Abstract
Objective
To determine whether lower socioeconomic status (SES), broadly defined, is associated with increased inflammation in adolescence and whether adiposity mediates these relationships.
Methods
Fasting blood samples from 941 non-Hispanic black and white 7–12 graders enrolled in a suburban, Midwestern school district were assayed for proinflammatory biomarkers [IL-6, TNF-α soluble receptor 2 (sTNFR2), fibrinogen]. A parent reported objective SES [parent education (E1 ≤ high school, E2 some college, E3 college graduate, E4 professional degree), household income] and youth indicated perceived SES (PSES). Multivariable linear regressions assessed the relationship of SES measures to biomarkers adjusting for age, race, gender, puberty status. In a final step, BMI z-score (BMIz) was added to models and Sobel tests performed to assess mediation by adiposity.
Results
Parent education was inversely associated with IL-6 (βE1=.11, βE2=.10 βE3=.02, p<.001). This association was attenuated but remained significant after adjusting for BMIz (p=.01). Sobel testing confirmed BMIz's partial mediating role (p<.001). Parent education was also inversely associated with sTNFR2 (βE1=.03, βE2=.02, βE3=.001, p=.01); this relationship was mediated by BMIz. Although no main effect was noted for PSES, PSES by race interactions were observed for sTNFR2 (p=.02) and IL-6 (p=.06). High PSES was associated with lower sTNFR2 and IL-6 for white, but not black youth. There were no associations with household income.
Conclusions
Social disadvantage, specifically low parent education, is associated with increased inflammation in adolescence. Adiposity explains some but not all associations, suggesting other mechanisms link lower SES to inflammation. High perceived SES is associated with lower inflammation for white, but not black youth.
Keywords: socioeconomic status, disparities, adolescence, inflammation, obesity
Cardiovascular disease (CVD) is a leading cause of death in the United States (1, 2) and worldwide (3). The link between socioeconomic status (SES) and CVD is well-established (4, 5). Individuals with low social status, regardless of whether social status is measured objectively or subjectively, have greater risk for CVD (4, 6). Health behaviors, many of which are socially patterned, account for only a part of this relationship (7–9). Thus, the psychological and physiological mechanisms underlying the SES gradient in CVD are largely unexplained.
Inflammation may serve as one of the physiological pathways linking SES to CVD. Proinflammatory biomarkers such as C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and fibrinogen are associated with CVD (10–14). CRP is a component of the hepatic acute phase response as is fibrinogen, which is involved in the development of thrombosis and hypercoagulable states (15, 16). IL-6 and TNF-α play a role in atherosclerosis by recruiting lipids and macrophages to vessel walls. SES gradients in these four biomarkers have been demonstrated in adults (17–21). However, few studies have investigated these associations in children or adolescents (22–27), which would be important to consider given that atherosclerotic CVD begins in early adolescence even though it manifests decades later (28). Childhood studies that have assessed the relationship between SES and inflammation have concentrated on CRP (22–25) despite the fact that CRP, a downstream, nonspecific inflammatory marker, is not an ideal biomarker to study in children. CRP’s distribution is highly skewed and most youth have undetectable levels with only a small group of sick children or adolescents manifesting levels associated with CVD risk in adults (25, 29). In addition to focusing on CRP, childhood studies of SES gradients in inflammation are also limited in that they have used only objective measures of SES even though subjective SES is an established, independent risk (6).
The current study addresses these two prior limitations. The study aims to determine if SES gradients exist in adolescence for IL-6, TNF-α, and fibrinogen. We use a broad definition of SES which includes objective and subjective domains of SES as both are important health correlates (6). Further, because SES is associated with adiposity (30) and adiposity is associated with inflammation in adolescence (31), we also explore whether adiposity plays a mediating role in any SES gradients we demonstrate. Our hypotheses are that SES will be inversely associated with the proinflammatory biomarkers and that these relationships will be partially mediated by adiposity. Finally, in consideration of the double jeopardy hypothesis (32), which posits that those who are socially disadvantage in more than one domain (ie. Black and low SES or female and low SES) have worse health effects than accounted for by either domain alone, we assess whether race or gender moderate any demonstrated relationships between SES and inflammation.
