Abstract
Ovarian carcinoma is the most deadly gynecological malignancy. Current chemotherapeutic drugs are only transiently effective and patients with advance disease often develop resistance despite significant initial responses. Mounting evidence suggests that anti-apoptotic proteins, including those of the inhibitor of apoptosis protein (IAP) family, play important roles in the chemoresistance. There has been a recent emergence of compounds that block the IAP functions. Here, we evaluated AT-406, a novel and orally active antagonist of multiple IAP proteins, in ovarian cancer cells as a single agent and in the combination with carboplatin for therapeutic efficacy and mechanism of action. We demonstrate that AT-406 has significant single agent activity in 60% of human ovarian cancer cell lines examined in vitro and inhibits ovarian cancer progression in vivo and that 3 out of 5 carboplatin-resistant cell lines are sensitive to AT-406, highlighting the therapeutic potential of AT-406 for patients with inherent or acquired platinum resistance. Additionally, our in vivo studies show that AT-406 enhances the carboplatin-induced ovarian cancer cell death and increases survival of the experimental mice, suggesting that AT-406 sensitizes the response of these cells to carboplatin. Mechanistically, we demonstrate that AT-406 induced apoptosis is correlated with its ability to down-regulate XIAP whereas AT-406 induces cIAP1 degradation in both AT-406 sensitive and resistance cell lines. Together, these results demonstrate, for the first time, the anti-ovarian cancer efficacy of AT-406 as a single agent and in the combination with carboplatin, suggesting that AT-406 has potential as a novel therapy for ovarian cancer patients, especially for patients exhibiting resistance to the platinum-based therapies.
Keywords: Smac mimetic, carboplatin, chemosensitization, ovarian cancer, therapeutic agent
Introduction
Although ovarian cancer only ranks 7th in prevalence of cancers among woman, nearly 60% of patients diagnosed will succumb to this disease.1 This is largely due to the lack of effective early detection methods which manifests in approximately 70% of patients presenting with late stage III or IV disease at the time of diagnosis. Because the efforts to enhance screening techniques have not yet achieved intended goals, focus has been shifted to optimize the treatment strategy to improve clinic outcomes for ovarian cancer patients.2 However, despite advances in surgical debulking, radiation, and chemotherapy over the past few decades, ovarian cancer prognosis still remains dismal. The current first line therapy for ovarian cancer is a combination of platinum based therapies (cisplatin or carboplatin) and paclitaxel, which is effective in patients with advanced ovarian cancer and approximately 80% of patients achieve a complete initial clinical response.3 However, the majority of these patients eventually develops drug resistance and relapse.
The sensitivity of a cancer cell to a chemotherapeutic agent is dependent on its ability to response to the drug-induced apoptosis. Mounting evidence suggests that disruption of the balance of key pro- and anti-apoptotic proteins is the major reason underlying the intrinsic and acquired chemoresistance.4-8 The regulators of apoptosis, including members of the inhibitors of apoptosis protein (IAP) family, are dysregulated in ovarian cancer and have been shown to contribute to its chemoresistance.4,9-11
Originally discovered in baculovirus, the IAP family consists of a group of eight proteins: XIAP (X-linked IAP), c-IAP1 (cellular IAP1), c-IAP2, LM-IAP (melanoma IAP)/Livin, ILP2 (IAPlike protein-2), NAIP (neuronal apoptosis-inhibitory protein), Bruce/Apollon, and survivin. Although their roles are not limited to apoptosis, XIAP is the most well-known IAP member for its tight and potent regulation of apoptosis. XIAP inhibits both the intrinsic and extrinsic apoptotic pathways by directly binding to caspase-3, caspase-7, and caspase-9, key effector caspases that drive apoptosis.
Downregulation of XIAP by antisense XIAP oligonucleotides increased caspase-3 activity and sensitized resistant ovarian cancer cells to chemotherapy.10,12,13 Furthermore, downregulation of XIAP expression inhibited proliferation4 and significantly increased the death of ovarian cancer cells in vitro as well as reduced tumor viability in vivo and prolonged survival of experimental mice.14 These studies suggest that interference of XIAP function primes cancer cells for death induced by cytotoxic agents.
Smac/DIABLO (second mitochondria-derived activator of caspase or direct IAP binding protein with low pI) is an endogenous antagonist of XIAP, cIAP1, and cIAP2.15,16 Efforts have been made to design small-molecule Smac mimetics as inhibitors of IAPs and novel cancer therapeutics. Previous observations suggested that the Smac mimetics may enhance efficacy of chemotherapy in ovarian cancer.17,18 AT-406 is an orally active Smac mimetic that been shown to effectively target XIAP and cIAP1/2.19 AT-406 has shown single agent activity in multiple cancer types including breast cancer.19 Little, however, is known about the efficacy of AT-406 in ovarian cancer as a single agent or in combination with front-line chemotherapy, such as carboplatin, and its mechanism of action.
