Abstract
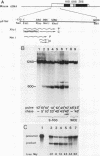
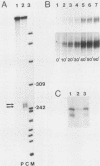
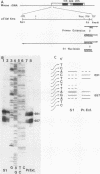
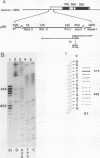
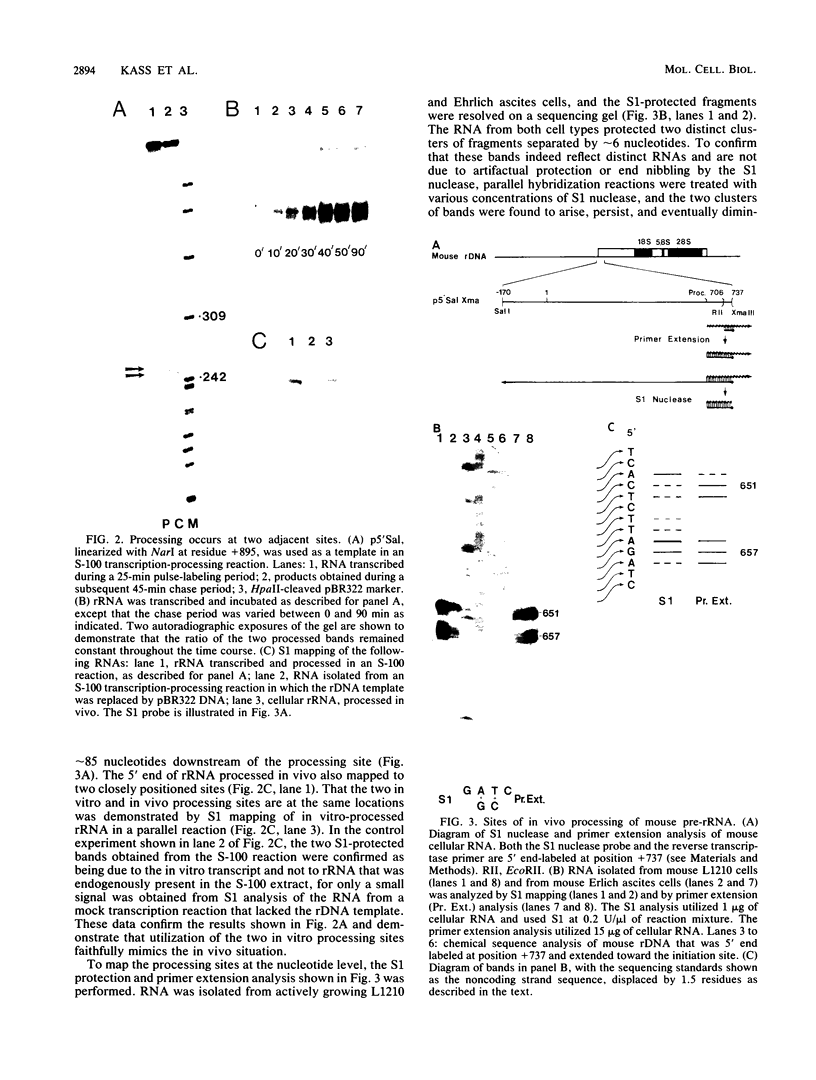
The primary transcript of the mouse rRNA gene is rapidly processed at nucleotide approximately +650 both in vivo and in vitro. Using run-off transcription in a mouse cell extract as well as S1 nuclease and primer extension analysis of cellular RNA, we demonstrated that this primary processing actually results in the formation of two species of downstream RNA which differ in length by approximately 6 nucleotides, indicating the existence of two closely positioned alternative processing sites. The 200-base-pair region just 3' to the mouse processing site has a striking 80% sequence homology with a region of the human rRNA external transcribed spacer, and S1 nuclease analysis of human cellular RNA has demonstrated that an analogous rRNA processing occurs at the 5' border of the homologous human region. Unlike rDNA transcriptional initiation, however, the primary rRNA processing is not highly species specific, for the transcript of a chimeric gene containing the human processing region adjacent to a mouse rDNA promoter was synthesized and correctly processed in a mouse cell extract. This result confirms that mouse and human rRNA undergo a common primary processing event which is evidently directed by sequences within the 200-base-pair conserved sequence region.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach R., Grummt I., Allet B. The nucleotide sequence of the initiation region of the ribosomal transcription unit from mouse. Nucleic Acids Res. 1981 Apr 10;9(7):1559–1569. doi: 10.1093/nar/9.7.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum B., Pierron G., Seebeck T., Braun R. Processing in the external transcribed spacer of ribosomal RNA from Physarum polycephalum. Nucleic Acids Res. 1986 Apr 25;14(8):3153–3166. doi: 10.1093/nar/14.8.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum B., Seebeck T., Braun R., Ferris P., Vogt V. Localization and DNA sequence around the initiation site of ribosomal RNA transcription in Physarum polycephalum. Nucleic Acids Res. 1983 Dec 10;11(23):8519–8533. doi: 10.1093/nar/11.23.8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman L. H., Goldman W. E., Goldberg G. I., Hebert M. B., Schlessinger D. Location of the initial cleavage sites in mouse pre-rRNA. Mol Cell Biol. 1983 Aug;3(8):1501–1510. doi: 10.1128/mcb.3.8.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig N., Kass S., Sollner-Webb B. Nucleotide sequence determining the first cleavage site in the processing of mouse precursor rRNA. Proc Natl Acad Sci U S A. 1987 Feb;84(3):629–633. doi: 10.1073/pnas.84.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Financsek I., Mizumoto K., Mishima Y., Muramatsu M. Human ribosomal RNA gene: nucleotide sequence of the transcription initiation region and comparison of three mammalian genes. Proc Natl Acad Sci U S A. 1982 May;79(10):3092–3096. doi: 10.1073/pnas.79.10.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman W. E., Goldberg G., Bowman L. H., Steinmetz D., Schlessinger D. Mouse rDNA: sequences and evolutionary analysis of spacer and mature RNA regions. Mol Cell Biol. 1983 Aug;3(8):1488–1500. doi: 10.1128/mcb.3.8.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt I., Roth E., Paule M. R. Ribosomal RNA transcription in vitro is species specific. Nature. 1982 Mar 11;296(5853):173–174. doi: 10.1038/296173a0. [DOI] [PubMed] [Google Scholar]
