Abstract
Cutaneous T-cell lymphoma (CTCL) displays immunosuppressive properties and phenotypic plasticity. The malignant T cells in CTCL can possess features of immunomodulating regulatory T cells (Treg) and IL-17-producing helper T cells (Th17) depending on the stimuli they receive from antigen presenting cells and other sources. IL-2-type cytokines activate STAT5 to promote expression of Treg-related FoxP3, while various cytokines can activate STAT3 to induce synthesis of IL-10 and IL-17. When the Treg phenotype is activated in the early stages of CTCL, “immune evasion” can occur, allowing the clonal T cells to expand. Late stages of CTCL lose the FoxP3 expression but continue to express an immunosuppressive cell-surface ligand PD-L1 suggesting that this and possibly other immunosuppressive proteins rather than FoxP3 are critical for the immunosuppressive state in the advanced stages of CTCL. Novel therapeutic agents may potentially exploit the phenotypic plasticity of CTCL such that the malignant T cells become vulnerable to antitumor immunity.
Keywords: CTCL, FoxP3, IL-10, IL-2, PD-L1, STAT3, STAT5, Th17, Treg
Introduction
T-cell lymphomas represent a heterogeneous group of lymphoproliferative disorders with most derived from the CD4 helper/inducer T cell subset.1,2 Of primary T-cell lymphoproliferative disorders of the skin, cutaneous T-cell lymphomas (CTCL) are the most common subtype. Early lesions of CTCL typically present as limited skin patches or plaques, called mycosis fungoides (MF), which can progress to tumor stage. At the tumor stage, the process may involve also extracutaneous sites, foremost lymph nodes and, less frequently, bone marrow and internal organs. Finally, MF lesions can undergo large cell transformation, which typically results in a highly aggressive clinical course. Sezary syndrome (SS) represents a leukemic form of CTCL in which the malignant T cells are present in the peripheral blood and has characteristic clinical manifestations, most distinctly the generalized erythroderma.
Regulatory T Cells
Regulatory T cells (Tregs) comprise a subset of CD4-positive T lymphocytes capable of inhibiting immune responses against a large spectrum of antigens including the ones expressed by malignant cells.3,4 The concept of lymphocytes with immunosuppressive qualities has existed for several decades, however their phenotypic attributes have only been elucidated fairly recently. Sakaguchi and colleagues identified T cells that control immune responses to non-self antigens by suppressing conventional T-cell activity later termed regulatory T cells (Treg cells or Tregs).5 Among the phenotypic hallmarks of these specialized helper T cells is the presence of the α-chain of the IL-2 (IL-2Rα), designated CD25, and the transcription factor FoxP3.6-11 It has been shown that the depletion of CD25+ T cells leads to a variety of autoimmune inflammatory diseases, whereas reconstitution with CD4+CD25+ T cells can inhibit the development of autoimmune conditions.12 Also, it has been shown that FoxP3 is not only a marker for Tregs, but plays an important role in the function of these cells, as loss or decreased expression of FoxP3 in Tregs cells has been shown to cause severe autoimmune diseases in both mice and humans.13-16 Experimental evidence indicates there are two subtypes of Tregs: those designated “natural” Treg cells that develop in the thymus and those labeled “induced” Tregs which acquire the Treg phenotype as mature post-thymic cells in response to an antigenic stimulation.3,4 Whereas all Treg cells typically express CD25, expression of the transcription factor FoxP3 is believed to be stable in “natural” Treg cells and more transient in “induced” Treg lymphocytes,17,18 reflecting to a large degree the differential DNA methylation status of key regulatory domains of the FoxP3 gene.19-21 It is quite possible that this perceived dichotomy will disappear over time, once the exact mechanisms of the Treg induction and differentiation are better understood.