In the present study, we investigated the therapeutic potential and underlying mechanism of AT-406 in human ovarian cancer. We demonstrate that AT-406 has significant single agent activity in 60% of ovarian cancer cell lines tested and inhibits ovarian cancer progression in vivo, as well as prolongs survival of experimental mice, suggesting the potential of AT-406 as a novel therapeutic agent against ovarian cancer. Moreover, 60% of carboplatin resistant ovarian cancer cell lines are sensitive to AT-406, highlighting the potential benefit of AT-406 as a therapeutic agent for the patients with inherent or acquired platinum resistance. In addition, our in vivo drug combination studies indicate that AT-406 enhances the carboplatin-induced ovarian cancer cell death and increases survival of the experimental mice, suggesting that AT-406 sensitizes the response of ovarian cancer cells to carboplatin. Mechanistically, we demonstrate that ovarian cancer cell apoptosis induced by AT-406 is correlated with the AT-406 mediated reduction of XIAP protein levels. Taken together, these results demonstrate for the first time the therapeutic efficacy of AT-406 as a single agent and in combination with carboplatin against ovarian cancer, and suggest that AT-406 has potential as a novel therapy for ovarian cancer patients, especially for the patients exhibiting resistance to the platinum-based therapies.
Results
XIAP is upregulated in human ovarian cancer
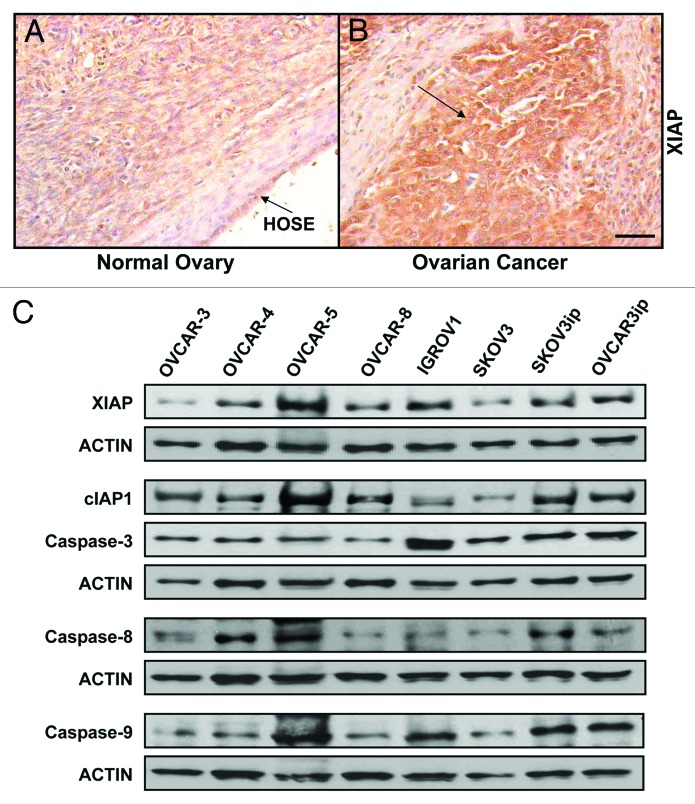
To determine the expression of XIAP by human ovarian cancers, we performed immunohistochemical analysis of paraffin sections of human ovarian cancers and normal ovary. Our results showed that human ovarian cancers express higher levels of XIAP comparing to their normal ovary counterparts (Fig. 1A and B). To assess the expression of endogenous apoptotic proteins by a panel of human ovarian cancer cells, we performed western blotting using protein lysates from eight human ovarian cancer cells and probed for the levels of XIAP, cIAP1, caspase-3, caspase-8, and caspase-9. Our results indicate that these ovarian cancer cells express these proteins, albeit at different levels (Fig. 1C).
Figure 1. XIAP is upregulated in human ovarian cancer. (A) XIAP levels in human ovarian cancer and normal ovary were assessed by immunohistochemistry using anti-human XIAP antibody (R&D). (B) Endogenous XIAP, cIAP1, Caspase-3, Caspase-8, and Caspase-9 expression in a panel of human ovarian cancer cell lines was determined by western blotting with anti-XIAP (Santa Cruz), -cIAP1, Caspase-3, -Caspase-8, or -Caspase-9 (Cell Signaling) antibodies. Actin (Neomarkers) was used as a loading control.
AT-406 displays single agent activity in ovarian cancer cell lines
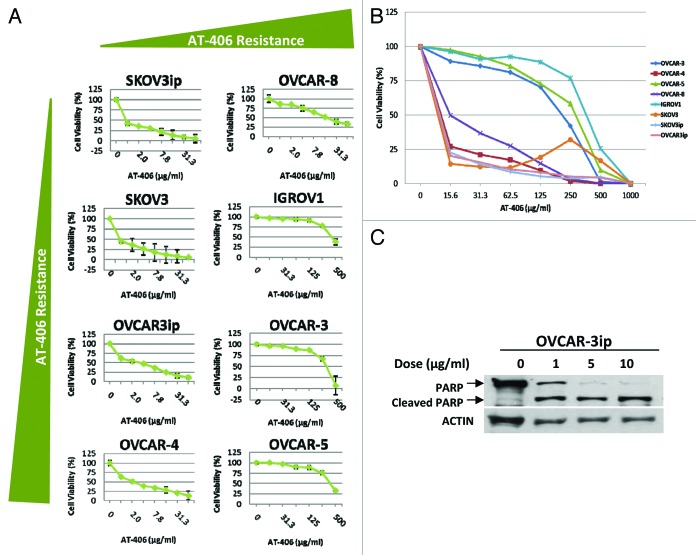
Smac mimetics have shown single agent activity in breast, leukemia, renal, and non-small cell lung carcinoma cells in vitro.20 In order to determine the sensitivity of our panel of ovarian cancer cells to AT-406, a newly developed and orally active Smac mimetic that targets XIAP and cIAP1/2,19 we treated these cells with increasing doses of AT-406 for 48 h. These results revealed that 60% of the ovarian cancer cell lines tested were sensitive to AT-406 as a single agent as evident by their IC50 < 10μg/ml (Fig. 2A and B). To obtain more accurate IC50 values, additional cell viability assays were performed with additional lower doses of AT-406. These results indicated that the IC50 values of AT-406 in these ovarian cancer cells range from 0.05–0.5 µg/ml, indicating significant single agent activity in these cells (Fig. S1).
