Abstract
Resveratrol (RSV), a natural compound present in the skin and seeds of red grapes, is considered a phytoestrogen and has structural similarity to the synthetic estrogen diethylstilbestrol. RSV inhibits tumor cell growth in estrogen receptor-positive (ER+) and negative (ER-) breast cancer cell lines resulting in cell specific regulation of the G1/S and G2/M stages of the cell cycle. However apoptotic cell death was only observed in ER+ MCF-7 cells. In this study, we designed and synthesized boronic acid derivative of RSV and evaluated their biological effects on ER+ MCF-7 breast cancer cells. The trans-4 analog inhibited the growth of MCF-7 cells and is not a substrate for p-glycoprotein. The trans-4 analog induces G1 cell cycle arrest, which coincides with marked inhibition of G1 cell cycle proteins and a greater pro-apoptotic effect. Finally, the trans-4 analog had no effect on the estrogen-stimulated growth of MCF-7 cells. Our results demonstrate that the trans-4 analog inhibits MCF-7 breast cancer cells by a different mechanism of action than that of RSV (S-phase arrest), and provides a new class of novel boronic acids of RSV that inhibit breast cancer cell growth.
Keywords: anticancer agent, boronic acid, estrogen receptor positive breast cancer, resveratrol
Introduction
Breast cancer is the second-most common cause of cancer-related death in women in the US; in 2009, this equated to over 207,000 new cases and 39,000 breast cancer deaths.1 Estrogens and the estrogen receptors α and β (ERα and ERβ) play an important role in the development of ~70% of breast cancer.2,3 One approach to inhibiting estrogen-responsive genes is to block the receptors with an antagonist of natural or semi-synthetic origin.4,5 This approach is the basis for endocrine or anti estrogen therapy, which is the most common first-line therapy for women with ER+ breast cancer.
RSV, a natural phytoalexin found in the skin and seeds of red grapes and other food products, has a wide spectrum of biological activities.6-8 Recently, RSV has gained interest in cancer chemoprevention based on its ability to inhibit cellular events associated with cancer initiation, promotion and progression.9-11 Previous studies on the in vitro anti proliferative effects of RSV suggest interactions with multiple molecular targets in several different cancer cells derived from breast, skin, gastric, colon, prostate, and leukemia.12 However, pharmacokinetic studies of RSV in humans conclude that even high doses of RSV might be insufficient to achieve in vivo concentrations that are required for the systemic prevention of cancer.13 This is in part due to low bioavailability and rapid metabolism to sulfate and glucuronide conjugates.13-16 Therefore, several studies have focused on the design and synthesis of novel RSV analogs with more potent antitumor activity and a better pharmacokinetic profile, and this remains an active field in the area of cancer drug discovery.17-20
In ER+ MCF-7 breast cancer cells, 17β-estradiol (E2) induces rapid transcriptional activation of ER and the activation of its target genes. This includes transient upregulation of cyclin D1, which leads to G1/S cell cycle transition.21-23 E2 also induces the anti-apoptotic effects via an increased Bcl2/Bax ratio. Therefore, E2 is likely to contribute to tumor promotion in breast cancer cells through its pro-proliferative and anti-apoptotic effects.
RSV is classified as a phytoestrogen and has structural similarity to the synthetic estrogen diethylstilbestrol and its ability to interact with estrogen receptors α and β (ERα and ERβ) in the micromolar range with an affinity lower than that of estradiol.24 Despite being a weak competitor of estradiol, RSV may act as a cooperative agonist in activating hormone receptor mediated gene transcription. Aside from cooperative agonism, RSV also exerts an anti-estrogen action by triggering the inhibition of estrogen induced cellular pathways associated with tumor proliferation and progression. However in the presence of estrogen (E2), RSV can have mixed agonist-antagonist action with respect to the growth of ER+ cells.25-27 Overall, RSV triggers multiple pathways that may or may not involve ER activation.28,29
Debate continues in regards to the direct molecular mechanism(s) and downstream effectors of RSV. Studies have implicated direct (phosphodiesterase (PDE) enzymes),30 indirect protein pathways (activation of sirtuin SIRT1 through a signaling cascade involving cAMP and AMPK),31 and transcription factors (STAT1).32 Taken together these studies support that multiple molecular targets have a role in RSV’s mechanism(s) of action.
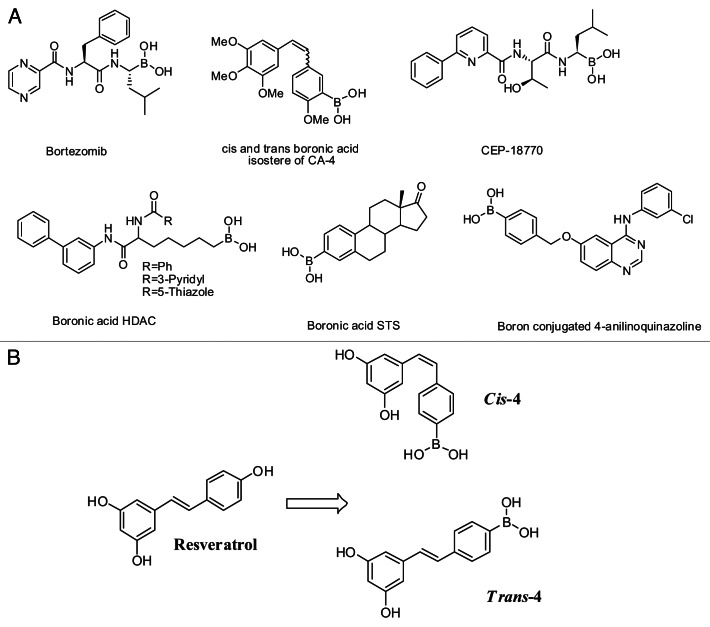
Previously we reported on a boronic acid bioisostere of combrestatin A-4 and chalcone analog of combrestatin A-4 as a potent anti cancer agent.33,34 Additionally, these chemotypes have displayed a variety of biological activities include proteosome inhibition,35 enzyme and protein inhibition,36 antibacterial and antiviral activity.37,38 Since boronic acid compounds have unique structural features, in this study we utilized this approach to synthesize both cis-and trans-novel boronic acid derivatives of RSV (Fig. 1B) and studied their effects on ER+ MCF-7 human breast cancer cells.
Figure 1. (A) Structures of known boronic acid compounds and (B) Design of boronic acid analogs of resveratrol: cis-4 and trans-4.