TREG Phenotypes in the T cells of CTCL
In principle, both malignant and reactive T cells can display phenotypic attributes of Tregs. However, only a subset of these T cells may display the phenotype at any given time. This phenomenon demonstrates the true functional heterogeneity of the lymphocytes implicated in mycosis fungoides and Sezary Syndrome, and is most likely reflective of their plasticity, as discussed later. The number of Tregs decreases with advancement of clinical stage in MF, perhaps indicating the importance of suppressing antitumor activity in its early stages. Furthermore, only a subset of malignant and, hence, clonal T cells as well as infiltrating non-malignant T cells are FoxP3+ Tregs. A study looking at 69 cases of MF and 17 unspecified lesions of CTCL showed stage-dependent FoxP3 expression in approximately 7%.22 Another study using immunohistochemistry found a much higher FoxP3 positivity, roughly 40%, in 16 cases of early stage MF while showing that FoxP3 expression decreased to 5% in tumor stage MF.23 A flow cytometric analysis showed that a majority of malignant T cells in early MF lesions express FoxP3, although at a relatively low expression when compared with non-malignant FoxP3+ lymphocytes.24 In SS, a similar heterogeneity is observed, as studies have shown variable amounts of phenotypic Tregs doubling as malignant Sezary cells. Several studies have shown that FoxP3 is expressed in the malignant T cells of SS, in approximately 40% of SS patients at levels comparable to normal Tregs.23,25 Surprisingly, a study by Heid et al. found FoxP3+ Treg expression to be dissociated from CD25 expression in the Sezary cells,26 possibly reflecting a low expression of CD25 in these cells. The observed variability of Treg phenotypes in both MF and SS raise questions about how subset populations acquire properties of regulatory T cells.
Phenotypic Plasticity in CTCL
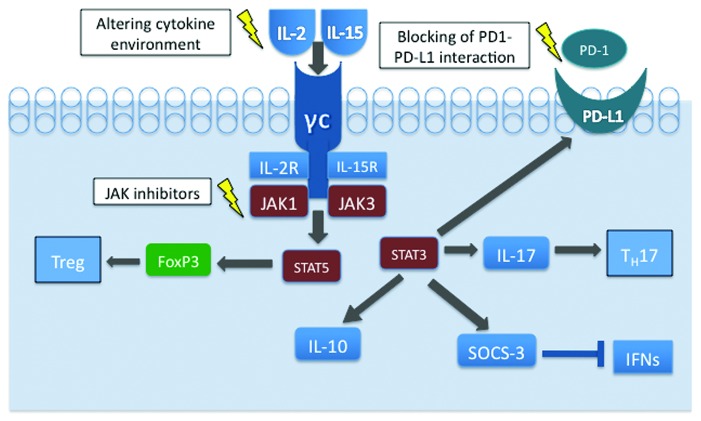
Perhaps explaining the phenomenon of phenotypic heterogeneity in MF and SS is the concept that the tumor microenvironment plays a large role in establishing these phenotypes (Fig. 1). It has been recently demonstrated that physiologic Th17 and induced Treg cells are phenotypically impressionable, which supports the notion of the phenotypic plasticity of their malignant T-cell counterparts.27,28 Indeed, the balance between the degree of STAT3 and STAT5 activation appears critical in determination of the T-cell differentiation toward the Th17 vs. Treg cells.29 Our findings indicate that cytokines IL-2 and IL-15 may play a critical role in the stimulation of malignant T-cell phenotypes.23,30 Both cytokines activate JAK1 and JAK3 kinases that phosphorylate and then activate their respective cytokine receptors, as well as the signaling proteins docked to the activated receptors, most notably STAT5. Out of seven CTCL cell lines all expressed CD25 and TGF-β, the expression of FoxP3 and IL-10 was restricted to two CTCL cell lines that are dependent on exogeneous IL-2. Both IL-2 and IL-15, signaling via receptors sharing the same β/γ chains, were able to induce expression of FoxP3. In contrast, IL-21, which signals through a structurally similar receptor also containing the common γ chain, was unable to induce FoxP3. The IL-2-mediated induction of IL-10 and FoxP3 expression occurred by signaling through STAT3 and STAT5, respectively.30 STAT3 is, indeed, an important mediator of plasticity, as the JAK3/STAT3 pathway promotes the secretion of IL-17 in CTCL cell lines31 and has also been implicated in other immunosuppressive mechanisms. Constitutive activation of STAT3 can cause expression of suppression of cytokine signaling-3 (SOCS-3) protein, which can attenuate effects of many different cytokines, including