- Gurney T., Jr Characterization of mouse 45S ribosomal RNA subspecies suggests that the first processing cleavage occurs 600 +/- 100 nucleotides from the 5' end and the second 500 +/- 100 nucleotides from the 3' end of a 13.9 kb precursor. Nucleic Acids Res. 1985 Jul 11;13(13):4905–4919. doi: 10.1093/nar/13.13.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominami R., Mishima Y., Urano Y., Sakai M., Muramatsu M. Cloning and determination of the transcription termination site of ribosomal RNA gene of the mouse. Nucleic Acids Res. 1982 Mar 25;10(6):1963–1979. doi: 10.1093/nar/10.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Arnheim N. Identification of the in vivo and in vitro origin of transcription in human rDNA. Nucleic Acids Res. 1982 Jul 10;10(13):3933–3949. doi: 10.1093/nar/10.13.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Sollner-Webb B. Transcription of mouse rRNA genes by RNA polymerase I: in vitro and in vivo initiation and processing sites. Cell. 1981 Nov;27(1 Pt 2):165–174. doi: 10.1016/0092-8674(81)90370-6. [DOI] [PubMed] [Google Scholar]
- Miller K. G., Tower J., Sollner-Webb B. A complex control region of the mouse rRNA gene directs accurate initiation by RNA polymerase I. Mol Cell Biol. 1985 Mar;5(3):554–562. doi: 10.1128/mcb.5.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y., Financsek I., Kominami R., Muramatsu M. Fractionation and reconstitution of factors required for accurate transcription of mammalian ribosomal RNA genes: identification of a species-dependent initiation factor. Nucleic Acids Res. 1982 Nov 11;10(21):6659–6670. doi: 10.1093/nar/10.21.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y., Yamamoto O., Kominami R., Muramatsu M. In vitro transcription of a cloned mouse ribosomal RNA gene. Nucleic Acids Res. 1981 Dec 21;9(24):6773–6785. doi: 10.1093/nar/9.24.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P. RNA processing comes of age. J Cell Biol. 1981 Dec;91(3 Pt 2):28s–38s. doi: 10.1083/jcb.91.3.28s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner-Webb B., Reeder R. H. The nucleotide sequence of the initiation and termination sites for ribosomal RNA transcription in X. laevis. Cell. 1979 Oct;18(2):485–499. doi: 10.1016/0092-8674(79)90066-7. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Tower J. Transcription of cloned eukaryotic ribosomal RNA genes. Annu Rev Biochem. 1986;55:801–830. doi: 10.1146/annurev.bi.55.070186.004101. [DOI] [PubMed] [Google Scholar]
- Sommerville J. RNA polymerase I promoters and transcription factors. Nature. 1984 Jul 19;310(5974):189–190. doi: 10.1038/310189a0. [DOI] [PubMed] [Google Scholar]
- Sutiphong J., Matzura C., Niles E. G. Characterization of a crude selective PolI transcription system from Tetrahymena pyriformis. Biochemistry. 1984 Dec 18;23(26):6319–6326. doi: 10.1021/bi00321a005. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Galibert F., Boiron M. Evidence for the existence of several molecular species in the "45S fraction" of mammalian ribosomal precursor RNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1117–1120. doi: 10.1073/pnas.68.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B. M., Giles N. H. Structure of a Neurospora RNA polymerase I promoter defined by transcription in vitro with homologous extracts. Nucleic Acids Res. 1985 Jun 25;13(12):4311–4332. doi: 10.1093/nar/13.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano Y., Kominami R., Mishima Y., Muramatsu M. The nucleotide sequence of the putative transcription initiation site of a cloned ribosomal RNA gene of the mouse. Nucleic Acids Res. 1980 Dec 20;8(24):6043–6058. doi: 10.1093/nar/8.24.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]