Results
Chemistry
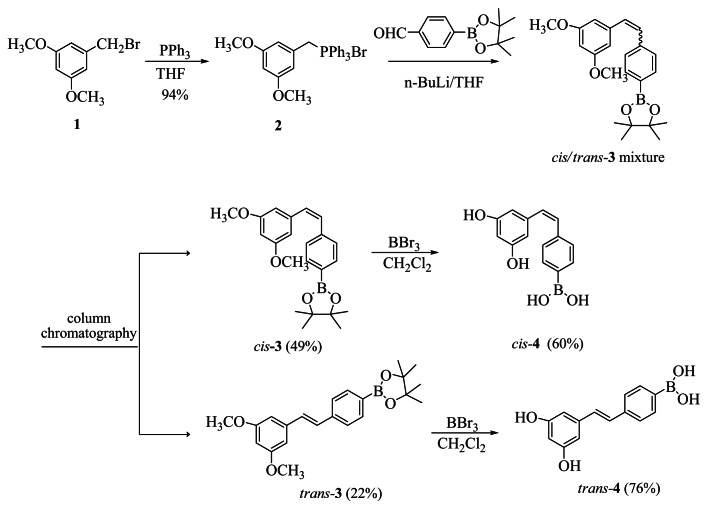
The strategy used to synthesize the boronic acid resveratrol analogs cis-4 and trans-4 is outlined in (Fig. 2). Briefly, 5-(bromomethyl)-1, 3-dimethoxybenzene was treated with triphenylphosphine to yield the phosphonium salt compound 2 in a 94% yield. This was followed by a Wittig coupling of compound 2 with 4-formylphenyl boronic acid pinacol ester in the presence of n-BuLi and resulted in a mixture of stilbenes 3 (in a 2:1 cis/trans ratio). The cis/trans mixture was purified and isomers were separated by flash column chromatography. Conversion of the separated cis-3 and trans-3 isomers to the final cis-4 and trans-4 boronic acid products was accomplished by treating cis-3 and trans-3 independently with borontribromide to afford the fully deprotected products cis-4 and trans-4. The purity of all the final target compounds was confirmed by reverse phase HPLC analysis using two different mobile phases (A: 10 - 40% acetonitrile in H2O(v/v), flow rate at 1 mL/min over 20 min; method B, 8% - 40% methanol in H2O(v/v), flow rate at 1 mL/min over 20 min).
Figure 2. Synthetic scheme of cis-4 and trans-4.
Biologic effect of trans-4 on MCF-7 human breast cancer cell growth inhibition
To examine the anti-proliferative effect of newly synthesized compounds, we first compared the effects of cis-4 and trans-4 to that of RSV on the growth of ER+ MCF-7 human breast cancer cells using the WST-1 assay. As shown in Table 1, the trans-4 analog has enhanced anti-proliferative activity on MCF-7 cell growth in a time and concentrated-dependent manner, with GI50 values of 36.6 µM ± 0.06 for 48 h and 31.1 µM ± 0.05 for 72 h. In contrast, the GI50 is nearly > 100 µM for trans-RSV and the cis-4 analog at 48 and 72 h (Table 1). These results are also consistent with studies published by Joe, AK et al.39, in which the GI50 for RSV is also > 100 µM in MCF-7 cells. Our study shows that trans-4 is a more potent inhibitor of cell proliferation than RSV, warranting further examination of its mechanism of action. To determine whether the cell growth inhibition of trans-4 require the ER, we performed a similar experiment in the ER-cell line MDA-MB-231. As shown in Figure S1 the GI50 of trans-4 is 46.0 µM ± 1.23 for 72 h supporting that the improved potency of trans-4 is independent of ER expression. Importantly, trans-4 has preferential toxicity toward cancer cells. As shown in Figure S2, the trans-4 has at least 2-fold lesser toxicity in immortalized “normal” human breast epithelial cells compare with normal cells.
Table 1. Effects of cis-4, trans-4 or RSV on growth inhibition of the ER+, estrogen-dependent human breast cancer MCF-7 cell line.
| MCF-7 Cell growth inhibition (WST-1 assay, G150, µM) |
|||
|---|---|---|---|
| Compound | 48 h | 72 h | |
| trans-4 |
36.6 ± 0.06 mM |
31.1 ± 0.05 mM |
|
| cis-4 |
155.8 ± 0.10 mM |
173.8 ± 0.10 mM |
|
| RSV | 97.7 ± 0.10 mM | 105.2 ± 0.09 mM | |
Note: cis-4, trans-4 and RSV were tested at various concentrations for effects on cell growth inhibition of MCF-7 breast cancer cells. Cell growth inhibition was estimated 48 and 72 h after the addition of each compound using the WST-1 reduction assay. The GI50 value (the concentration yielding 50% growth inhibition) was interpolated from the graph of the log of compound concentration vs. the fraction of surviving cells. The GI50 was calculated using Graph Pad Prism. Data are expressed as mean (± SEM) of triplicate experiments.
Growth inhibitory effects of trans-4 on a multidrug resistant variant of MCF-7 cells
During chemotherapy, breast tumor cells commonly develop resistance40 through a phenomenon known as multidrug resistance (MDR). Overexpression of P glycoprotein 170 (Pgp-170, the product of the MDR1 gene) is one of the most common causes of MDR and is expressed in many types of cancer including breast and ovary.41 We evaluated trans-4 for effects on the growth of CL10.3 cells, a derivative of MCF-7 that overexpresses the P-glycoprotein,42 to assess its susceptibility to MDR. As shown in Table 2, trans-4 is equipotent in CL 10.3 and MCF-7 cells. This is in contrast to paclitaxel, a known substrate of MDR1,43 which strongly inhibits MCF-7 cell growth but has no effect on CL 10.3 cells. These results clearly demonstrate that trans-4 is effective in multidrug resistant breast cancer cells and therefore may have potential therapeutic utility in this important clinical context.
Table 2. Effects of trans-4 or paclitaxel on the growth inhibition of multi-drug resistant breast cancer cell lines.
| CL 10.3 Cell growth inhibition (WST-1 assay, GI50, µM 72 h) |
||||
|---|---|---|---|---|
| Cell line | Origin | trans-4 | Paclitaxel | |
| MCF-7 |
breast |
31.10 ± 0.05 mM |
0.002 ± 1.12 mM |
|
| CL 10.3 |
breast (MDR) |
49.09 ± 0.001 mM |
> 50 mM |
|
| MDR GI50/non-MDF GI50 | 1.57 mM | > 25,000 mM | ||
Note:trans-4 or Paclitaxel were tested at various concentrations for effects on cell growth inhibition of breast cancer cells. Cell growth inhibition was estimated 72 h after the addition of each compound using the WST-1 reduction assay. The GI50 value (the concentration yielding 50% growth inhibition) was interpolated from the graph of the log of compound concentration vs. the fraction of surviving cells. The GI50 was calculated using Graph Pad. Data are expressed as mean (± SEM) of triplicate experiments.
