Abstract
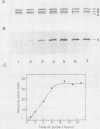
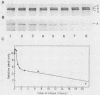
The membrane skeletal protein ankyrin was shown to be continuously acylated and deacylated with long-chain fatty acids in mature erythrocytes. At least a fraction of the lipid bound to ankyrin turned over rapidly (half-life, approximately 50 min) compared with the polypeptide backbone, which was stable throughout the erythrocyte life. This indicates a regulatory significance of the fatty acid modification for the function of ankyrin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam M., Rodriguez A., Turbide C., Larrick J., Meighen E., Johnstone R. M. In vitro acylation of the transferrin receptor. J Biol Chem. 1984 Dec 25;259(24):15460–15463. [PubMed] [Google Scholar]
- Bennett V. The membrane skeleton of human erythrocytes and its implications for more complex cells. Annu Rev Biochem. 1985;54:273–304. doi: 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Sefton B. M. Myristic acid is attached to the transforming protein of Rous sarcoma virus during or immediately after synthesis and is present in both soluble and membrane-bound forms of the protein. Mol Cell Biol. 1984 Dec;4(12):2697–2704. doi: 10.1128/mcb.4.12.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Sefton B. M. Direct identification of palmitic acid as the lipid attached to p21ras. Mol Cell Biol. 1986 Jan;6(1):116–122. doi: 10.1128/mcb.6.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. Q., Ulsh L. S., DuBois G., Shih T. Y. Posttranslational processing of p21 ras proteins involves palmitylation of the C-terminal tetrapeptide containing cysteine-186. J Virol. 1985 Nov;56(2):607–612. doi: 10.1128/jvi.56.2.607-612.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeg J. M., Meng M. S., Ronan R., Fairwell T., Brewer H. B., Jr Human apolipoprotein A-I. Post-translational modification by fatty acid acylation. J Biol Chem. 1986 Mar 25;261(9):3911–3914. [PubMed] [Google Scholar]
- Klockmann U., Deppert W. Acylated simian virus 40 large T-antigen: a new subclass associated with a detergent-resistant lamina of the plasma membrane. EMBO J. 1983;2(7):1151–1157. doi: 10.1002/j.1460-2075.1983.tb01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockmann U., Deppert W. Evidence for transmembrane orientation of acylated simian virus 40 large T antigen. J Virol. 1985 Nov;56(2):541–548. doi: 10.1128/jvi.56.2.541-548.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockmann U., Staufenbiel M., Deppert W. Membrane interactions of simian virus 40 large T-antigen: influence of protein sequences and fatty acid acylation. Mol Cell Biol. 1984 Aug;4(8):1542–1550. doi: 10.1128/mcb.4.8.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee A. I., Courtneidge S. A. Two classes of fatty acid acylated proteins exist in eukaryotic cells. EMBO J. 1985 May;4(5):1137–1144. doi: 10.1002/j.1460-2075.1985.tb03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T. Stabilizing infrastructure of cell membranes. Annu Rev Cell Biol. 1985;1:531–561. doi: 10.1146/annurev.cb.01.110185.002531. [DOI] [PubMed] [Google Scholar]
- McIlhinney R. A., Pelly S. J., Chadwick J. K., Cowley G. P. Studies on the attachment of myristic and palmitic acid to cell proteins in human squamous carcinoma cell lines: evidence for two pathways. EMBO J. 1985 May;4(5):1145–1152. doi: 10.1002/j.1460-2075.1985.tb03752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Spizz G. Fatty acylation of cellular proteins. Temporal and subcellular differences between palmitate and myristate acylation. J Biol Chem. 1986 Feb 15;261(5):2458–2466. [PubMed] [Google Scholar]
- Olson E. N., Towler D. A., Glaser L. Specificity of fatty acid acylation of cellular proteins. J Biol Chem. 1985 Mar 25;260(6):3784–3790. [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Biosynthesis of the human transferrin receptor in cultured cells. J Biol Chem. 1981 Dec 25;256(24):12888–12892. [PubMed] [Google Scholar]
- Schlesinger M. J. Fatty acid acylation of eukaryotic cell proteins. Methods Enzymol. 1983;96:795–801. doi: 10.1016/S0076-6879(83)96067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Bracha M., Schlesinger M. J. Evidence for covalent attachment of fatty acids to Sindbis virus glycoproteins. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1687–1691. doi: 10.1073/pnas.76.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979 Aug;17(4):813–819. doi: 10.1016/0092-8674(79)90321-0. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Relation of fatty acid attachment to the translation and maturation of vesicular stomatitis and Sindbis virus membrane glycoproteins. J Biol Chem. 1980 Apr 25;255(8):3334–3339. [PubMed] [Google Scholar]
- Sefton B. M., Trowbridge I. S., Cooper J. A., Scolnick E. M. The transforming proteins of Rous sarcoma virus, Harvey sarcoma virus and Abelson virus contain tightly bound lipid. Cell. 1982 Dec;31(2 Pt 1):465–474. doi: 10.1016/0092-8674(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Staufenbiel M., Lazarides E. Ankyrin is fatty acid acylated in erythrocytes. Proc Natl Acad Sci U S A. 1986 Jan;83(2):318–322. doi: 10.1073/pnas.83.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willumsen B. M., Christensen A., Hubbert N. L., Papageorge A. G., Lowy D. R. The p21 ras C-terminus is required for transformation and membrane association. Nature. 1984 Aug 16;310(5978):583–586. doi: 10.1038/310583a0. [DOI] [PubMed] [Google Scholar]