Figure 2. AT-406 displays single agent activity in ovarian cancer cell lines. (A) Eight human ovarian cancer cell lines were treated for 48 h with increasing doses of AT-406 as depicted in the graphs. (B) Graphical representation of the cell viability curves for all eight ovarian cancer cell lines distinguishing AT-406 sensitive vs. insensitive cell lines. (C) OVCAR-3ip ovarian cancer cell line was treated with increasing doses of AT-406 for 24 h as indicated in the figure. Cells were lysed and probed for cleaved-PARP levels by western blotting. Actin was used as a loading control.
To determine the type of cell death induced by AT-406, we treated the panel of ovarian cancer cells with increasing doses of AT-406 for 24 h and assessed the level of cleaved-PARP, a directly downstream substrate of caspase-3, which has been used routinely as an indicator of apoptosis. These results indicate that AT-406 promotes PARP cleavage in a dose-dependent manner (Fig. 2C), suggesting that AT-406 activates the apoptotic pathway.
AT-406 sensitizes the in vitro responses of ovarian cancer cells to carboplatin
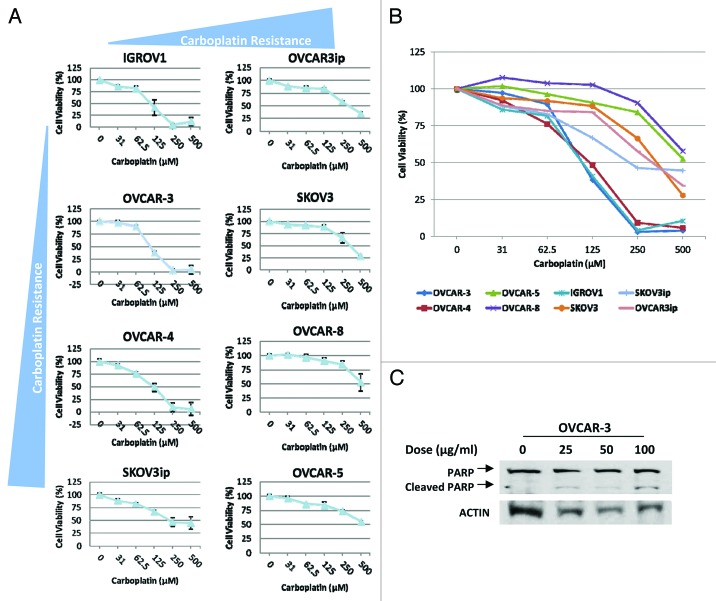
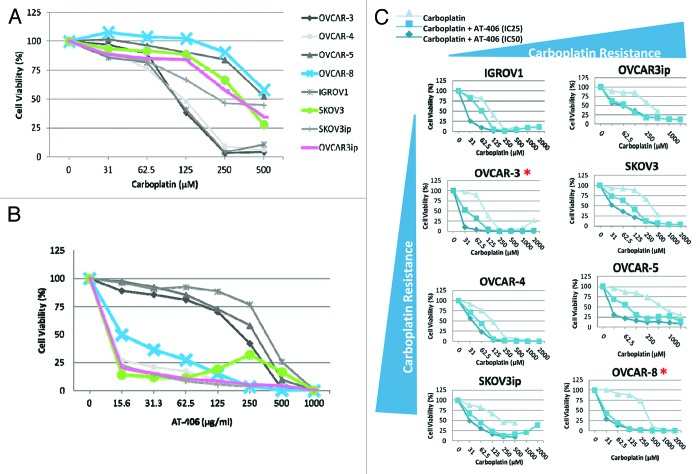
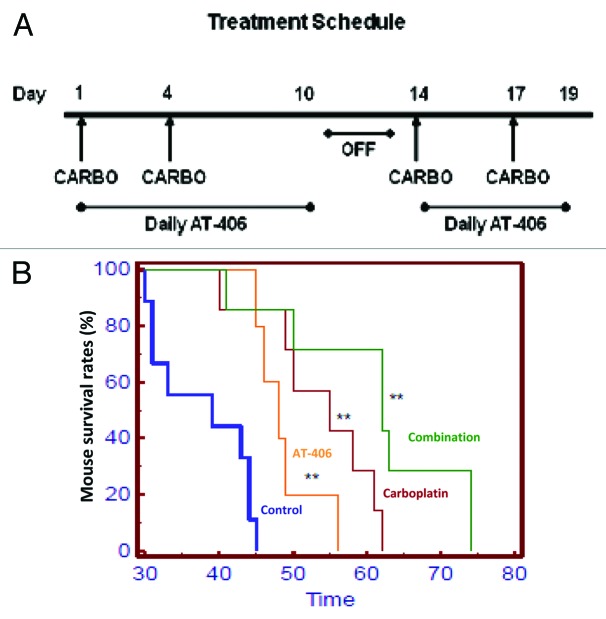
Carboplatin is the standard first-line chemotherapy for ovarian cancer. To determine whether AT-406 can sensitize the response of ovarian cancer cells to carboplatin, we first assessed the single agent activity of carboplatin on our panel of ovarian cancer cells by treating the panel of ovarian cancer cells with increasing doses of carboplatin for 48 h and assessed the cell death via cell viability assays. We found that 50 percent of the cells tested showed responses to carboplatin at a dose range of 100–250 μM (Fig. 3A and B). Consistent with the previous reports, we found that carboplatin induced apoptosis of these ovarian cancer cells as judged by the increased levels of cleaved-PARP upon the treatment with increased doses of carboplatin (Fig. 3C). Our results also showed that 3 out of 5 carboplatin resistance ovarian cancer cell lines, notably OVCAR-8, SKOV-3 and OVCAR-3ip, are sensitive to AT-406 treatment alone (Fig. 4A and B, blue, green, and pink curves), suggesting that AT-406 may have potential as a single agent for the patients with carboplatin refractory tumors.