Effect of trans-4 on cell cycle distribution in MCF-7 cells
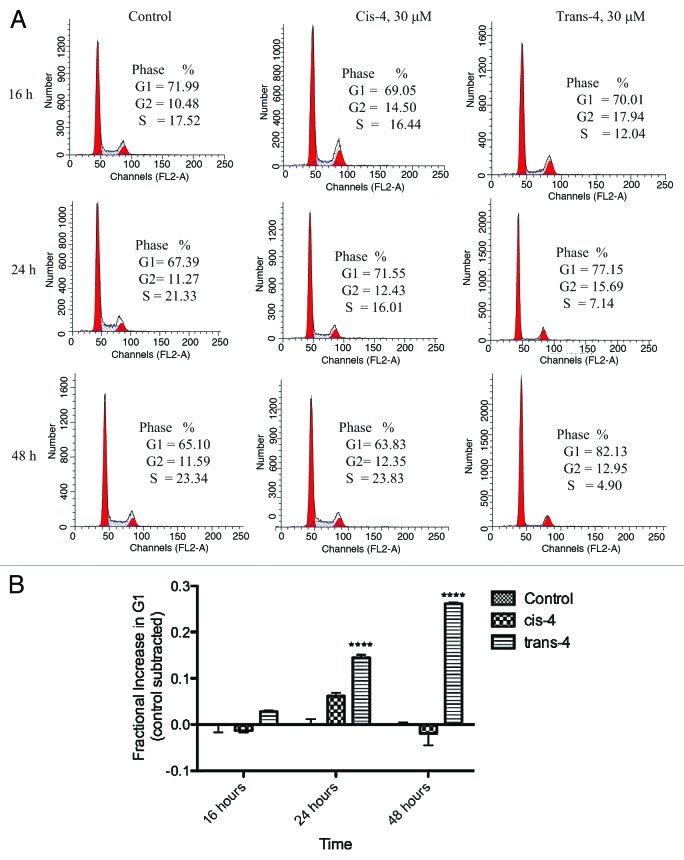
To determine whether the growth inhibitory effect of trans-4 occurs through blockade of the cell cycle, we analyzed the DNA content in each phase of the cell cycle by flow cytometry. MCF-7 cells treated with DMSO (vehicle control), cis-4 and trans-4 for 16, 24, or 48 h were subjected to flow cytometric analysis. As shown in Figure 3A, trans-4 induced a time-dependent accumulation of cells in the G1 compartment relative to vehicle control. The G1 phase accumulation was paralleled by a marked reduction in the percentage of cells in the S phase. Trans-4 at 30 µM reached the highest level of G1 arrest with 26% more cells in G1 as compared with control at 48 h (Fig. 3B). These data suggest trans-4 blocks MCF-7 cell cycle progression in the G1 phase, which likely contributes to its growth inhibition effects.
Figure 3. A. Effect of compounds on cell cycle distribution in MCF-7 breast cancer cell lines. Asynchronous MCF-7 cells were treated with 30 µM of cis-4, trans-4, or vehicle control for different time intervals (16, 24, and 48 h), fixed in 70% ethanol and DNA content was determined by labeling with PI followed by flow cytometric analysis. B. Fraction of compound treated cells with trans-4 in G1 relative to control-treated cells. Results are represented as a mean of triplicate samples.
Irreversible effect of trans-4 on MCF-7 cell growth inhibition
Because trans-4 was a more potent inhibitor of MCF-7 cell growth as compared with RSV, we examined whether the growth inhibition was irreversible or reversible. Toward this end, we treated cells with trans-4 under three different conditions as described in the materials and methods using the WST-1 assay. As shown in Figure 4, the GI50 for trans-4 after 48 h of treatment is 45 μM (method 1). Treatment of cells with trans-4 for 48 h followed by recovery in fresh media without trans-4 for an additional 48 h (method 2) gave a GI50 of 22 μM which is 2-fold lower than method 1. The effects were selective to cancer cells as compared with normal cells (Fig. S2) indicate that the initial anti-proliferative effect of trans-4 in MCF-7 cells is maintained, or even enhanced, in the absence of drug, i.e., irreversible. The irreversible anti-proliferative effect of trans-4 was further confirmed by FACS analysis by measuring the percentage of DNA in each cell cycle phase (Fig. S3). In addition, treatment of cells with trans-4 for 72 h with media changes (containing trans-4) after every 24 h (method 3) gave a GI50 of 10 μM. This result suggests that sequential dosing with trans-4 enhances its effect on growth inhibition.
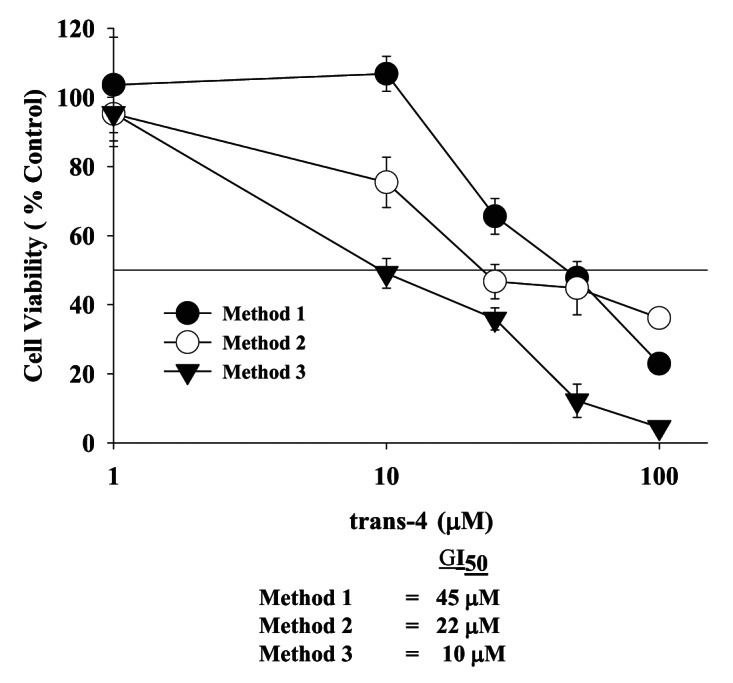
Figure 4. Irreversible of trans-4 on MCF-7 breast cancer cell growth inhibition under different conditions. After 24 h post incubation, cells were treated with increasing concentrations of trans-4 (1–100 µM) under three different conditions. In the first method, cells were exposed for 48 h to trans-4. In the second method cells were exposed to trans-4 for 48 h, washed with serum free media and cultured for additional 48 h without compound. In the third method, cells were treated every 24 h to fresh media with trans-4 for 72 h. Cells were harvested at the indicated times and analyze for cell growth inhibition by the WST-1 method. Values represent the mean ± SD of triplicate wells. The experiment was repeated thrice with identical results.
