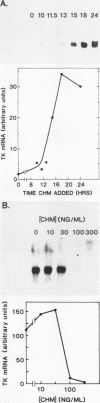
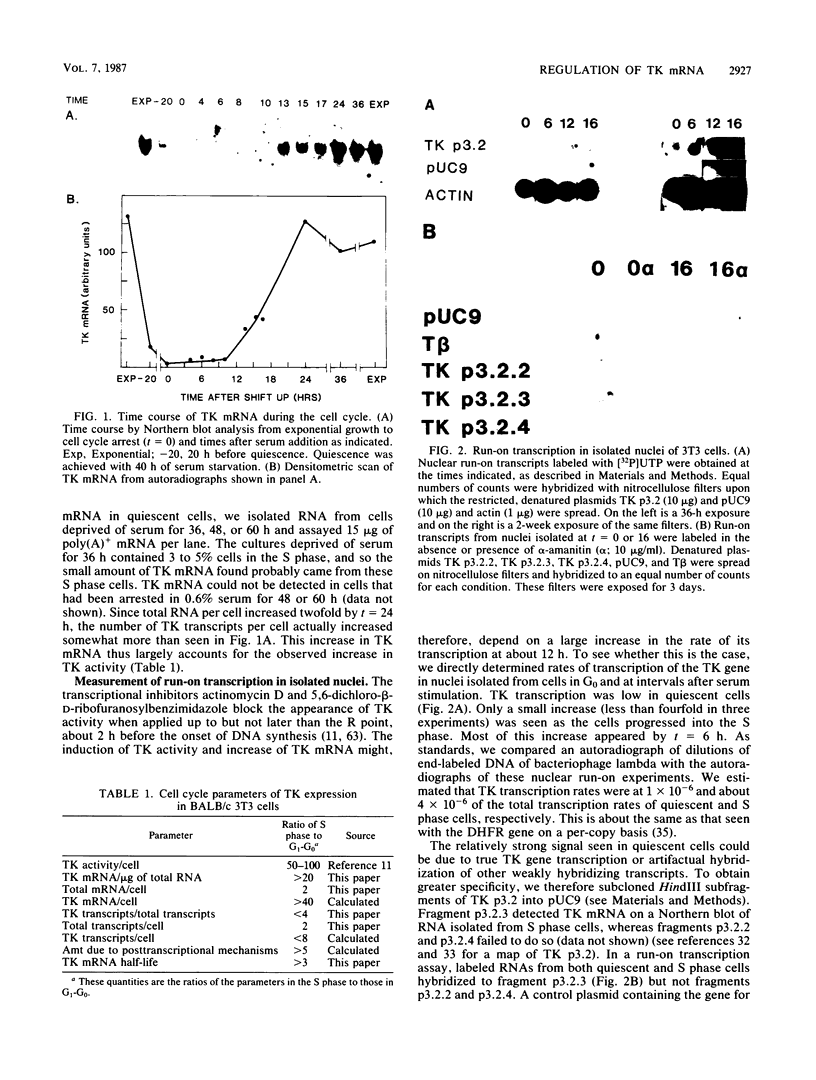
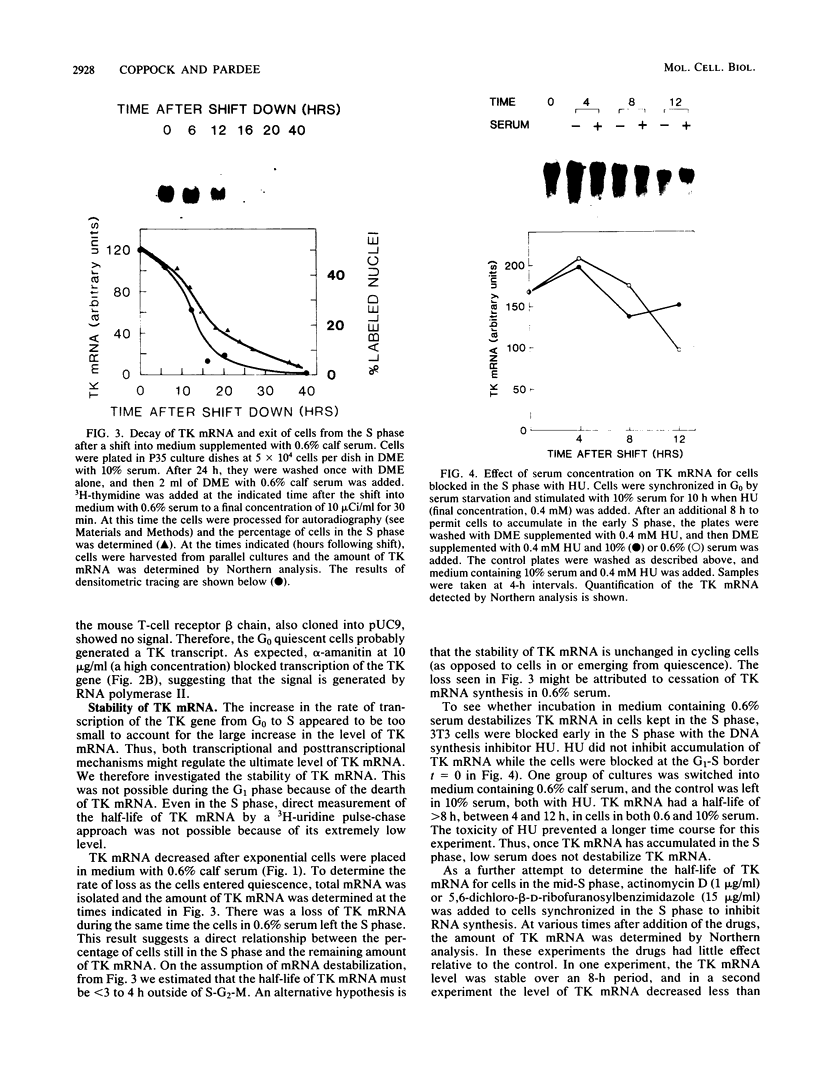
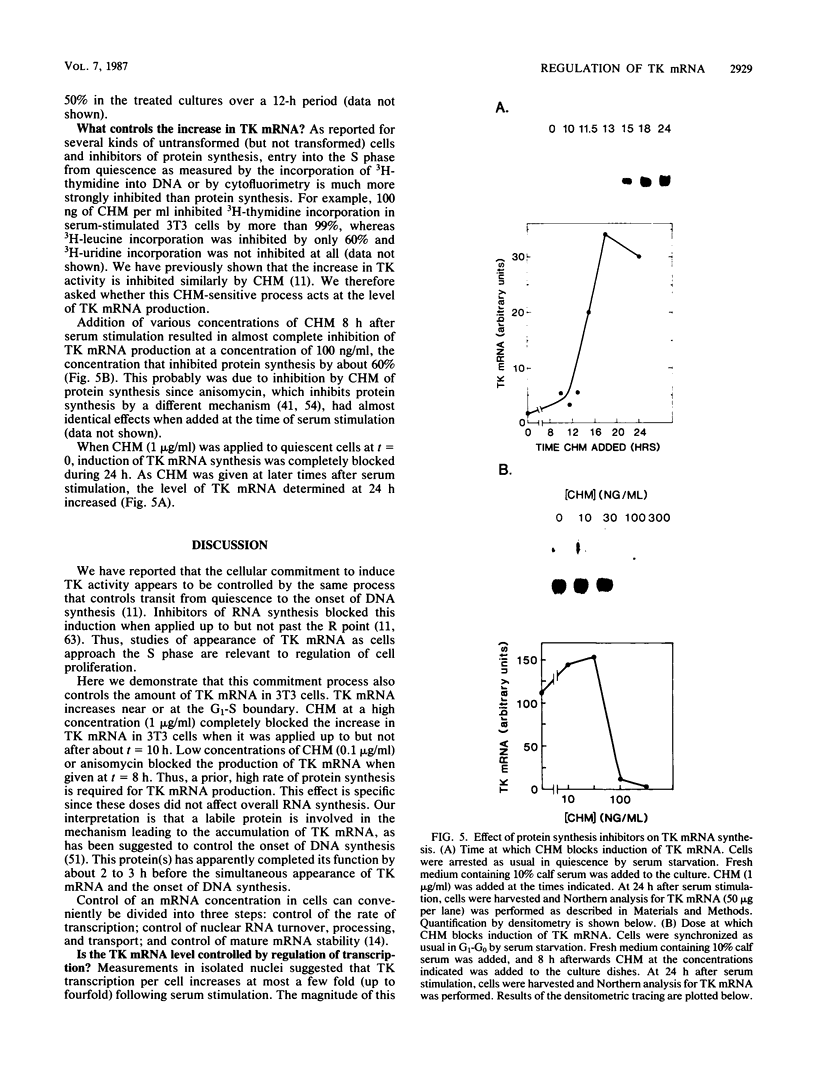
Abstract
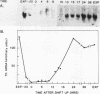
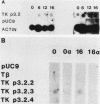
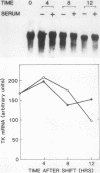
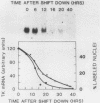
To investigate the mechanism which controls the onset of DNA synthesis, we examined the regulation of thymidine kinase (TK) and its mRNA in the cell cycle. TK activity provides a useful marker for the onset of the S phase in mammalian cells. The present analysis of regulation of TK mRNA in BALB/c 3T3 cells showed that (i) the increase in TK activity depended on the availability of TK mRNA, (ii) the level of TK mRNA between G0 and S increased more than 20-fold, (iii) the rate of run-on TK transcription increased at most 2- to 4-fold between the G0 and S phases, (iv) the half-life of TK mRNA was greater than 8 to 12 h in the S and M phases and decreased as cells entered quiescence, (v) the TK mRNA increase was fully blocked by inhibition of protein synthesis by only 60%, (vi) this inhibition was completely effective for up to about 10 h following serum addition and progressively much less effective when the drugs were added later. These results suggest that the appearance of TK mRNA at the beginning of the S phase in serum-stimulated 3T3 cells is controlled not only by the rate of gene transcription but importantly also by the decreased rate of mRNA degradation. Similar mechanisms may be involved in regulation of the onset of DNA synthesis and the increase in TK mRNA since both are controlled in a manner consistent with a requirement for a labile protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., McAuslan B. R. Expression of thymidine kinase variants is a function of the replicative state of cells. Cell. 1974 Jun;2(2):113–117. doi: 10.1016/0092-8674(74)90100-7. [DOI] [PubMed] [Google Scholar]
- Baumbach L. L., Marashi F., Plumb M., Stein G., Stein J. Inhibition of DNA replication coordinately reduces cellular levels of core and H1 histone mRNAs: requirement for protein synthesis. Biochemistry. 1984 Apr 10;23(8):1618–1625. doi: 10.1021/bi00303a006. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986 Jun 12;321(6071):702–706. doi: 10.1038/321702a0. [DOI] [PubMed] [Google Scholar]
