Abstract
Autophagy is a lysosomal degradation pathway for bulk cytosolic proteins and damaged organelles, and is well known to act as a cell survival mechanism. Acetaminophen (APAP) overdose can cause liver injury in animals and humans by inducing necrosis due to mitochondrial damage. We recently found that pharmacological induction of autophagy by rapamycin protects against, whereas pharmacological suppression of autophagy by chloroquine exacerbates, APAP-induced liver injury in mice. Autophagy is induced to remove APAP-induced damaged mitochondria and thus attenuates APAP-induced hepatocyte necrosis. To our surprise, we found that liver-specific Atg5 knockout mice are not more susceptible, but are resistant to APAP-induced liver injury due to compensatory effects. Our work suggests that pharmacological modulation of autophagy is a novel therapeutic approach to ameliorate APAP-induced liver injury. Moreover, our work also suggests that caution needs to be exercised when using genetic autophagy gene knockout mice for pathophysiological studies.
Keywords: Atg5 liver-specific knockout mice, acetaminophen, autophagy, liver injury
The liver is a vital organ that has a wide range of functions. One of the major functions of the liver is to metabolize and detoxify drugs. Consequently, liver is often the major target to be damaged by drugs. Drug-induced liver injury is one of the most frequent reasons for stopping the development of drugs and also for the withdrawal of approved drugs from the market. Drug hepatotoxicity accounts for more than 50% of acute liver failure cases in the United States. Acetaminophen (APAP) is a safe drug at therapeutic levels, but an overdose can cause severe liver injury in animals and man. Currently, APAP hepatotoxicity is the most frequent cause of acute liver failure of any etiology in the US, and APAP is one of the most studied hepatotoxic drugs.
The mechanisms that contribute to APAP-induced liver injury have been extensively studied. The majority of a therapeutic dose of APAP is directly conjugated with glucuronic acid or sulfate, and these conjugates are further excreted into bile through the canalicular multidrug resistance-associated protein 2 (ABCC2/MRP2) or into blood through ABCC3/MRP3. The remaining part of the dose is metabolized through the cytochrome P450 system, mainly via CYP2E1, to generate N-acetyl-p-benzoquinone imine (NAPQI), a highly reactive metabolite. NAPQI reacts with glutathione (GSH) to form a GSH-adduct, which is excreted into the bile. Therefore, the intracellular GSH levels are a critical factor in determining the pathogenesis of APAP toxicity. When GSH is exhausted, NAPQI then binds to cellular proteins including mitochondrial proteins to trigger mitochondrial dysfunction. APAP-induced mitochondrial dysfunction is illustrated by the inhibition of the mitochondrial respiratory chain, selective mitochondrial reactive oxygen and peroxynitrite formation, and the onset of mitochondrial membrane permeability transition resulting in the collapse of the membrane potential and necrotic cell death.
Because mitochondrial damage is the key feature in APAP-induced cell death, it is not surprising that we found that APAP overdose triggers autophagy as a defense mechanism to remove damaged mitochondria, a process referred to as mitophagy. Most importantly, cotreatment or post-treatment with rapamycin to stimulate autophagy, almost completely suppresses APAP-induced liver injury. We found that rapamycin does not affect APAP metabolism, suggesting that its protective effects are downstream of APAP metabolism and may mainly involve mitophagy. The findings from the post-treatment withrapamycin (2 h after APAP administration) may have particular clinical relevance because most patients in the emergency room have already passed the APAP metabolism stage and are in the process of developing liver injury. With the rapid progress in the discovery of autophagy inducers rather than rapamycin, targeting autophagy could be a novel avenue for treating APAP overdose patients.