Effect of trans-4 on the expression level of G1 cell cycle proteins in MCF-7 cells
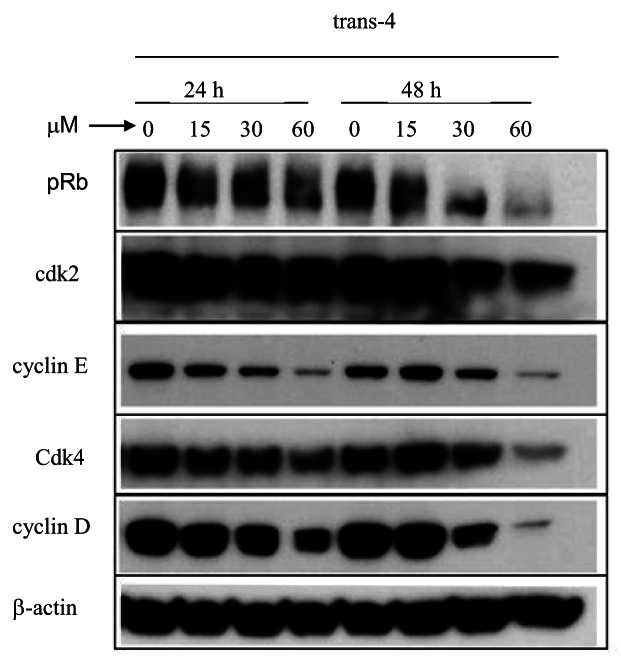
We next investigated whether the cell cycle arrest in the G1 phase induced by trans-4 was related to the expression of G1 cell cycle positive regulatory proteins that control the G1-to-S transition; these include cyclin D1 and cyclin E, their associated cyclin-dependent kinases (cdk4, cdk2), and the phosphorylation state of pRb. MCF-7 cells were treated with the indicated concentrations of trans-4 for 24 and 48 h, and then harvested for immunoblotting. As shown in Figure 5, trans-4 decreases the expression level of cdk4, cdk2, cyclin E, cyclin D1 and pRb, which are responsible for cell cycle progression early in the G1 phase, with the greatest effect observed at 48 h exposure. This downregulation of G1-S positive regulatory proteins correlates with the observed accumulation of cells in G1 phase in trans-4 treated MCF-7 cells.
Figure 5. Effect of trans-4 on the expression of G1-S transition regulatory proteins. MCF-7 cells were treated with the indicated concentrations of trans-4 for 24 and 48 h. Cell lysates were subjected to immunoblotting to detect cyclin D1, cdk4, cyclin E, cdk2 and pRb proteins by their specific antibodies. Human -actin was used as a loading control. Twenty-micrograms of lysate was used for each experimental condition.
Apoptotic changes in MCF-7 cells in response to trans-4
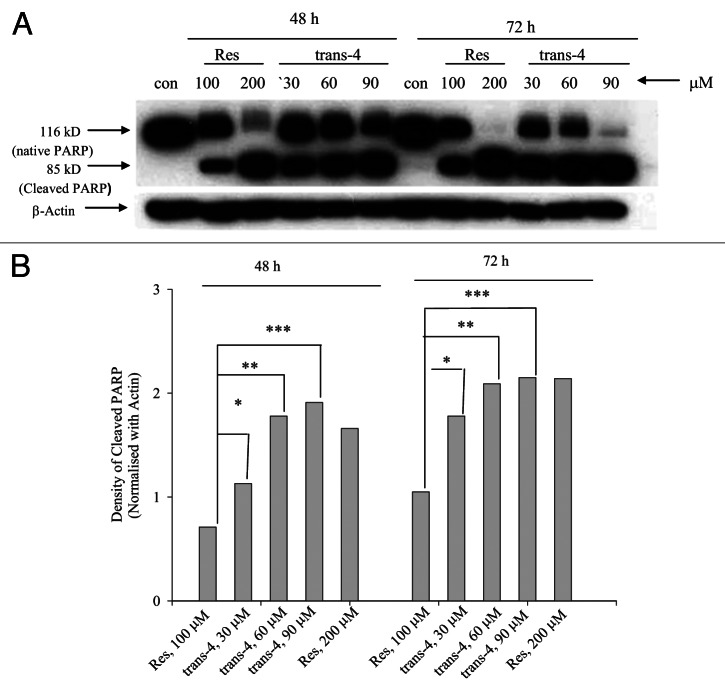
To characterize whether trans-4 could induce apoptotic cell death in MCF-7 cells, we examined cleavage of the caspase substrate PARP by immunoblotting. As shown in Figure 6A and B cells treated with 30 µM trans-4 showed robust expression of the 85-kd a cleavage fragment of PARP. This band is more intense in trans-4 treated MCF7 cells compare with RSV, which included similar levels of PARP cleavage at 200 µM. These results demonstrate that the stronger anti-proliferative effect of trans-4 is also associated with a more potent induction of apoptosis when compared with RSV.
Figure 6. western blot analysis of PARP cleavage. (A) MCF7 cells were treated with trans-4 for 48 and 72 h and equal amounts of cell lysate were resolved using SDS-PAGE and analyzed by immunoblot using anti-PARP antibody. The blots were re-probed with anti -actin antibody to confirm equal protein loading. (B) Comparison of density of cleaved PARP band (normalized with actin) of trans-4 and resveratrol were determined by densitometry NIH image analysis.
Effect of trans-4 on E2-mediated stimulation of MCF-7 cell growth
The 4’ hydroxyl group of RSV mimics E2, which activates ER and stimulates down-stream signaling pathways in ER+ breast cancer cell lines.25,26 However, RSV can have mixed agonist/antagonist activity toward E2-mediated MCF-7 cell growth. We therefore examined the effect of trans-4 on E2-mediated cell growth to check trans-4 action against ER and determine whether it acts as an agonist or antagonist. MCF-7 cells were seeded in a phenol-red free cell culture media and after 24 h, cells were treated with the indicated concentrations of RSV or trans-4 in the presence of a constant E2 concentration (10-9M). As shown in Figure 7A, trans-4 does not have any effect on E2induced cell growth and therefore appears to have neither agonist nor antagonist activity toward ER activity in MCF-7 cells. In contrast, RSV antagonizes E2-mediated MCF-7 cell growth (Fig. 7B). These results convincingly demonstrate that, due to structural alteration, trans-4 boronic acid utilizes a different mechanism to inhibit MCF-7 cell growth which is independent of the ER.
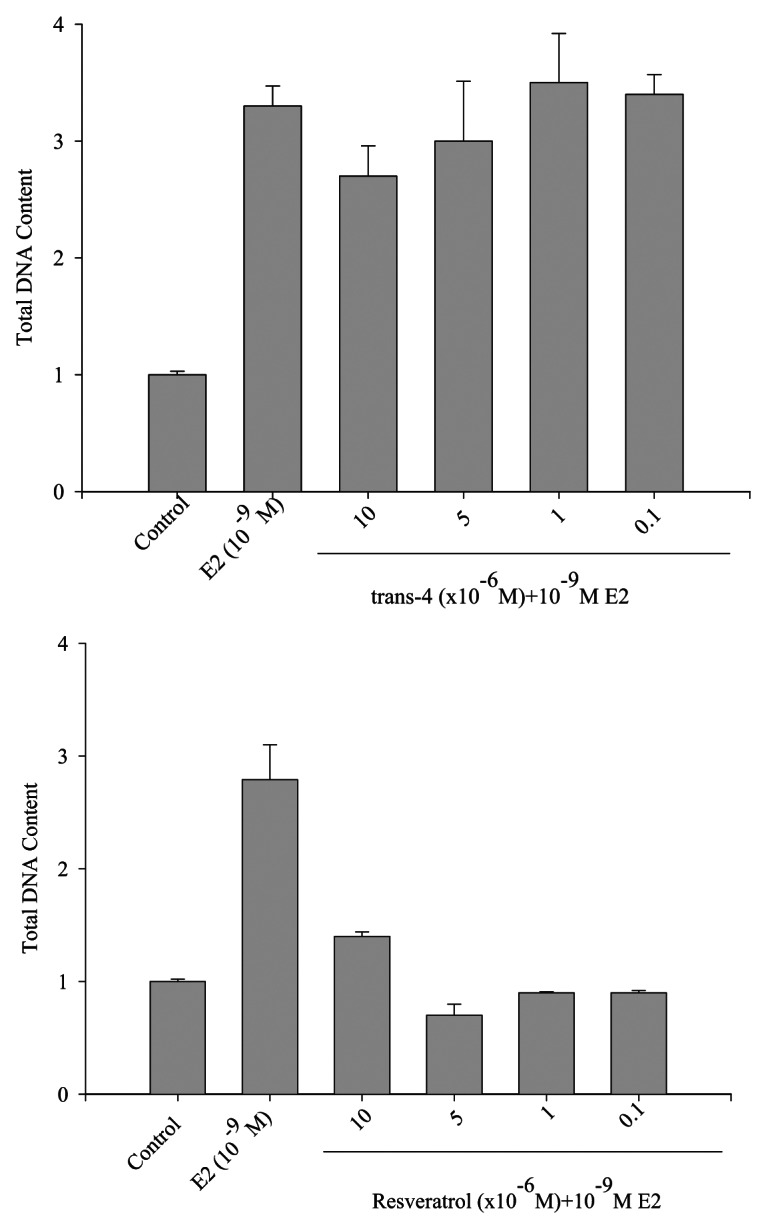
Figure 7. Effect of trans-4 or RSV on E2-mediated MCF-7 cell growth. MCF-7 cells were treated with estradiol (E2, 10-9M) alone or in combination with the indicated concentrations of trans-4 or RSV. After 5 d incubation, the DNA content of the treated cells was measured using a DNA fluorescence kit (BioRad # 170–2480). Results are shown as the mean of triplicate samples ± SD. Repeated the experiment in twice with identical results.