Figure 3. A panel of human ovarian cancer cells displays a range of IC50 to carboplatin. (A) Eight human ovarian cancer cell lines were treated for 48 h with increasing doses of carboplatin. Each graph represents the viability curve of an ovarian cancer cell line as detailed in the panel. (B) The graphical representation of cell viability curves for all eight ovarian cancer cell lines demonstrates the carboplatin-sensitive vs. -insensitive cell lines. (C) OVCAR3 ovarian cancer cell lines were treated with increasing doses of carboplatin for 24 h as indicated in the panel. The cells were then lysed and probed for cleaved-PARP (Cell Signaling) via western blotting.
Figure 4. AT-406 sensitizes the in vitro responses of ovarian cancer cells to carboplatin. (A and B) Graphical representation of ovarian cancer cells treated with carboplatin and AT-406 as single agents, highlighting that the carboplatin resistant cell lines are concurrently sensitive to AT-406 treatment (blue, green, and pink curves). (C) The Cell viability curves of ovarian cancer cells treated with increasing doses of carboplatin in the presence or absence of the IC25 or IC50 of AT-406. *Denotes ovarian cancer cell lines in which AT-406 sensitizes the carboplatin induced cell death.
Previous studies have suggested that reduction of XIAP levels sensitizes cancer cells to chemotherapeutic agent such as cisplatin.21 In order to determine whether AT-406 sensitizes the response of ovarian cancer cells to carboplatin, we first performed in vitro cell viability assays using the drug combination. Briefly, ovarian cancer cell lines were plated in triplicate (2 × 105 cells per well) in 96-well plates and treated for 48 h with increasing doses of carboplatin in the presence or absence of IC25 or IC50 doses of AT-406. Results from these experiments suggest that AT-406 sensitizes a cohort of ovarian cancer cells to carboplatin treatment (Fig. 4C).
AT-406 exhibits anti-ovarian cancer efficacy both as a single agent and in combination with carboplatin
To determine the in vivo anti-ovarian cancer efficacy of AT-406 as a single agent and when used in combination with carboplatin, we treated Rag-1 mice bearing intraperitoneally implanted OVCAR-3ip cells (Fig. S2) with different agents and the combination as detailed in the “Materials and Methods” section. Our results showed that both carboplatin and AT-406 as single agents significantly inhibited the progression of ovarian cancer and prolonged survival of the experimental mice (Fig. 5). In addition, AT-406 sensitized the anti-ovarian cancer activity of carboplatin and further extended the mouse survival (Fig. 5).
