring expression of TUSC1 in homozygously deleted cells was achieved by generating stably transfected cells. The expression levels of TUSC1 were verified by RT-PCR analysis and western blot (Figure 1B). Results from cell proliferation experiments showed the cell growth rate in the stably transfected cells, Nu6-1 and H290, with TUSC1 expressing vector grew slower than cells transfected with empty vector (Figure 4A, C). These results suggest that exogenous expression of the TUSC1 gene can reduce tumor cell line growth in vitro.
Figure 4. Overexpression of TUSC1 reduces cell growth and tumorigenicity of lung cancer cells.
(A, B) In vitro cell growth of cancer cell lines (Nu6-1 and H290) transfected with TUSC1. (C, D) Tumor volume of subcutaneously injected stably transfected cancer cells with TUSC1 in nude mice in vivo. Restoration of TUSC1 expression in cell lines with TUSC1 homozygous deletion suppressed cell growth and tumor development compared to the parental cell lines transfected with the empty vector (p<0.05 ).
We further determined whether the inhibitory effects of the TUSC1 gene on tumor cell proliferation in vitro could be demonstrated through tumor growth in vivo in nude mice. We generated tumor xenografts by subcutaneously injecting 1×106 TUSC1 stably transfected Nu6-1 and H290 cells or empty vector containing cells into nude mice to evaluate the efficacy of the gene in suppressing tumor growth. TUSC1 transfected cells were injected on the left side of each animal and the corresponding vector control cells were injected on the right side of the same animal. The xenografts were monitored for three months for tumor size and the growth of tumors. Average tumor volumes from the cells transfected with TUSC1 were compared with the average tumor volume from the parental cell line transfected with the empty expression vector. The results show that TUSC1 significantly suppresses tumor growth (p<0.05, Figure 4B). These data are not only consistent with the data from the in vitro study, but also demonstrate that TUSC1 is effective in reducing tumor cell line growth in vitro and in vivo.
Discussion
It is well known that genetic alterations of chromosome 9p occur in multiple types of human cancers including lung. Identification of functional tumor suppressor(s) on chromosome 9p will provide opportunities to develop biomarkers and innovative therapeutic strategies urgently needed for cancer diagnosis, prognosis and treatment. We had previously reported a putative tumor suppressor gene, TUSC1, which resides in a region of homozygous deletion at marker D9S126 and we had demonstrated reduced expression of TUSC1 mRNA in human lung cancer cell lines [14]. In the current study, we were able to show reduced and differential expression of TUSC1 protein in lung cancer cell lines and primary lung cancer tissue samples using a TUSC1 specific polyclonal antibody (Figures 1, 2). Most importantly, introducing the TUSC1 gene into tumor cell lines harboring a homozygous deletion of TUSC1 (Nu6-1 and H290) had the effect of inhibiting cell proliferation in vitro and reducing tumor growth in vivo (Figure 4). These results further support our previous hypothesis that TUSC1 may play an important role in lung tumorigenesis and function as a tumor suppressor gene (14). Moreover, our data indicate higher levels of TUSC1 expression is correlated with increased survival times for lung cancer patients (Figure 2). This lead us to suggest that expression levels of TUSC1 may be a potential biomarker for prognosis, although, more studies will be required with larger sample sizes and detailed clinical data to support this concept. In addition, we also demonstrated TUSC1 resides in both the cytoplasm and nucleus of normal cells, tumor cell lines and tumor tissue microarrays (Figures 2, 3) suggesting TUSC1 may participate in multiple functional pathways for its physiological function.
The mechanism by which TUSC1 expression is reduced in lung cancer cells and tumor tissues, and the mechanism by which TUSC1 governs the inhibition of NSCLC cell growth remain to be fully elucidated. To date we have not detected hypermethylation of the promoter region of the TUSC1 gene in the cell lines with the diminished expression of TUSC1 or increased apoptosis by FACS analysis through over-expression of TUSC1 (data not shown). Moreover, we and others did not detect somatic mutations in the TUSC1 ORF in the samples tested or as reported in the literature [32]–[34]. However we were able confirm five SNPs also reported in SNP database (The Wellcome Trust Sanger Institute, http://www.sanger.ac.uk/resources/databases). Additionally, the relationship between the SNPs and effects on protein structure or function of the gene remain to be determined. Interestingly, one SNP, rs13290968, in the TUSC1 gene was recently reported to be associated with a pigmentation phenotype and tanning ability among cutaneous malignant melanoma patients [17]. Future directions include testing whether nonsynonymous SNPs can alter the protein structure and/or the binding ability of TUSC1 to its potential protein partner(s) as well as cell growth characteristics by overexpressing the mutated ORF in TUSC1 homozygously deleted cells. These results may help to explain diminished TUSC1 expression and why mutation of TUSC1 was not seen as a frequent event in tumorigenesis. Additionally, we noted a significant reduction in tumor frequency and volume in the animals subcutaneously injected with TUSC1 stably transfected Nu6-1 cells as opposed to H290 cells and restoration of TUSC1 expression appears to have more effects in vivo than in vitro (Figure 4). These results suggest that the reduction of tumor and cell growth by TUSC1 might not be solely due to the exogenous TUSC1 expression. One possible explanation may be that the in vivo microenvironment activates potential TUSC1 pathway(s) more effectively than in the in vitro setting. Toward this goal, we are identifying and validating potential binding partners by yeast two-hybrid analysis.