Discussion
RSV, a naturally occurring phytoalexin present in the skin and seeds of red grapes and other medicinal plants, has received broad attention due to its potential as a chemotherapeutic and cancer prevention agent.9,10 Previously RSV has been shown to have growth inhibitory effects in different human cancer cell lines of both hematological and epithelial origin including breast, colorectal, leukemia, and epidermoid carcinoma. However depending on concentrations of RSV, it behaves as estrogenic/anti-estrogenic effect in ER+ and ER- breast cancer cells and involved in growth inhibition by different target in cell proliferation and apoptotic pathways.28 Therefore the ability of RSV to interact with ER and mechanism of tumor growth inhibition in breast cancer cells is not conclusive. Due to these conflicting data, much attention has been focused on the design and synthesis of novel structural analogs of RSV that have more activity than RSV at inhibiting cancer cell growth.
Previously we have shown that a set of CA-4 and chalcone derivatives of CA-4 containing a boronic acid modification have potent anti-proliferative effects.33,34 In the present study, we designed and synthesize the boronic acid biomimetics of RSV by replacing the 4’-OH with to boronic acid resulting in the two analogs shown in Figure 1B. Trans-4 has enhanced activity in inhibiting MCF-7 cell growth compared with RSV (Table 1). Previous studies of RSV on MCF-7 cell growth inhibition were accompanied by S-phase cell cycle arrest at much higher doses (300 µM).39 This also raises the possibility that growth inhibition by trans-4 might also be attributed to cell cycle disruption. We observed that trans-4 induced a G1 cell cycle phase arrest at 30 µM in a time dependent manner as shown in Figure 3. This different mechanism (G1 vs S phase arrest) might be the underlying cause for the more potent cell inhibitory effect induced by trans-4 as compared with RSV. The G1/S transition is a key regulatory point where a cell decides whether or not to enter into DNA replication (S phase).44 Hence, molecules that inhibit the G1/S phase transition often have potential therapeutic significance. Importantly, trans-4 also showed an irreversible growth inhibitory effect (Fig. 4).
The growth inhibitory action of trans-4 (Table 2) and RSV (Fig. S4) were also assessed on MDR human breast cancer cells (CL 10.3, derived from MCF-7 cells transfected with the human mdr 1 gene). Trans-4 possessed significant inhibitory activity against the MDR cell line and is likely not a substrate for P-glycoprotein. Inhibition of the expression level of G1 phase regulatory proteins (cyclins D1 and E, CDK2 and CDK4, pRb) in MCF-7 cells (Fig. 5), further supports the idea that trans-4 acts by halting cell division at the G1/S check point. Treatment of cancer cells with the trans-4 also strongly induces apoptosis as measured by the 85 Kd cleaved PARP fragment. This effect occurred at a much lower concentration than RSV (Fig. 6). Finally, it appears that the molecular mechanism of trans-4 action does not involve the ER or the inhibition of E2-induced cell growth (Fig. 7).
In summary, we have designed and synthesized a new class of boronic acid biomimetics of RSV that have enhanced growth inhibition with G1 cell cycle arrest and pro-apoptotic capabilities in MCF-7 cells. Furthermore, treatment of MCF-7 cells with trans-4 resulted in irreversible growth inhibition. These promising results support the trans-4 analog for the continued investigation of preclinical toxicity and efficacy.
Methods and Materials
General Methods
NMR spectra were recorded using a Varian-400 spectrometer for 1H (400 MHz) and 13C (100 MHz). Chemical shifts (δ) are given in ppm downfield from tetramethylsilane, as internal standard, and coupling constants (J-values) are in hertz (Hz). Purifications by flash chromatography were performed. Analytical high pressure liquid chromatography (HPLC) and liquid chromatography/ mass spectrometry (LC/MS) analyses were conducted using Shimadzu LC-20AD pumps and a SPD20A UV-vis detector. Reverse phase HPLC was performed on Restek’s Ultra IBD C18 (5 μm, 4.6 × 50 mm) using two Shimadzu LC-20AD pumps and a SPD-20A-vis detector set at 330 nm: Method A, 10–40% acetonitrile in H2O (v/v), flow rate at 1 mL/min over 20 min; Method B, 8–40% methanol in H2O (v/v), flow rate at 1 mL/min over 20 min. High-resolution mass spectra (HRMS) were recorded on a QSTAR Elite mass spectrometer.
Chemical synthesis
3,5-Dimethoxybenzyltriphenylphosphonium bromide (2)
Triphenylphosphine (6.5 g, 24.7 mmol) was added to a solution of 5-(bromomethyl)-1, 3-dimethoxybenzene (4.4 g, 19.0 mmol) in dry THF (30 ml). The mixture was refluxed with stirring for 24 h. The resulting white solid was filtered and washed with ether/hexane to afford a white solid 2 (8.8 g, 94%). 1H NMR (400 MHz, CDCl3) 8 7.70 (m, 9H), 7.59 (m, 6H), 6.29 (m, 2H), 6.23 (m, 1H), 5.22 (d, 2H, J = 14.4), 3.46 (s, 6H).