Figure 5. AT-406 displayed anti-ovarian cancer efficacy as a single agent and when used in combination with carboplatin, and prolonged survival of experimental mice. (A) Schematic representation of in vivo treatment schedule. Briefly, 5 × 106 OVCAR3ip cells were injected i.p. into a cohort of immunocompromised B6.129S7-Rag1tmMom mice. The tumors were allowed to grow for a week and these mice were then randomly divided into the following treatment groups: Control (n = 9), Carboplatin (n = 7), AT-406 (n = 5), and Combination (n = 7). These groups were treated as depicted in the schematic and the followings: Control treatment, the vehicle (200μl) by oral gavage everyday for 10 d, followed by a 3 d break, and 6 subsequent treatments for a total of 16 treatments; AT-406 treatment, AT-406 at 100 mg/kg by oral gavage as described for the vehicle treatment; carboplatin treatment, 40 mg/kg intraperitoneal injection of carboplatin twice weekly for two cycles; and the combination treatment, treating with AT-406 and carboplatin simultaneously adhering to the protocol described above for individual agents. Survival analysis was performed and mice were sacrificed when they appeared moribund or displayed signs of distress. (B) Kaplan-Meier survival curves that compare the control vehicle-treated (n = 9), carboplatin-treated (n = 7), AT-406-treated (n = 5), or AT-406 plus carboplatin-treated (n = 7) mice. Group 1 = Control, Group 2 = Carboplatin, Group 3 = AT-406, Group 4 = AT-406 plus Carboplatin. **, p > 0.005
AT-406 induced degradation of XIAP in the drug sensitive ovarian cancer cell lines
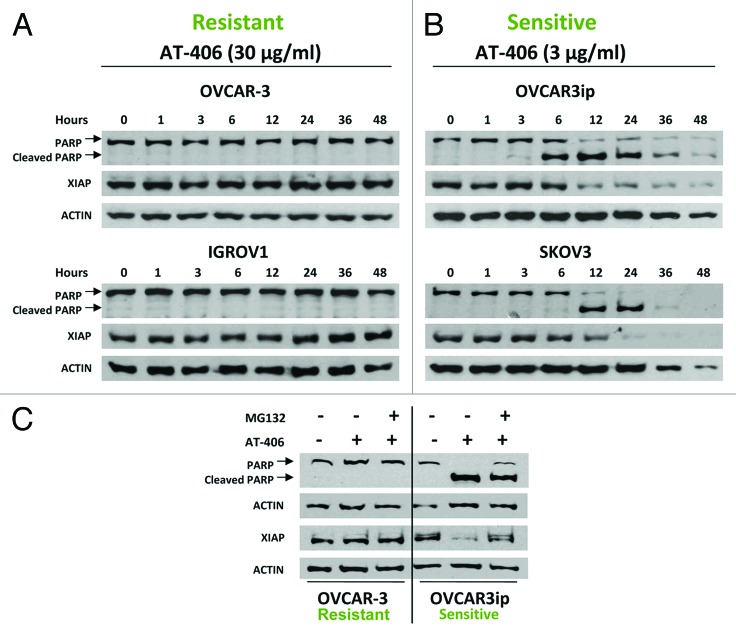
To better our understanding of the mechanism underlying the anti-ovarian cancer activity of AT-406 and the mechanism mediating the differential responses of ovarian cancer cells to AT-406, we first assessed the ability of AT-406 to induce the PARP cleavage, an indicator of apoptosis, in different ovarian cancer cell lines. We found that the AT-406 insensitive cell lines, OVCAR-3 and IGROV1, displayed no PARP cleavage when they were treated with 30μg/ml of AT-406 for 48 h, whereas the AT-406 sensitive cell lines, OVCAR-3ip and SKOV-3, responded with significant PARP cleavage at 6 and 12 h, respectively, post treatment with 3 µg/ml of AT-406 (Fig. 6A and B).
Figure 6. AT-406 reduces XIAP protein levels and induces PARP cleavage in the AT-406 sensitive but not AT- 406 resistant ovarian cancer cell lines. A-B, Ovarian cancer cells, OVCAR-3, IGROV1, OVCAR-3ip and SKOV-3, were treated with indicated amounts of AT-406 for 48 h. Cells were harvested at varying time points as indicated in the panels and probed for cleaved-PARP and XIAP. Actin was used as a loading control. C, OVCAR3 and OVCAR-3ip ovarian cancer cell lines were treated with or without AT-406 (3 μg/ml or 30 μg/ml, respectively) for 4 h. Cells treated with AT-406 were then treated with a vehicle (DMSO) or MG132 (10 μM/ml) for an additional 4 h before the cells were lysed and extracted for western blotting with anti-XIAP and cleaved-PARP antibodies.
Smac mimetics have been shown to induce apoptosis through promoting the degradation of IAP family members.22 To investigate the mechanism of action of AT-406, we examined the levels of XIAP, cIAP1, and cleavage of PARP by western blotting. Our results showed that AT-406 induced downregulation of XIAP expression in AT-406 sensitive but not resistant cell lines (Fig. 6A and B). Consistent with a previous report,19 however, AT-406 treatment induced a rapid downregulation of cIAP1 in both sensitive and insensitive cell lines examined, suggesting that reduction of cIAP1 protein levels alone in these ovarian cancer cells is not sufficient to induce apoptosis (Fig. S3) and that the AT-406 sensitivity in ovarian cancer cell lines is associated with AT-406 mediated downregulation of XIAP but not cIAP1.
To elucidate whether the reduction of XIAP expression was mediated through the proteasome-mediated degradation, we treated AT-406 treated cells with MG132, a proteasome inhibitor, and assessed the XIAP levels in these cells. Our results demonstrated that the reduction of XIAP protein is inhibited in the AT-406 sensitive cells concomitantly treated with AT-406 and MG132, indicating that the downregulation of XIAP is indeed mediated by proteasome (Fig. 6C).