Taken together, we have provided further evidence that TUSC1 expression is downregulated in lung cancer cell lines and a trend towards higher expression of TUSC1 is correlated with longer survival times for lung cancer patients. Restoration of TUSC1 expression in lung cancer cells is followed by the suppression of tumorigenicity in vivo and slows cell growth in vitro, suggesting TUSC1 functions as a tumor suppressor gene. Further study into TUSC1’s activity by interacting with key components of survival pathways and other interacting proteins, and generating a knock-out mouse model for TUSC1, will provide more evidence to support TUSC1’s function as a tumor suppressor gene and help us to address its physiological functions. Future studies to elucidate the effect of nonsynonymous SNPs will be interesting to understand if there is a cancer-associated SNP(s) and their effects on TUSC1 activity. The results may also provide a strategy for the prevention, early detection, diagnosis, and treatment for lung cancer and other human cancers related to the loss of chromosome 9p21 region.
Acknowledgments
The authors would like to thank Nan Roche (Laboratory of Cancer Biology and Genetics, NCI) for critical comments on the manuscript, thank Dr. Christophe Cataisson (Laboratory of Cancer Biology and Genetics, NCI) for his help with the xenograft experiments. We would like to thank Dr. Steve Belinsky (Lovelace Respiratory Research Institute) for H290, Nu6-1, NE18 and SKMES-1 cell lines. We would also like to thank Dr. Jyotsna Pandey (Laboratory of Cancer Biology and Genetics, NCI) for her help on Figure 4.
Funding Statement
This research was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.American Cancer Society. Cancer Facts & Figures 2012 (2012) Atlanta: American Cancer Society.
- 2. Zochbauer-Muller S, Gazdar AF, Minna JD (2002) Molecular pathogenesis of lung cancer. Ann Rev Physiol 64: 681–708. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, et al. (2012) SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations), National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site.
- 4. Cheng JQ, Jhanwar SC, Lu YY, Testa JR (1993) Homozygous deletions within 9p21-p22 identify a small critical region of chromosomal loss in human malignant mesotheliomas Cancer Res. 53: 4761–3. [PubMed] [Google Scholar]
- 5. Holland EA, Beaton SC, Edwards BG, Kefford RF, Mann GJ (1994) Loss of heterozygosity and homozygous deletions on 9p21–22 in melanoma. Oncogene 9: 1361–5. [PubMed] [Google Scholar]
- 6. Coleman A, Fountain JW, Nobori T, Olopade OI, Robertson G, et al. (1994) Distinct deletions of chromosome 9p associated with melanoma versus glioma, lung cancer, and leukemia. Cancer Res 54: 344–8. [PubMed] [Google Scholar]
- 7. Kamb A, Gruis NA, Weaver-Feldhaus J, Liu Q, Harshman K, et al. (1994) A cell cycle regulator potentially involved in genesis of many tumor types. Science 264: 436–440. [DOI] [PubMed] [Google Scholar]
- 8. An HX, Niederacher D, Picard F, van Roeyen C, Bender HG, et al. (1996) Frequent allele loss on 9p21–22 defines a smallest common region in the vicinity of the CDKN2 gene in sporadic breast cancer. Genes Chromosomes Cancer 17: 14–20. [DOI] [PubMed] [Google Scholar]
- 9. Mead LJ, Gillespie MT, Hung JY, Rane US, Rayeroux KC, et al. (1997) Frequent loss of heterozygosity in early non-small cell lung cancers at chromosome 9p21 proximal to the CDKN2a gene. Int J Cancer 71: 213–7. [DOI] [PubMed] [Google Scholar]
- 10. Wiest JS, Franklin WA, Otstot JT, Forbey K, Varella-Garcia M, et al. (1997) Identification of a novel region of homozygous deletion on chromosome 9p in squamous cell carcinoma of the lung: the location of a putative tumor suppressor gene. Cancer Res 57: 1–6. [PubMed] [Google Scholar]
- 11. Takeuchi S, Koike M, Seriu T, Bartram CR, Slater J, et al. (1997) Homozygous deletions at 9p21 in childhood acute lymphoblastic leukemia detected by microsatellite analysis. Leukemia 11: 1636–40. [DOI] [PubMed] [Google Scholar]
- 12. Sheu JC, Lin YW, Chou HC, Huang GT, Lee HS, et al. (1999) Loss of heterozygosity and microsatellite instability in hepatocellular carcinoma in Taiwan. Br J Cancer 80: 468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pollock PM, Welch J, Hayward NK (2001) Evidence for three tumor suppressor loci on chromosome 9p involved in melanoma development. Cancer Res 61: 1154–61. [PubMed] [Google Scholar]