- Bradshaw H. D., Jr, Deininger P. L. Human thymidine kinase gene: molecular cloning and nucleotide sequence of a cDNA expressible in mammalian cells. Mol Cell Biol. 1984 Nov;4(11):2316–2320. doi: 10.1128/mcb.4.11.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw H. D., Jr Molecular cloning and cell cycle-specific regulation of a functional human thymidine kinase gene. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5588–5591. doi: 10.1073/pnas.80.18.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent T. P., Butler J. A., Crathorn A. R. Variations in phosphokinase activities during the cell cycle in synchronous populations of HeLa cells. Nature. 1965 Jul 10;207(993):176–177. doi: 10.1038/207176a0. [DOI] [PubMed] [Google Scholar]
- Brooks R. F. Continuous protein synthesis is required to maintain the probability of entry into S phase. Cell. 1977 Sep;12(1):311–317. doi: 10.1016/0092-8674(77)90209-4. [DOI] [PubMed] [Google Scholar]
- Campisi J., Medrano E. E., Morreo G., Pardee A. B. Restriction point control of cell growth by a labile protein: evidence for increased stability in transformed cells. Proc Natl Acad Sci U S A. 1982 Jan;79(2):436–440. doi: 10.1073/pnas.79.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coppock D. L., Pardee A. B. Regulation of thymidine kinase activity in the cell cycle by a labile protein. J Cell Physiol. 1985 Aug;124(2):269–274. doi: 10.1002/jcp.1041240215. [DOI] [PubMed] [Google Scholar]
- Croy R. G., Pardee A. B. Enhanced synthesis and stabilization of Mr 68,000 protein in transformed BALB/c-3T3 cells: candidate for restriction point control of cell growth. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4699–4703. doi: 10.1073/pnas.80.15.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani C., Blanchard J. M., Piechaczyk M., El Sabouty S., Marty L., Jeanteur P. Extreme instability of myc mRNA in normal and transformed human cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7046–7050. doi: 10.1073/pnas.81.22.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Das M. Initiation of nuclear DNA replication: evidence for formation of committed prereplicative cellular state. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5677–5681. doi: 10.1073/pnas.78.9.