IFN-γ and IFN-α, and thus weaken the antitumor immunity.32,33 The composition of the malignant T cells changes during the course of the disease, as Kupper and colleagues have shown a depletion in the complexity of the T-cell repertoire that was most pronounced in patients with advanced CTCL.34 Another factor, the interaction of PD-1 (Programmed Death 1) and its ligand, PD-L1, also appears to contribute to the immune evasion. Interactions between PD-1 and its ligands control the induction and maintenance of peripheral T-cell tolerance during normal immune responses, since PD-1 expression tends to be upregulated on tumor infiltrating lymphocytes35 and cancer cells of various types aberrantly express PD-L1 and may escape antitumor immunity.36 In the large transformed T cells of late-stage CTCL, PD-1 expression virtually undetectable in these cells while PD-L1 expression is strong and large cells seem to have stronger PD-L1 expression compared with smaller tumor cells.37 While the mechanisms of PD-L1 induction in CTCL are currently unknown, they may well involve STAT3-mediated activation of the PD-L1 gene, as we have found in a systemic anaplastic large cell lymphoma expressing ALK kinase.38 Indeed, activated STAT3 is expressed by CTCL cells39 and its expression is the most pronounced at the advanced, tumor stage of MF.40 A recent observation that normal antigen-presenting cells become tolerogenic due to the STAT3-driven PD-L1 expression strongly supports this notion.41 In the aggregate, the above-cited data suggest that the PD-L1 mediated inhibition of immune response, rather than the FoxP3-dependent immunosuppression, is critical in the advanced stages of CTCL. Furthermore, these results support the notion that the malignant T-cell phenotype is not static but constantly changing, with considerable input from the cytokine milieu of its environment as well stage of the disease. Importantly, this phenotypic plasticity can be possibly exploited by novel therapeutics, such as cytokine-related therapy attempting to mold T-cell phenotypes away from the immunosuppressive properties, inhibition of Jak activity by small-molecule kinase inhibitors and direct blocking of PD1-PD-L1 interaction (Fig. 1). By modifying the phenotype, these diverse therapeutic approaches may interfere with the key defense mechanisms of the malignant CTCL cells and abrogate their ability to evade the immune system. Consequently, they may indirectly affect survival and growth of the malignant CTCL cells and either eliminate them or attenuate the clinical course of the lymphoma.
Figure 1. Phenotypic plasticity of malignant T cells in CTCL cells. By activating STAT5, IL-2 and IL-15 play a major role in inducing FoxP3 expression and a development of Treg phenotype. Activation of STAT3 leads to IL-17, IL-10, PD-L1 and SOCS-3 expression fostering the TH17 phenotype and immune evasion by diverse, FoxP3-independent mechanisms. Targeting of the key cell signaling nodes may modulate the cytokine-dependent CTCL cell phenotype and, consequently, exert a therapeutic effect.
Conclusion
The heterogeneity of the protein expression and function of the malignant T cells in CTCL is reflected in ability of these cells to not only evade detection by normal immune cells seeking to destroy them, but in becoming more prevalent over time, leading to the late-stage disease and poor patient outcomes. By better understanding of how cytokine signaling in the tumor microenvironment affects the fluent phenotype of these neoplastic cells, may lead to targeted therapeutics able to modify the cytokine milieu and either eliminate the malignant CTCL cells or induce transition of the malignant T cells into a more innocuous type.
Acknowledgments
Supported in part by the NCI grant R01-CA89194.