Discussion
Resistance to current chemotherapies is the major obstacle for successful ovarian cancer treatment. Several recent studies have shown that dysregulation of the balance of pro- and anti-apoptotic proteins can lead to resistance to chemotherapeutic agents. XIAP, a potent inhibitor of cell death and a member of the IAP family, has been shown to be upregulated in chemoresistant ovarian cancer cells.10,23 Furthermore, downregulation of XIAP can induce apoptosis in ovarian cancer cells, as well as reverse chemoresistance.4,14 These studies suggested therapeutic potential of Smac mimetics for ovarian cancer patients through concurrently targeting of multiple IAP proteins. In our present study, we establish for the first time the therapeutic efficacy of a novel Smac mimetic, AT-406, for ovarian cancer. We demonstrate that AT-406 displays significant single agent activity in 60% of ovarian cancer cell lines examined (Fig. 3). Furthermore, induction of cell death in the AT-406 sensitive cell lines required less than 1 μg of AT-406 (Fig. S1) and is associated with downregulation of XIAP but not cIAP1. This finding emphasizes the addiction of these cell lines to XIAP and is consistent with a previous study in which antisense oligonucleotide against XIAP alone was sufficient to induced apoptosis of ovarian cancer cell lines in vitro.14 Furthermore, our studies demonstrated that 3 out of 5 carboplatin resistant human ovarian cancer cell lines are concurrently sensitive to AT-406 treatment. Since 20 to 30 percent of ovarian cancers exhibit inherent resistance to platinum therapy,3 this result suggests that AT-406 has potential to be used as a novel frontline agent for this group of platinum resistant patients.
Despite high rates of the platinum resistance in ovarian cancer patients, the initial responses to platinum-based drugs, such as carboplatin, are substantial. Over 80% of ovarian cancer patients treated with carboplatin display significant responses.3 Therefore, we were interested in determining whether AT-406 can sensitize ovarian cancer cells to carboplatin. Our in vitro studies indicate that AT-406 indeed enhances ovarian cancer cell death induced by carboplatin (Fig. 4). The fact that AT-406 is capable of sensitizing carboplatin responses in a number of carboplatin resistant cell lines suggests that XIAP contributes to the platinum resistance in these cell lines. In addition to our findings in vitro, our in vivo experiments utilizing the orthotopic ovarian cancer model demonstrated that AT-406 displayed significant single agent activity as evident by prolonged mouse survival as compared with the control group. Additionally, AT-406 in combination with carboplatin further prolonged mouse survival compared with the single agents, suggesting a potential utility of AT-406 in the ovarian cancer patients who have become refractory to platinum-based therapies (Fig. 5). Lastly, our study reveals the difference between AT-406 sensitive and resistant ovarian cancer cells at a molecular level. AT-406 markedly downregulated XIAP protein levels in the AT-406 sensitive but not resistant ovarian cancer cells whereas AT-406 induces rapid cIAP1 degradation in both sensitive and resistant cells, suggesting that reduction of XIAP mediates anti-ovarian cancer efficacy of AT-406.
Our data provides clear evidence for the single agent activity of AT-406 in ovarian cancer as well as the efficacy of AT-406 to sensitize the response of ovarian cancers to carboplatin, therefore increasing therapeutic efficacy of this front-line agent. Collectively, these studies suggest that AT-406 is an effective novel agent for a broad range of ovarian cancers.
Materials and Methods
Patient ovarian samples, cells and reagents
Ovarian cancer and normal ovarian tissues were obtained from the Cooperative Human Tissue Network (CHTN) at University of Pennsylvania and the Ohio State University. Human ovarian surface epithelial (HOSE) cells were from ScienCell Res Lab. OVCAR-3, OVCAR-4, OVCAR-5, OVCAR-8, IGROV1, and SKOV-3 cells were from the NCI (DTP, DCTD Tumor Repository). OVCAR-3ip cells were derived from the in vivo selection of parental OVCAR-3 cells that were capable of forming ascites tumors in immuno-compromised mice. These cells were maintained according to the providers’ and manufacturers’ instructions. Anti-XIAP (Santa Cruz), -cleaved PARP (Cell Signaling), and -Actin (Neomarkers) antibodies, as well as Cell Titer-Glo Luminescent Cell Viability Assay kit (Promega) were used in the experiments.
Immunoblotting
Cells were extracted with 4x SDS Laemmli sample buffer without the dye. Protein concentrations of all samples were determined using Bio-Rad Dc Protein Assay Reagents. Equal amounts of proteins were analyzed by western blotting as described previously.24 Actin was used as a loading control.
The Effects of AT-406 on XIAP levels and PARP cleavage
SKOV-3, OVCAR-3, IGROV1, and OVCAR-3ip cells were cultured until subconfluence in 6-well plates. The cells were treated with fresh media containing 3µg/ml or 30 µg/ml of AT-406 as detailed in the figure legends. The cells were then lysed at various time points over a 48 h period and equal amounts of extracted proteins were analyzed by western blotting with anti-XIAP or anti-cleaved PARP antibodies.
The Effect of MG132 on the AT-406 induced downregulation of XIAP
OVCAR-3 and OVCAR-3ip cells were cultured until subconfluence in 6-well plates. The cells were then treated with a vehicle (DMSO) or AT-406 (3 µg/ml or 30 µg/ml). After 4 h, MG132 (10µM/ml) was applied to these cells. After an additional 4 h, the cells were lysed and equal amounts of extracted proteins were analyzed by western blotting with anti-XIAP and anti-cleaved PARP and antibodies.
Cell viability assays
Cell viability assays were performed using the Cell Titer-Glo Luminescent Cell Viability Assay kit (Promega) as described previously.25 Briefly, ovarian cancer cell lines were plated in triplicate (2 × 105 cells per well) in 96-well plates and treated for 48 h with different doses of AT-406 and/or carboplatin as detailed in the figure legends. The cell viability was measured by the Modulus™ Microplate Multimode Reader (Turner Biosystems).