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle A. J., Graves R. A., Marzluff W. F., Johnson L. F. Regulation of histone mRNA production and stability in serum-stimulated mouse 3T6 fibroblasts. Mol Cell Biol. 1983 Nov;3(11):1920–1929. doi: 10.1128/mcb.3.11.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström Y., Eriksson S., Jildevik I., Skog S., Thelander L., Tribukait B. Cell cycle-dependent expression of mammalian ribonucleotide reductase. Differential regulation of the two subunits. J Biol Chem. 1985 Aug 5;260(16):9114–9116. [PubMed] [Google Scholar]
- Eriksson S., Martin D. W., Jr Ribonucleotide reductase in cultured mouse lymphoma cells. Cell cycle-dependent variation in the activity of subunit protein M2. J Biol Chem. 1981 Sep 25;256(18):9436–9440. [PubMed] [Google Scholar]
- Farnham P. J., Schimke R. T. Transcriptional regulation of mouse dihydrofolate reductase in the cell cycle. J Biol Chem. 1985 Jun 25;260(12):7675–7680. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Casimir C. Post-transcriptional regulation of the chicken thymidine kinase gene. Nucleic Acids Res. 1984 Feb 10;12(3):1427–1446. doi: 10.1093/nar/12.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson S. L., Wu J. S., Johnson L. F. Cell cycle regulation of dihydrofolate reductase mRNA metabolism in mouse fibroblasts. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5140–5144. doi: 10.1073/pnas.77.9.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer R., Müllner E., Seiser C., Wintersberger E. Cell cycle regulated synthesis of stable mouse thymidine kinase mRNA is mediated by a sequence within the cDNA. Nucleic Acids Res. 1987 Jan 26;15(2):741–752. doi: 10.1093/nar/15.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenh C. H., Geyer P. K., Johnson L. F. Control of thymidylate synthase mRNA content and gene transcription in an overproducing mouse cell line. Mol Cell Biol. 1985 Oct;5(10):2527–2532. doi: 10.1128/mcb.5.10.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenh C. H., Rao L. G., Johnson L. F. Regulation of thymidylate synthase enzyme synthesis in 5-fluorodeoxyuridine-resistant mouse fibroblasts during the transition from the resting to growing state. J Cell Physiol. 1985 Jan;122(1):149–154. doi: 10.1002/jcp.1041220122. [DOI] [PubMed] [Google Scholar]
- Johnson L. F., Fuhrman C. L., Wiedemann L. M. Regulation of dihydrofolate reductase gene expression in mouse fibroblasts during the transition from the resting to growing state. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):397–306. doi: 10.1002/jcp.1040970314. [DOI] [PubMed] [Google Scholar]