Glossary
Abbreviations:
- CTCL
cutaneous T-cell lymphoma
- MF
mycosis fungoides (skin-predominant form of CTCL)
- SS
Sezary syndrome (leukemic form of CTCL)
- Treg
regulatory T cells
- Th17
IL-17-producing helper T cells
- SOCS-3
suppression of suppressor of cytokine signaling-3
- PD-L1
programmed death ligand 1
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/18144
References
- 1.Dummer R. Future perspectives in the treatment of cutaneous T-cell lymphoma (CTCL) Semin Oncol. 2006;33:S33–6. doi: 10.1053/j.seminoncol.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 2.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–85. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 3.Chattopadhyay S, Chakraborty NG, Mukherji B. Regulatory T cells and tumor immunity. Cancer Immunol Immunother. 2005;54:1153–61. doi: 10.1007/s00262-005-0699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–5. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 6.Tran DQ, Ramsey H, Shevach EM. Induction of FoxP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–90. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 8.Soper DM, Kasprowicz DJ, Ziegler SF. IL-2Rbeta links IL-2R signaling with FoxP3 expression. Eur J Immunol. 2007;37:1817–26. doi: 10.1002/eji.200737101. [DOI] [PubMed] [Google Scholar]
- 9.Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, Nomura T, et al. FoxP3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–9. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–87. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 11.Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, et al. Single-cell analysis of normal and FoxP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci USA. 2006;103:6659–64. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Sakaguchi S. Homeostatic maintenance of natural FoxP3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–35. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams LM, Rudensky AY. Maintenance of the FoxP3-dependent developmental program in mature regulatory T cells requires continued expression of FoxP3. Nat Immunol. 2007;8:277–84. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 14.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated FoxP3 expression. Nature. 2007;445:766–70. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 15.Wildin RS, Freitas A. IPEX and FoxP3: clinical and research perspectives. J Autoimmun. 2005;25(Suppl):56–62. doi: 10.1016/j.jaut.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FoxP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 17.Randolph DA, Fathman CG. Cd4+Cd25+ regulatory T cells and their therapeutic potential. Annu Rev Med. 2006;57:381–402. doi: 10.1146/annurev.med.57.121304.131337. [DOI] [PubMed] [Google Scholar]
- 18.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, et al. IL-10-secreting regulatory T cells do not express FoxP3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–93. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 19.Baron U, Floess S, Wieczorek G, Baumann K, Grützkau A, Dong J, et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FoxP3(+) conventional T cells. Eur J Immunol. 2007;37:2378–89. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 20.Janson PC, Winerdal ME, Marits P, Thörn M, Ohlsson R, Winqvist O. FOXP3 promoter demethylation reveals the committed Treg population in humans. PLoS One 2008; 3:e1612, 2008. [DOI] [PMC free article] [PubMed]
- 21.Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, et al. Epigenetic regulation of FoxP3 expression in regulatory T cells by DNA methylation. J Immunol. 2009;182:259–73. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gjerdrum LM, Woetmann A, Odum N, Burton CM, Rossen K, Skovgaard GL, et al. FOXP3+ regulatory T cells in cutaneous T-cell lymphomas: association with disease stage and survival. Leukemia. 2007;21:2512–8. doi: 10.1038/sj.leu.2404913. [DOI] [PubMed] [Google Scholar]