In vivo efficacy studies of AT-406
AT-406 was dissolved in DMSO as a stock solution at 200mg/ml. The stock solution was diluted in the vehicle solution, which consists of 10mg/ml hypromellose (Sigma) and 1μl of Tween 80 in PBS, to achieve the final concentration of 10 mg/ml. 5 × 106 OVCAR-3ip cells were injected intraperitoneally (i.p.) into each immunocompromised B6.129S7-Rag1tmMom mouse (Jackson Lab). Seven days after tumor implantation, the tumor bearing mice were randomly divided into the following treatment groups: control (n = 9), carboplatin (n = 7), AT-406 (n = 5), and combination of AT-406 and carboplatin (n = 7). These groups of mice were treated as follows: each control mouse received 0.2ml of vehicle solution alone by oral gavage every day for 10 d, followed by a 3 d break, and 6 subsequent oral gavage treatments for a total of 16 treatments. AT-406 treatment mice received AT-406 (100 mg/kg) by oral gavage as described for the control treatment mice and the carboplatin treatment mice received carboplatin (40 mg/kg) through intraperitoneal injection twice weekly for two cycles. The combination group received AT-406 and carboplatin treatments simultaneously adhering to the protocols described above for the individual treatment groups. Following an approved IACUC protocol, mouse survival analysis was performed and mice were sacrificed when they appeared moribund or displayed signs of distress, at which time, the mice were considered as dead. At the conclusion of the experiment, mouse tumors and vital organs were removed, fixed, and sectioned for further analyses.
Histology and Immunohistochemistry (IHC)
Histology was performed as described previously.26,27 Paraffin sections derived from ovarian cancer patients and normal ovaries were stained with H&E (hematoxylin and eosin) or reacted with anti-XIAP antibody (R&D).
Statistics
Other than survival experiments, one tailed student’s t-tests were used to analyze statistical differences between the control and experimental groups. For mouse survival experiments, LogRank statistical analysis (SigmaPlot) was used to calculate the statistical differences between control and experimental groups. Differences were considered statistically significant at p < 0.05.
Supplementary Material
Disclosure of Potential Conflicts of Interest
Dr Shaomeng Wang owns stocks and stock options in Ascenta Therapeutics and serves as a consultant for Ascenta, which has licensed AT-406 from the University of Michigan for development. Dr Wang is an inventor on AT-406 and receives royalties from the University of Michigan. Other authors have no conflicts of financial interest to declare.
Acknowledgments
We thank excellent technical support of Ms. Rong Lu and Mr. Yin Xu at Mount Sinai School of Medicine, the Cooperative Human Tissue Network (CHTN) for providing human ovarian tissues. This work is supported by the funds from NIH-NCI (R01CA135158 to Q.Y.). Melissa Brunckhorst was supported by an institutional training grant (5T32CA078207–12). Dimitry Lerner is a fellow at the Gynecologic Oncology Program at Mount Sinai School of Medicine.
Glossary
Abbreviations:
- CHTN
Cooperative Human Tissue Network
- c-IAP1/2
cellular IAP1/2
- H&E
hematoxylin and eosin
- ILP2
IAP-like protein-2
- IAP
inhibitor of apoptosis protein
- IHC
Immunohistochemistry
- i.p.
intraperitoneally
- LM-IAP
melanoma IAP
- NAIP
neuronal apoptosis-inhibitory protein
- XIAP
X-linked IAP
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/20563
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Bell R, Petticrew M, Luengo S, Sheldon TA. Screening for ovarian cancer: a systematic review. Health Technol Assess. 1998;2:i–iv, 1-84. [PubMed] [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N Engl J Med. 1993;329:1550–9. doi: 10.1056/NEJM199311183292108. [DOI] [PubMed] [Google Scholar]
- 4.Ma JJ, Chen BL, Xin XY. XIAP gene downregulation by small interfering RNA inhibits proliferation, induces apoptosis, and reverses the cisplatin resistance of ovarian carcinoma. Eur J Obstet Gynecol Reprod Biol. 2009;146:222–6. doi: 10.1016/j.ejogrb.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Tirrò E, Consoli ML, Massimino M, Manzella L, Frasca F, Sciacca L, et al. Altered expression of c-IAP1, survivin, and Smac contributes to chemotherapy resistance in thyroid cancer cells. Cancer Res. 2006;66:4263–72. doi: 10.1158/0008-5472.CAN-05-3248. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, Ding F, Luo A, Chen A, Yu Z, Ren S, et al. XIAP is highly expressed in esophageal cancer and its downregulation by RNAi sensitizes esophageal carcinoma cell lines to chemotherapeutics. Cancer Biol Ther. 2007;6:973–80. doi: 10.4161/cbt.6.6.4195. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Jian Z, Xia K, Li X, Lv X, Pei H, et al. XIAP is related to the chemoresistance and inhibited its expression by RNA interference sensitize pancreatic carcinoma cells to chemotherapeutics. Pancreas. 2006;32:288–96. doi: 10.1097/01.mpa.0000218314.67111.fb. [DOI] [PubMed] [Google Scholar]