Z-2[4-(3,5-dimethyoxystyryl)phenyl]-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (Cis-3)
Phosphonium bromide 2 (3.0 g, 6.08 mmol) was suspended in dry THF and cooled to -78°C. n-BuLi (3.8 ml, 6.08 mmol, 1.6 M in hexane) was added slowly with stirring. The mixture was stirred at -78°C for 3 h, and 4-formylphenyl boronic acid pinacol ester (1.41 g, 6.08 mmol) in 5 ml THF was added dropwise. The reaction temperature was maintained at -78°C for another hour, and the mixture was warmed to room temperature. The reaction mixture was stirred overnight. The mixture was poured into saturated NH4Cl (20 ml) and extracted with EtOAc. The extracts were combined, washed with brine and dried over Na2SO4. The organic layer was filtered and rotary concentrated to give the crude mixture of cis/trans stilbene 3. Flash column chromatography using 5% EtOAc/ hexane eluted the cis-stilbene 3 (Rf = 0.52, Hex / EtOAc = 5 / 1) as a white semi solid (1.1 g, 49%). 1H NMR (400 MHz, CDCl3): 8 7.74 (d, 2H, J = 8.0 Hz), 7.32 (d, 2H, J = 8.4 Hz), 6.62 (d, 1H, J = 12.4 Hz), 6.56 (d, 1H, J = 12.4 Hz), 6.44 (m, 2H)., 6.34 (m, 1H), 3.63 (s, 6H), 1.35 (s, 12H).
E-2[4-(3,5-dimethyoxystyryl)phenyl]-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (Trans-3)
Flash column as described above yielded stilbene trans-3 (Rf = 0.49, Hex / EtOAc = 5 / 1) as a pale yellow semi solid (0.5 g, 22%). 1H NMR (400 MHz, CDCl3): 8 7.82 (d, 2H, J = 8.0), 7.51 (d, 2H, J = 8.0 Hz), 7.10 (dd, 2H, J = 16.8, 16.8 Hz), 6.68 (m, 2H). 6.72 (m, 1H), 3.82 (s, 6H), 1.36 (s, 12H).
(Z)-4-(3,5-dihydroxystyryl)phenylboronic acid (Cis-4)
Cis-3 (0.4 g, 1.09 mmol) was dissolved in dry CH2Cl2 (5 ml) and cooled to -78°C. BBr3 (10 ml, 10 mmol, 1.0 M in CHCl2) was added dropwise. The resulting mixture was stirred at -78°C for another 1.5 h, then warmed to room temperature and stirred overnight. The reaction was quenched with H2O (10 ml). Concentrated and applied to flash column chromatography using 10% CH2Cl2/ MeOH eluted the cis-4 (Rf = 0.5, CHCl2 / MeOH = 9 / 1) as a soft solid (0.16 g, 60%). 1H NMR (400 MHz, DMSO-d6): 8 9.11 (s, 2H), 7.97 (s, 2H), 7.62 (d, 2H, J = 8.4), 7.17 (d, 2H, J = 8.0 Hz), 6.45 (dd, 2H, J = 12.4, 12.8 Hz), 6.05 (m, 3H) HPLC: Method A, retention time = 13.28 min; Method B, retention time = 11.32 min; HRMS: 257.1059 (MH+). The 1H NMR spectrum (Fig. S5) for cis-4 is provided.
(E)-4-(3,5-dihydroxystyryl)phenylboronic acid (Trans-4)
Trans-4 was obtained in 76% yield (Rf = 0.4, CHCl2 / MeOH = 9/1) as a semi-solid from the trans-3 following the same procedure as described above. 1H NMR (400 MHz, DMSO-d6) 8 9.22 (s, 2H), 7.98 (s, 2H), 7.74 (d, 2H, J = 8.0), 7.50 (d, 2H, J = 8.0 Hz), 7.03 (dd, 2H, J = 16.4, 16.4 Hz), 6.41 (m, 1H) Anal. Calcd for C14H13BO4.0.5 H2O: C, 63.38; H, 5.28. Found: C, 63.30, H, 5.07. HPLC: Method A, retention time = 11.10 min; Method B, retention time = 8.54 min; HRMS: 256.9723 (MH+). The HPLC chromatogram (Fig. S6) and 1H NMR spectrum (Fig. S7) for trans-4 are provided.
Biological assays cell lines and culture
The human breast cell lines MCF-7 (HTB-22), MDA-MB-231 (HTB-26) and MCF-10A were provided by the tissue culture core facility at Lombardi Comprehensive Cancer Center, Georgetown University Medical Center. MCF-7MDR (CL 10.3) cells were a gift from Dr. Robert Clarke from the Georgetown University Medical Center.42 MCF-7 and CL 10.3 cells were maintained in DMEM (Gibco, 12491) supplemented with 10% heat inactivated fetal bovine serum (FBS) (Hyclone, SH30396.03), 2 mM L-glutamine (Gibco, 25030), and 50 µg/mL each of antibiotics, namely penicillin, streptomycin, and neomycin at 37°C in a humidified incubator containing 5% CO2. MCF-10A were maintained in Dulbecco's modified Eagle's medium-F12 (DMEM/F12) (Invitrogen, 21041025) supplemented with 5% horse serum (Gibco, 16050), 1% penicillin/streptomycin (Gibco, 15140), 0.5 µg/ml hydrocortisone (Sigma, H6909), 10 µg/ml insulin (Sigma, 15500), and 20 ng/ml recombinant human EGF (Gibco, PHG0311).
Cell growth assay (WST-1 assay)
The effect of the cis-4 and trans-4 analogs RSV on cell growth was determined by WST-1 assay. Briefly, cells were seeded into a 96-well plate at 3,500 cells per well in DMEM containing 10% FBS. After overnight incubation, cells were treated with the compounds (1–100 µM) for 48 and 72 h at 37°C. Control cells were treated with an equal amount of DMSO. After the indicated incubation time, cell viability was measured by WST-1 assay according to the manufacturer’s instructions (Roche). Briefly, 20 µL of WST-1 solution was added in each well and incubated for 2–4 h. The water-soluble tetrazolium salt of WST-1 is converted into orange formazan by dehydrogenase in the mitochondria of living cells. Formazan absorbance, which correlates to the number of living cells, was recorded at wavelengths of 450 nm and 630 nm using a microplate reader (Ultramark, Microplate Imaging System, Bio-Rad). The GI50 was calculated from the graph of the log of compound concentration vs. the fraction of the surviving cells.
Cell cycle analysis
The effect of cis-4 and trans-4 on cell cycle progression was analyzed by flow cytometry. Cells were seeded in 6-well plates and treated with 30 µM of cis-4 and trans-4 at different time intervals (16, 24, and 48 h). Cells were trypsinized, centrifuged at 2000 rpm and cell pellets were collected. Pellets were washed with 1X PBS, permeabilized with 70% (v/v) ethanol, re-suspended in 1 ml of PBS containing 1 mg/ml RNase and 50 mg/ml propidium iodide, incubated in the dark for 30 min at room temperature, and analyzed by a FACSort Flow Cytometer (Becton Dickinson). The cell cycle distribution was evaluated on DNA plots using the Modfit software (Verity softwarehouse).