- 14. Shan Z, Parker T, Wiest JS (2004) Identifying novel homozygous deletions by microsatellite analysis and characterization of tumor suppressor candidate 1 gene, TUSC1, on chromosome 9p in human lung cancer. Oncogene 23: 6612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. La Rochelle J, Klatte T, Dastane A, Rao N, Seligson D, et al. (2010) Chromosome 9p deletions identify an aggressive phenotype of clear cell renal cell carcinoma. Cancer 16: 4696–702. [DOI] [PubMed] [Google Scholar]
- 16. Ploussard G, Dubosq F, Soliman H, Verine J, Desgrandchamps F, et al. (2010) Prognostic value of loss of heterozygosity at chromosome 9p in non-muscle-invasive bladder cancer. Urology 76: 513.e13–8. [DOI] [PubMed] [Google Scholar]
- 17.Yang XR, Liang X, Pfeiffer RM, Wheeler W, Maeder D, et al.. (2010) Associations of 9p21 variants with cutaneous malignant melanoma, nevi, and pigmentation phenotypes in melanoma-prone families with and without CDKN2A mutations. Fam Cancer 625–33. [DOI] [PMC free article] [PubMed]
- 18. Serrano M, Hannon GJ, Beach D (1993) A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366: 704–707. [DOI] [PubMed] [Google Scholar]
- 19. Quelle DE, Zindy F, Ashmun RA, Sherr CJ (1995) Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell 83: 993–1000. [DOI] [PubMed] [Google Scholar]
- 20. Cairns P, Polascik TJ, Eby Y, Tokino K, Califano J, et al. (1995) Frequency of homozygous deletion at p16/CDKN2 in primary human tumours. Nat Genet 11: 210–2. [DOI] [PubMed] [Google Scholar]
- 21. Merlo A, Herman JG, Mao L, Lee DJ, Gabrielson E, et al. (1995) 5′ CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med 7: 686–92. [DOI] [PubMed] [Google Scholar]
- 22. Xiao S, Li D, Corson JM, Vijg J, Fletcher JA (1995) Codeletion of p15 and p16 genes in primary non-small cell lung carcinoma. Cancer Res 55: 2968–71. [PubMed] [Google Scholar]
- 23. Castellano M, Pollock PM, Walters MK, Sparrow LE, Down LM, et al. (1997) CDKN2A/p16 is inactivated in most melanoma cell lines. Cancer Res. 57: 4868–75. [PubMed] [Google Scholar]
- 24. Hamada K, Kohno T, Kawanishi M, Ohwada S, Yokota J (1998) Association of CDKN2A(p16)/CDKN2B(p15) alterations and homozygous chromosome arm 9p deletions in human lung carcinoma. Cancer 22: 232–40. [DOI] [PubMed] [Google Scholar]
- 25. Hamada K, Kohno T, Takahashi M, Yamazaki M, Yamazaki M, et al. (2000) Two regions of homozygous deletion clusters at chromosome band 9p21 in human lung cancer. Genes Chromosomes Cancer 27: 308–18. [PubMed] [Google Scholar]
- 26.Fogh J, Trempe G (1975) New human tumor cell lines. In: Fogh J, ed. Human tumor cells in vitro. New York and London: Plenum, 115–159.
- 27. Carney DN, Gazdar AF, Bepler G, Guccion JG, Marangos PJ, et al. (1985) Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res 45(6): 2913–23. [PubMed] [Google Scholar]
- 28. Pettijohn DE, Pfenninger O, Brown J, Duke R, Olsson L (1988) Tumorigenic human squamous lung cancer cells have defined cell surface carbohydrates that are absent from nontumorigenic cells. Proc Natl Acad Sci U S A 85(3): 802–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldsmith P, Gierschik P, Milligan G, Unson CG, Vinitsky R, et al. (1987) Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J Biol Chem 262: 14683–8. [PubMed] [Google Scholar]
- 30. Fukuoka J, Fujii T, Shih JH, Dracheva T, Meerzaman D, et al. (2004) Chromatin remodeling factors and BRM/BRG1 expression as prognostic indicators in non-small cell lung cancer. Clin Cancer Res 10: 4314–24. [DOI] [PubMed] [Google Scholar]
- 31. Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, et al. (2007) The genomic landscapes of human breast and colorectal cancers. Science 16: 1108–13. [DOI] [PubMed] [Google Scholar]
- 32. Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, et al. (2008) Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 26: 1801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, et al. (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 26: 1807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parsons DW, Li M, Zhang X, Jones S, Leary RJ, et al. (2011) The genetic landscape of the childhood cancer medulloblastoma. Science 331: 435. [DOI] [PMC free article] [PubMed] [Google Scholar]