- 8.Konopleva M, Zhao S, Hu W, Jiang S, Snell V, Weidner D, et al. The anti-apoptotic genes Bcl-X(L) and Bcl-2 are over-expressed and contribute to chemoresistance of non-proliferating leukaemic CD34+ cells. Br J Haematol. 2002;118:521–34. doi: 10.1046/j.1365-2141.2002.03637.x. [DOI] [PubMed] [Google Scholar]
- 9.Sapi E, Alvero AB, Chen W, O’Malley D, Hao XY, Dwipoyono B, et al. Resistance of ovarian carcinoma cells to docetaxel is XIAP dependent and reversible by phenoxodiol. Oncol Res. 2004;14:567–78. doi: 10.3727/0965040042707943. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki H, Sheng Y, Kotsuji F, Tsang BK. Down-regulation of X-linked inhibitor of apoptosis protein induces apoptosis in chemoresistant human ovarian cancer cells. Cancer Res. 2000;60:5659–66. [PubMed] [Google Scholar]
- 11.Williams J, Lucas PC, Griffith KA, Choi M, Fogoros S, Hu YY, et al. Expression of Bcl-xL in ovarian carcinoma is associated with chemoresistance and recurrent disease. Gynecol Oncol. 2005;96:287–95. doi: 10.1016/j.ygyno.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Xing H, Gao Q, Chen G, Lu Y, Wang S, et al. Regulation of HtrA2/Omi by X-linked inhibitor of apoptosis protein in chemoresistance in human ovarian cancer cells. Gynecol Oncol. 2005;97:413–21. doi: 10.1016/j.ygyno.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 13.He XP, Ota T, Liu P, Su CQ, Chien J, Shridhar V. Downregulation of HtrA1 promotes resistance to anoikis and peritoneal dissemination of ovarian cancer cells. Cancer Res. 2010;70:3109–18. doi: 10.1158/0008-5472.CAN-09-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw TJ, Lacasse EC, Durkin JP, Vanderhyden BC. Downregulation of XIAP expression in ovarian cancer cells induces cell death in vitro and in vivo. Int J Cancer. 2008;122:1430–4. doi: 10.1002/ijc.23278. [DOI] [PubMed] [Google Scholar]
- 15.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/S0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 16.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/S0092-8674(00)00009-X. [DOI] [PubMed] [Google Scholar]
- 17.Hu W, Wang F, Tang J, Liu X, Yuan Z, Nie C, et al. Proapoptotic protein Smac mediates apoptosis in cisplatin-resistant ovarian cancer cells when treated with the anti-tumor agent AT101. J Biol Chem. 2012;287:68–80. doi: 10.1074/jbc.M111.271205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrucci E, Pasquini L, Petronelli A, Saulle E, Mariani G, Riccioni R, et al. A small molecule Smac mimic potentiates TRAIL-mediated cell death of ovarian cancer cells. Gynecol Oncol. 2007;105:481–92. doi: 10.1016/j.ygyno.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Cai Q, Sun H, Peng Y, Lu J, Nikolovska-Coleska Z, McEachern D, et al. A potent and orally active antagonist (SM-406/AT-406) of multiple inhibitor of apoptosis proteins (IAPs) in clinical development for cancer treatment. J Med Chem. 2011;54:2714–26. doi: 10.1021/jm101505d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Probst BL, Liu L, Ramesh V, Li L, Sun H, Minna JD, et al. Smac mimetics increase cancer cell response to chemotherapeutics in a TNF-α-dependent manner. Cell Death Differ. 2010;17:1645–54. doi: 10.1038/cdd.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong QS, Zheng LD, Wang L, Zeng FQ, Chen FM, Dong JH, et al. Downregulation of XIAP expression induces apoptosis and enhances chemotherapeutic sensitivity in human gastric cancer cells. Cancer Gene Ther. 2005;12:509–14. doi: 10.1038/sj.cgt.7700813. [DOI] [PubMed] [Google Scholar]
- 22.Lu J, Bai L, Sun H, Nikolovska-Coleska Z, McEachern D, Qiu S, et al. SM-164: a novel, bivalent Smac mimetic that induces apoptosis and tumor regression by concurrent removal of the blockade of cIAP-1/2 and XIAP. Cancer Res. 2008;68:9384–93. doi: 10.1158/0008-5472.CAN-08-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansouri A, Zhang Q, Ridgway LD, Tian L, Claret FX. Cisplatin resistance in an ovarian carcinoma is associated with a defect in programmed cell death control through XIAP regulation. Oncol Res. 2003;13:399–404. doi: 10.3727/096504003108748410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai Y, Liu YJ, Wang H, Xu Y, Stamenkovic I, Yu Q. Inhibition of the hyaluronan-CD44 interaction by merlin contributes to the tumor-suppressor activity of merlin. Oncogene. 2007;26:836–50. doi: 10.1038/sj.onc.1209849. [DOI] [PubMed] [Google Scholar]
- 25.Brunckhorst MK, Wang H, Lu R, Yu Q. Angiopoietin-4 promotes glioblastoma progression by enhancing tumor cell viability and angiogenesis. Cancer Res. 2010;70:7283–93. doi: 10.1158/0008-5472.CAN-09-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau YK, Murray LB, Houshmandi SS, Xu Y, Gutmann DH, Yu Q. Merlin is a potent inhibitor of glioma growth. Cancer Res. 2008;68:5733–42. doi: 10.1158/0008-5472.CAN-08-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y, Stamenkovic I, Yu Q. CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. 2010;70:2455–64. doi: 10.1158/0008-5472.CAN-09-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.