Western blot analysis
Western blotting was performed as previously published.45 In brief, cell pellets were collected at the indicated times after treatment with compounds, suspended in 100 µL of lysis buffer (50 mM TRIS-HCl, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 20 mM NaF, 100 mM Na3VO4, 0.5% NP-40, 1% Triton X-100, 1 mM PMSF, 5 µg/ml aprotinin, 5 µg/ml leupeptin), vortexed twice and incubated in an ice bath for 30 min. Lysates were cleared by centrifugation at 12000 rpm for 15 min at 4°C and protein was estimated by detergent compatible BCA protein assay kit (Pierce). Equivalent amounts of total proteins were resolved by SDS-PAGE (10%) and transferred to PVDF membranes. Membranes were blocked by 5% non-fat powdered milk in TBST overnight. Membranes were incubated with the indicated primary antibodies (Rabbit polyclonal antibodies Cdk2 (SC-163) and Cdk4 (SC-260), mouse monoclonal Cyclin E was obtained from Santa Cruz, mouse monoclonal Cyclin D was obtained from Santa Cruz, mouse monoclonal pRb was obtained from BD PharMingen, Anti–actin was obtained from Sigma) for 2 h followed by HRP-conjugated secondary antibodies for 1 h and developed using enhanced chemiluminescence (Perkin Elmer). For pRb protein, we used 6% acrylamide SDS-PAGE.
Cell growth assay (reversible/irreversible) for trans-4
To determine whether the effect of trans-4 on growth inhibition was reversible or irreversible, MCF-7 cells were treated with trans-4 under three different conditions. In method 1, cells were treated with trans-4 continuously for 48 h. In Method 2, cells were treated with trans-4 for 48 h and incubated further in fresh media without trans-4 for an additional 48 h (a total of 96 h). In method 3, cells were treated with trans-4 for 72 h with a change in media containing fresh trans-4 after every 24 h. Cell viability was measured at the indicated times by WST-1 assay according to the above-mentioned protocol.
Measuring the DNA content in estrogen mediated MCF-7 cell growth
To examine the effect of resveratrol or trans-4 on E2-mediated MCF-7 cell growth, MCF-7 cells were cultured in estrogen-depleted media (phenol-red free modified Eagle’s medium supplemented with 10% charcoal stripped FBS) for 4 d, changing the media every 24 h. Cells were then seeded in 24 well plates at 10,000 cells per well in 1 ml of estrogen-depleted media. After 24 h incubation, media were changed and contained the indicated concentration of RSV, trans-4, E2 alone, or combinations of RSV with E2 or trans-4 with E2. Cells were incubated at 37°C for an additional 4 d. Media were removed and cells were frozen at -78°C overnight and the DNA content in each well was measured according to the manufacturer’s protocol (DNA Quantification Kit, Bio-Rad). Results are represented as mean of triplicate samples.
Test compound
RSV was purchased from Sigma (St. Louis, MO) and the cis and trans boronic acid derivatives of RSV were prepared as described in the chemical synthesis section. Compounds were dissolved in DMSO at a 50 mM stock concentration, stored at –20oC, and diluted in serum-free medium immediately before use.
Statistical analysis
All experiments were repeated three times, and the data expressed as mean ± standard deviation (SD). Two-tailed student t test was used for statistical analysis of the data. A p < 0.05 was taken as the level of significance.
Supplementary Material
Disclosure of Potential Conflicts of Interest
A patent application has been filed by Georgetown University on behalf of the inventors that are listed as authors in this article.
Acknowledgments
We thank Dr. Karen Creswell for flow cytometry support. Flow Cytometry, Microscopy and Imaging, and Tissue Culture Shared Resources are supported by P30-CA051008–18 (LCCC Cancer Center Support Grant). We sincerely thank Dr. Youhong Wang, Department of Oncology, Georgetown University Medical Center, for the generous gift of the pRb antibody. Financial support was provided by the Drug Discovery Program at Georgetown University Medical Center.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/20845
References
- 1.Jemal A, Siegel R, Xu J, Ward E, Thun MJ. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Service RF. New role for estrogen in cancer? Science. 1998;279:1631–3. doi: 10.1126/science.279.5357.1631. [DOI] [PubMed] [Google Scholar]
- 3.Sommer S, Fuqua SA. Estrogen receptor and breast cancer. Semin Cancer Biol. 2001;11:339–52. doi: 10.1006/scbi.2001.0389. [DOI] [PubMed] [Google Scholar]
- 4.Jordan VC. A century of deciphering the control mechanisms of sex steroid action in breast and prostate cancer: the origins of targeted therapy and chemoprevention. Cancer Res. 2009;69:1243–54. doi: 10.1158/0008-5472.CAN-09-0029. [DOI] [PubMed] [Google Scholar]
- 5.Peng J, Sengupta S, Jordan VC. Potential of selective estrogen receptor modulators as treatments and preventives of breast cancer. Anticancer Agents Med Chem. 2009;9:481–99. doi: 10.2174/187152009788451833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goswami SK, Das DK. Resveratrol and chemoprevention. Cancer Lett. 2009;284:1–6. doi: 10.1016/j.canlet.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 7.Leifert WR, Abeywardena MY. Cardioprotective actions of grape polyphenols. Nutr Res. 2008;28:729–37. doi: 10.1016/j.nutres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Gautam R, Jachak SM. Recent developments in anti-inflammatory natural products. Med Res Rev. 2009;29:767–820. doi: 10.1002/med.20156. [DOI] [PubMed] [Google Scholar]
- 9.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 10.Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 2008;269:243–61. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 11.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 12.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, et al. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–83. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–52. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 14.Niles RM, Cook CP, Meadows GG, Fu YM, McLaughlin JL, Rankin GO. Resveratrol is rapidly metabolized in athymic (nu/nu) mice and does not inhibit human melanoma xenograft tumor growth. J Nutr. 2006;136:2542–6. doi: 10.1093/jn/136.10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr., Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–82. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 16.Wenzel E, Soldo T, Erbersdobler H, Somoza V. Bioactivity and metabolism of trans-resveratrol orally administered to Wistar rats. Mol Nutr Food Res. 2005;49:482–94. doi: 10.1002/mnfr.200500003. [DOI] [PubMed] [Google Scholar]
- 17.Pan MH, Lin CL, Tsai JH, Ho CT, Chen WJ. 3,5,3′,4′,5′-pentamethoxystilbene (MR-5), a synthetically methoxylated analogue of resveratrol, inhibits growth and induces G1 cell cycle arrest of human breast carcinoma MCF-7 cells. J Agric Food Chem. 2010;58:226–34. doi: 10.1021/jf903067g. [DOI] [PubMed] [Google Scholar]