While induction of autophagy by rapamycin protects against APAP-induced hepatotoxicity, it is not unexpected that pharmacological suppression of autophagy by chloroquine further exacerbates APAP-induced liver injury. In addition to the pharmacological modulation of autophagy, we also used a genetic approach by challenging the Atg5 liver-specific knockout mice with APAP. To our surprise, we found that Atg5 liver-specific knockout mice are highly resistant to APAP-induced liver injury. It was previously shown that deletion of Atg7, another essential autophagy gene, in the mouse liver leads to severe liver injury with hepatomegaly. Lack of autophagy in the Atg5-knockout mouse liver also results in the accumulation of SQSTM1/p62 and subsequent persistent activation of nuclear factor (erythroid-derived 2)-like 2 (NFE2L2/NRF2) by releasing kelch-like ECH-associated protein 1 (KEAP1), which functions as a cytosolic inhibitor of NFE2L2, from this protein. Similar to Atg7 liver-specific knockout mice, hepatomegaly and liver injury were also observed in Atg5 liver-specific knockout mice. However, liver injury in the Atg5 liver-specific knockout mice is less severe compared with the Atg7 liver-specific knockout mice based on glutamic pyruvate transaminase/serum alanine aminotransferase (GPT/ALT) activities. The different extent of liver injury between Atg5- and Atg7 liver-specific knockout mice is not clear, but it is possible that ATG5 and ATG7 may have different functions in addition to regulating autophagy. Nevertheless, similar to Atg7 liver-specific knockout mice, we also found persistent activation of NFE2L2 in mouse livers with loss of ATG5. NFE2L2 is a transcription factor critical for protection against electrophilic and oxidative stress by regulating the expression of many cytoprotective genes such as NAD(P)H dehydrogenase quinone 1 (NQO1), glutamate-cysteine ligase, catalytic subunit (GCLC) and glutamate-cysteine ligase, modifier subunit (GCLM). Indeed, we found that all these enzymes are highly upregulated in Atg5-deficient mouse livers compared with wild-type mouse livers resulting in a two-fold increased hepatic GSH content. The higher basal hepatic GSH content in the Atg5-knockout mouse livers renders these mice more resistant to APAP-induced toxicity by promoting APAP detoxification and limiting protein binding of the reactive metabolite (W.X. Ding, unpublished data). Furthermore, we also found increased apoptosis and caspase-3 activation and subsequent compensatory hepatocyte proliferation likely due to the basal apoptosis in Atg5 liver-specific knockout mice. Compensatory hepatocyte proliferation may also attenuate APAP-induced liver injury by increasing the capacity of liver repair and regeneration.
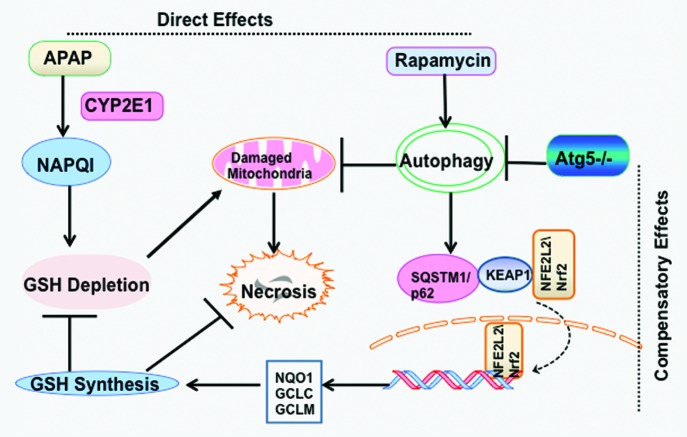
In conclusion, our studies indicate that pharmacological induction of autophagy may provide a novel therapeutic approach for drug-induced liver injury. Caution needs to be exercised when conducting physiological and pathological studies using autophagy gene knockout mice because of the compensatory effects due to the lack of autophagy (Fig. 1).
Figure 1. Scheme of autophagy in APAP-induced liver injury. (A) Direct effects. APAP is first metabolized by CYP2E1 and generates NAPQI. NAPQ1 depletes cellular GSH and binds to mitochondrial proteins to initiate mitochondrial damage resulting in necrotic cell death. Induction of autophagy by rapamycin protects against APAP-induced necrosis by removing damaged mitochondria. (B) Compensatory effects. Genetic deletion of Atg5 in the mouse liver leads to the suppression of autophagy and accumulation of hepatic SQSTM1/p62. Increased p62 protein causes persistent activation of NFE2L2 in the mouse liver by releasing the inhibition of KEAP1 on NFE2L2, which allows increased nuclear translocation of NFE2L2. Persistent activation of NFE2L2 increases the expression of GSH synthesis enzymes resulting in an increased basal GSH content in the mouse liver, which protects against APAP-induced liver injury by removing the toxic metabolite NAPQI.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/19659