- Johnson L. F., Rao L. G., Muench A. J. Regulation of thymidine kinase enzyme level in serum-stimulated mouse 3T6 fibroblasts. Exp Cell Res. 1982 Mar;138(1):79–85. doi: 10.1016/0014-4827(82)90093-3. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., FREARSON P. M. DECLINE OF THYMIDINE KINASE ACTIVITY IN STATIONARY PHASE MOUSE FIBROBLAST CELLS. J Biol Chem. 1965 Jun;240:2565–2573. [PubMed] [Google Scholar]
- Kit S., Jorgensen G. N. Formation of thymidine kinase and deoxycytidylate deaminase in synchronized cultures of chinese hamster cells temperature-sensitive for DNA synthesis. J Cell Physiol. 1976 May;88(1):57–64. doi: 10.1002/jcp.1040880108. [DOI] [PubMed] [Google Scholar]
- Lau Y. F., Kan Y. W. Direct isolation of the functional human thymidine kinase gene with a cosmid shuttle vector. Proc Natl Acad Sci U S A. 1984 Jan;81(2):414–418. doi: 10.1073/pnas.81.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Shimizu K., Zipser D. Isolation and preliminary characterization of the Chinese hamster thymidine kinase gene. Mol Cell Biol. 1983 Oct;3(10):1815–1823. doi: 10.1128/mcb.3.10.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A. Structure and expression of the Chinese hamster thymidine kinase gene. Mol Cell Biol. 1986 Jun;6(6):1998–2010. doi: 10.1128/mcb.6.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys E. J., Crouse G. F., Kellems R. E. Dihydrofolate reductase gene expression in cultured mouse cells is regulated by transcript stabilization in the nucleus. J Cell Biol. 1984 Jul;99(1 Pt 1):180–187. doi: 10.1083/jcb.99.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys E. J., Kellems R. E. Control of dihydrofolate reductase messenger ribonucleic acid production. Mol Cell Biol. 1981 Nov;1(11):961–971. doi: 10.1128/mcb.1.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. F., Lieberman H. B., Yeh D. B., Xu T., Zhao S. Y., Ruddle F. H. Molecular cloning and structural analysis of murine thymidine kinase genomic and cDNA sequences. Mol Cell Biol. 1985 Nov;5(11):3149–3156. doi: 10.1128/mcb.5.11.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. F., Zhao S. Y., Ruddle F. H. Genomic cloning and preliminary characterization of the human thymidine kinase gene. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6528–6532. doi: 10.1073/pnas.80.21.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield J. W. The periodic synthesis of thymidine kinase in mouse fibroblasts. Biochim Biophys Acta. 1966 Feb 21;114(2):398–403. doi: 10.1016/0005-2787(66)90319-4. [DOI] [PubMed] [Google Scholar]