- 23.Kasprzycka M, Zhang Q, Witkiewicz A, Marzec M, Potoczek M, Liu X, et al. Gamma c-signaling cytokines induce a regulatory T cell phenotype in malignant CD4+ T lymphocytes. J Immunol. 2008;181:2506–12. doi: 10.4049/jimmunol.181.4.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark RA, Shackelton JB, Watanabe R, Calarese A, Yamanaka K, Campbell JJ, et al. High-scatter T cells: a reliable biomarker for malignant T cells in cutaneous T-cell lymphoma. Blood. 2011;117:1966–76. doi: 10.1182/blood-2010-05-287664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krejsgaard T, Gjerdrum LM, Ralfkiaer E, Lauenborg B, Eriksen KW, Mathiesen AM, et al. Malignant Tregs express low molecular splice forms of FOXP3 in Sézary syndrome. Leukemia. 2008;22:2230–9. doi: 10.1038/leu.2008.224. [DOI] [PubMed] [Google Scholar]
- 26.Heid JB, Schmidt A, Oberle N, Goerdt S, Krammer PH, Suri-Payer E, et al. FOXP3+CD25- tumor cells with regulatory function in Sézary syndrome. J Invest Dermatol. 2009;129:2875–85. doi: 10.1038/jid.2009.175. [DOI] [PubMed] [Google Scholar]
- 27.Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011;117:6532–41. doi: 10.1182/blood-2010-11-317321. [DOI] [PubMed] [Google Scholar]
- 28.Schmidl C, Hansmann L, Andreesen R, Edinger M, Hoffmann P, Rehli M. Epigenetic reprogramming of the RORC locus during in vitro expansion is a distinctive feature of human memory but not naïve Treg. Eur J Immunol. 2011;41:1491–8. doi: 10.1002/eji.201041067. [DOI] [PubMed] [Google Scholar]
- 29.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–54. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marzec M, Halasa K, Kasprzycka M, Wysocka M, Liu X, Tobias JW, et al. Differential effects of interleukin-2 and interleukin-15 versus interleukin-21 on CD4+ cutaneous T-cell lymphoma cells. Cancer Res. 2008;68:1083–91. doi: 10.1158/0008-5472.CAN-07-2403. [DOI] [PubMed] [Google Scholar]
- 31.Krejsgaard T, Ralfkiaer U, Clasen-Linde E, Eriksen KW, Kopp KL, Bonefeld CM, et al. Malignant cutaneous T-cell lymphoma cells express IL-17 utilizing the Jak3/Stat3 signaling pathway. J Invest Dermatol. 2011;131:1331–8. doi: 10.1038/jid.2011.27. [DOI] [PubMed] [Google Scholar]
- 32.Brender C, Lovato P, Sommer VH, Woetmann A, Mathiesen AM, Geisler C, et al. Constitutive SOCS-3 expression protects T-cell lymphoma against growth inhibition by IFNalpha. Leukemia. 2005;19:209–13. doi: 10.1038/sj.leu.2403610. [DOI] [PubMed] [Google Scholar]
- 33.Brender C, Nielsen M, Kaltoft K, Mikkelsen G, Zhang Q, Wasik M, et al. STAT3-mediated constitutive expression of SOCS-3 in cutaneous T-cell lymphoma. Blood. 2001;97:1056–62. doi: 10.1182/blood.V97.4.1056. [DOI] [PubMed] [Google Scholar]
- 34.Yawalkar N, Ferenczi K, Jones DA, Yamanaka K, Suh KY, Sadat S, et al. Profound loss of T-cell receptor repertoire complexity in cutaneous T-cell lymphoma. Blood. 2003;102:4059–66. doi: 10.1182/blood-2003-04-1044. [DOI] [PubMed] [Google Scholar]
- 35.Blank C, Brown I, Marks R, Nishimura H, Honjo T, Gajewski TF. Absence of programmed death receptor 1 alters thymic development and enhances generation of CD4/CD8 double-negative TCR-transgenic T cells. J Immunol. 2003;171:4574–81. doi: 10.4049/jimmunol.171.9.4574. [DOI] [PubMed] [Google Scholar]
- 36.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kantekure K, Yang Y, Raghunath P, Schaffer A, Woetmann A, Zhang Q, et al. Expression patterns of the immunosuppressive proteins PD-1/CD279 and PD-L1/CD274 at different stages of cutaneous T-cell lymphoma (CTCL)/mycosis fungoides (MF) Am J Dermatopathol. doi: 10.1097/DAD.0b013e31821c35cb. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci USA. 2008;105:20852–7. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Q, Nowak I, Vonderheid EC, Rook AH, Kadin ME, Nowell PC, et al. Activation of Jak/STAT proteins involved in signal transduction pathway mediated by receptor for interleukin 2 in malignant T lymphocytes derived from cutaneous anaplastic large T-cell lymphoma and Sezary syndrome. Proc Natl Acad Sci USA. 1996;93:9148–53. doi: 10.1073/pnas.93.17.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witkiewicz A, Raghunath P, Wasik A, Junkins-Hopkins JM, Jones D, Zhang Q, et al. Loss of SHP-1 tyrosine phosphatase expression correlates with the advanced stages of cutaneous T-cell lymphoma. Hum Pathol. 2007;38:462–7. doi: 10.1016/j.humpath.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Wölfle SJ, Strebovsky J, Bartz H, Sähr A, Arnold C, Kaiser C, et al. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol. 2011;41:413–24. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]