- 18.Ma Z, Molavi O, Haddadi A, Lai R, Gossage RA, Lavasanifar A. Resveratrol analog trans 3,4,5,4′-tetramethoxystilbene (DMU-212) mediates anti-tumor effects via mechanism different from that of resveratrol. Cancer Chemother Pharmacol. 2008;63:27–35. doi: 10.1007/s00280-008-0704-z. [DOI] [PubMed] [Google Scholar]
- 19.Heynekamp JJ, Weber WM, Hunsaker LA, Gonzales AM, Orlando RA, Deck LM, et al. Substituted trans-stilbenes, including analogues of the natural product resveratrol, inhibit the human tumor necrosis factor alpha-induced activation of transcription factor nuclear factor kappaB. J Med Chem. 2006;49:7182–9. doi: 10.1021/jm060630x. [DOI] [PubMed] [Google Scholar]
- 20.Minutolo F, Sala G, Bagnacani A, Bertini S, Carboni I, Placanica G, et al. Synthesis of a resveratrol analogue with high ceramide-mediated proapoptotic activity on human breast cancer cells. J Med Chem. 2005;48:6783–6. doi: 10.1021/jm050528k. [DOI] [PubMed] [Google Scholar]
- 21.Altucci L, Addeo R, Cicatiello L, Dauvois S, Parker MG, Truss M, et al. 17beta-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G(1)-arrested human breast cancer cells. Oncogene. 1996;12:2315–24. [PubMed] [Google Scholar]
- 22.Prall OW, Sarcevic B, Musgrove EA, Watts CK, Sutherland RL. Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased cyclin D1 expression and decreased cyclin-dependent kinase inhibitor association with cyclin E-Cdk2. J Biol Chem. 1997;272:10882–94. doi: 10.1074/jbc.272.16.10882. [DOI] [PubMed] [Google Scholar]
- 23.Sakamoto T, Horiguchi H, Oguma E, Kayama F. Effects of diverse dietary phytoestrogens on cell growth, cell cycle and apoptosis in estrogen-receptor-positive breast cancer cells. J Nutr Biochem. 2010;21:856–64. doi: 10.1016/j.jnutbio.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Lappano R, Rosano C, Madeo A, Albanito L, Plastina P, Gabriele B, et al. Structure-activity relationships of resveratrol and derivatives in breast cancer cells. Mol Nutr Food Res. 2009;53:845–58. doi: 10.1002/mnfr.200800331. [DOI] [PubMed] [Google Scholar]
- 25.Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci U S A. 1997;94:14138–43. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levenson AS, Gehm BD, Pearce ST, Horiguchi J, Simons LA, Ward JEIII, 3rd, et al. Resveratrol acts as an estrogen receptor (ER) agonist in breast cancer cells stably transfected with ER alpha. Int J Cancer. 2003;104:587–96. doi: 10.1002/ijc.10992. [DOI] [PubMed] [Google Scholar]
- 27.Lu R, Serrero G. Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. J Cell Physiol. 1999;179:297–304. doi: 10.1002/(SICI)1097-4652(199906)179:3<297::AID-JCP7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 28.Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001;61:7456–63. [PubMed] [Google Scholar]
- 29.Basly JP, Marre-Fournier F, Le Bail JC, Habrioux G. Chulia, A. Estrogenic/antiestrogenic and scavenging properties of (E)- and (Z)-resveratrol. J Life Sci. 2000;66:769–77. doi: 10.1016/S0024-3205(99)00650-5. [DOI] [PubMed] [Google Scholar]
- 30.Tennen RI, Michishita-Kioi E, Chua KF. Finding a target for resveratrol. Cell. 2012;148:387–9. doi: 10.1016/j.cell.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 31.Chung EY, Kim BH, Hong JT, Lee CK, Ahn B, Nam SY, et al. Resveratrol down-regulates interferon-γ-inducible inflammatory genes in macrophages: molecular mechanism via decreased STAT-1 activation. J Nutr Biochem. 2011;22:902–9. doi: 10.1016/j.jnutbio.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Vakana E, Platanias LC. AMPK in BCR-ABL expressing leukemias. Regulatory effects and therapeutic implications. Oncotarget. 2011;2:1322–8. doi: 10.18632/oncotarget.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong Y, Grembecka J, Edler MC, Hamel E, Mooberry SL, Sabat M, et al. Structure-based discovery of a boronic acid bioisostere of combretastatin A-4. Chem Biol. 2005;12:1007–14. doi: 10.1016/j.chembiol.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Kong Y, Wang K, Edler MC, Hamel E, Mooberry SL, Paige MA, et al. A boronic acid chalcone analog of combretastatin A-4 as a potent anti-proliferation agent. Bioorg Med Chem. 2010;18:971–7. doi: 10.1016/j.bmc.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorsey BD, Iqbal M, Chatterjee S, Menta E, Bernardini R, Bernareggi A, et al. Discovery of a potent, selective, and orally active proteasome inhibitor for the treatment of cancer. J Med Chem. 2008;51:1068–72. doi: 10.1021/jm7010589. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki N, Suzuki T, Ota Y, Nakano T, Kurihara M, Okuda H, et al. Design, synthesis, and biological activity of boronic acid-based histone deacetylase inhibitors. J Med Chem. 2009;52:2909–22. doi: 10.1021/jm900125m. [DOI] [PubMed] [Google Scholar]
- 37.Ness S, Martin R, Kindler AM, Paetzel M, Gold M, Jensen SE, et al. Structure-based design guides the improved efficacy of deacylation transition state analogue inhibitors of TEM-1 beta-Lactamase(,) Biochemistry. 2000;39:5312–21. doi: 10.1021/bi992505b. [DOI] [PubMed] [Google Scholar]
- 38.Venkatraman S, Wu W, Prongay A, Girijavallabhan V, George Njoroge F. Potent inhibitors of HCV-NS3 protease derived from boronic acids. Bioorg Med Chem Lett. 2009;19:180–3. doi: 10.1016/j.bmcl.2008.10.124. [DOI] [PubMed] [Google Scholar]
- 39.Joe AK, Liu H, Suzui M, Vural ME, Xiao D, Weinstein IB. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin Cancer Res. 2002;8:893–903. [PubMed] [Google Scholar]
- 40.Tan B, Piwnica-Worms D, Ratner L. Multidrug resistance transporters and modulation. Curr Opin Oncol. 2000;12:450–8. doi: 10.1097/00001622-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Ferry DR. Testing the role of P-glycoprotein expression in clinical trials: applying pharmacological principles and best methods for detection together with good clinical trials methodology. Int J Clin Pharmacol Ther. 1998;36:29–40. [PubMed] [Google Scholar]
- 42.Clarke R, Currier S, Kaplan O, Lovelace E, Boulay V, Gottesman MM, et al. Effect of P-glycoprotein expression on sensitivity to hormones in MCF-7 human breast cancer cells. J Natl Cancer Inst. 1992;84:1506–12. doi: 10.1093/jnci/84.19.1506. [DOI] [PubMed] [Google Scholar]
- 43.O’Shaughnessy JA, Cowan KH. Current status of paclitaxel in the treatment of breast cancer. Breast Cancer Res Treat. 1995;33:27–37. doi: 10.1007/BF00666068. [DOI] [PubMed] [Google Scholar]
- 44.Reddy GP. Cell cycle: regulatory events in G1-->S transition of mammalian cells. J Cell Biochem. 1994;54:379–86. doi: 10.1002/jcb.240540404. [DOI] [PubMed] [Google Scholar]
- 45.Deb TB, Coticchia CM, Dickson RB. Calmodulin-mediated activation of Akt regulates survival of c-Myc-overexpressing mouse mammary carcinoma cells. J Biol Chem. 2004;279:38903–11. doi: 10.1074/jbc.M405314200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.