- McKeehan W., Hardesty B. The mechanism of cycloheximide inhibition of protein synthesis in rabbit reticulocytes. Biochem Biophys Res Commun. 1969 Aug 15;36(4):625–630. doi: 10.1016/0006-291x(69)90351-9. [DOI] [PubMed] [Google Scholar]
- Merrill G. F., Hauschka S. D., McKnight S. L. tk Enzyme expression in differentiating muscle cells is regulated through an internal segment of the cellular tk gene. Mol Cell Biol. 1984 Sep;4(9):1777–1784. doi: 10.1128/mcb.4.9.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. L., Zokas L., Schreiber R. D., Verma I. M. Rapid induction of the expression of proto-oncogene fos during human monocytic differentiation. Cell. 1985 Jan;40(1):209–217. doi: 10.1016/0092-8674(85)90324-1. [DOI] [PubMed] [Google Scholar]
- Mittermayer C., Bosselmann R., Bremerskov V. Initiation of DNA synthesis in a system of synchronized L-cells: rhythmicity of thymidine kinase activity. Eur J Biochem. 1968 May;4(4):487–489. doi: 10.1111/j.1432-1033.1968.tb00238.x. [DOI] [PubMed] [Google Scholar]
- Navalgund L. G., Rossana C., Muench A. J., Johnson L. F. Cell cycle regulation of thymidylate synthetase gene expression in cultured mouse fibroblasts. J Biol Chem. 1980 Aug 10;255(15):7386–7390. [PubMed] [Google Scholar]
- Noguchi H., Prem veer Reddy G., Pardee A. B. Rapid incorporation of label from ribonucleoside disphosphates into DNA by a cell-free high molecular weight fraction from animal cell nuclei. Cell. 1983 Feb;32(2):443–451. doi: 10.1016/0092-8674(83)90464-6. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Coppock D. L., Yang H. C. Regulation of cell proliferation at the onset of DNA synthesis. J Cell Sci Suppl. 1986;4:171–180. doi: 10.1242/jcs.1986.supplement_4.11. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Peterson T. A., Prakash L., Prakash S., Osley M. A., Reed S. I. Regulation of CDC9, the Saccharomyces cerevisiae gene that encodes DNA ligase. Mol Cell Biol. 1985 Jan;5(1):226–235. doi: 10.1128/mcb.5.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow P. W., Riddle V. G., Pardee A. B. Synthesis of labile, serum-dependent protein in early G1 controls animal cell growth. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4446–4450. doi: 10.1073/pnas.76.9.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- Schaefer J. P., Wheatley P. J. Structure of anisomycin. J Org Chem. 1968 Jan;33(1):166–169. doi: 10.1021/jo01265a031. [DOI] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadari S., Weissbach A. The interrelation between DNA synthesis and various DNA polymerase activities in synchronized HeLa cells. J Mol Biol. 1974 Jun 15;86(1):11–20. doi: 10.1016/s0022-2836(74)80003-3. [DOI] [PubMed] [Google Scholar]
- Stewart C. J., Ito M., Conrad S. E. Evidence for transcriptional and post-transcriptional control of the cellular thymidine kinase gene. Mol Cell Biol. 1987 Mar;7(3):1156–1163. doi: 10.1128/mcb.7.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart P., Ito M., Stewart C., Conrad S. E. Induction of cellular thymidine kinase occurs at the mRNA level. Mol Cell Biol. 1985 Jun;5(6):1490–1497. doi: 10.1128/mcb.5.6.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubblefield E., Mueller G. C. Thymidine kinase activity in synchronized HeLa cell cultures. Biochem Biophys Res Commun. 1965 Aug 16;20(4):535–538. doi: 10.1016/0006-291x(65)90613-3. [DOI] [PubMed] [Google Scholar]
- Szyf M., Kaplan F., Mann V., Giloh H., Kedar E., Razin A. Cell cycle-dependent regulation of eukaryotic DNA methylase level. J Biol Chem. 1985 Jul 25;260(15):8653–8656. [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Levels of c-myc oncogene mRNA are invariant throughout the cell cycle. 1985 Mar 28-Apr 3Nature. 314(6009):363–366. doi: 10.1038/314363a0. [DOI] [PubMed] [Google Scholar]
- Wu J. S., Johnson L. F. Regulation of dihydrofolate reductase gene transcription in methotrexate-resistant mouse fibroblasts. J Cell Physiol. 1982 Feb;110(2):183–189. doi: 10.1002/jcp.1041100212. [DOI] [PubMed] [Google Scholar]
- Yang H. C., Pardee A. B. Insulin-like growth factor I regulation of transcription and replicating enzyme induction necessary for DNA synthesis. J Cell Physiol. 1986 Jun;127(3):410–416. doi: 10.1002/jcp.1041270309. [DOI] [PubMed] [Google Scholar]
- Yen A., Pardee A. B. Exponential 3T3 cells escape in mid-G1 from their high serum requirement. Exp Cell Res. 1978 Oct 1;116(1):103–113. doi: 10.1016/0014-4827(78)90068-x. [DOI] [PubMed] [Google Scholar]