METHODS
Cohort
This study uses data from the Princeton School District (PSD) Study, which was based in a suburban school district of Ohio. All procedures for the PSD Study were approved by the Institutional Review board of the participating institutions and participants had parental written informed consent and teen assent if under age 18 and written participant informed consent if age 18 and older (30, 33). The study began in the 2001–2002 school year and 1646 non-Hispanic black and white students (51% female, 56% non-Hispanic white) participated. Study procedures included a survey, a physical exam to measure height and weight and phlebotomy and a baseline parent survey. Fibrinogen was assayed from the blood sample and, when available, plasma was banked for future assays. In 2008, funding was obtained to perform additional assays for a subsample of 1207 participants. This subsample included those for whom a parent had reported information on socioeconomic status in 2001–2002 (N=1339, 81%) and who had continued the study. Of these 1207, 941 (78%) had available banked plasma which was assayed for biomarkers. There were no gender differences between these 941 with inflammatory biomarker data and the 705 who completed baseline assessments but did not have these additional assays. However, those with available inflammatory biomarker information were more likely to be non-Hispanic white than non-Hispanic black, (64.3% vs 50.2%, p<0.001) and were slightly younger on average at baseline (15.0 yrs vs 15.8 yrs, p<0.001).
Socioeconomic Status Measures
Parent Education
A parent reported his/her current education level as well as spouse/current partner’s education level. For analyses, responses were collapsed into four categories reflecting the highest level of parental education: E1= less than or equal to high school/GED, E2= some college or vocational training, E3= college graduate, and E4= professional degree.
Household Income
The baseline parent survey also asked the parent to report current household pre-tax income from all sources by selecting a response from 9 ordered options (< $5000 to ≥ $100,000). As the ranges of these response options varied, the midpoints were used in analyses and $150,000 was used for ≥ $100,000 category. Household income was imputed for participants missing this variable (13.7%) using multiple imputation based upon parent education and race (30).
Perceived Socioeconomic Status (PSES)
The Subjective Social Status Scale-Youth Version was used to measure PSES (34, 35). This validated pictorial scale uses a 10-rung ladder and asks youth to choose the rung which best represents their family’s position in American society (34, 35). Higher scores represent higher perceived SES.
Proinflammatory Biomarkers
All assays were performed on banked plasma. IL-6 was measured using an ELISA assay from R & D Systems that employed the quantitative sandwich enzyme immunoassay technique. The assay had a sensitivity of 0.094 pg/mL, and the day-to-day variabilities of the assay at concentrations of 0.66, 1.97 and 8.16 pg/mL were 12.2, 7.6 and 9.9%. TNF-α was measured by its soluble TNF-α receptor 2 (sTNFR2). sTNFR2 remains elevated in plasma for a longer period of time than TNF-α, is easy to detect, and correlates with soluble TNF-α. sTNFR2 was also measured using an ELISA assay from R & D Systems. Day-to-day variabilities at concentrations of 89.9, 197, and 444 pg/mL were 5.1, 3.5, and 3.6%, respectively. Fibrinogen concentration was assessed using an immunoturbidimetric assay on the Hitachi 911 analyzer (Roche Diagnostics - Indianapolis, IN). The day-to-day variabilities of the assay at concentrations of 167.4, 323.6 and 554.1 mg/dL were 0.94, 1.06, and 1.50%, respectively.
Adiposity
Body mass index (BMI) was derived from measured height and weight. The protocols for collection of height and weight have been described previously (30). In brief, trained staff measured students’ heights and weights two times and if these measurements differed by a preset amount, a third measurement was taken and the averaged value used. Students were asked to remove shoes and items from their pockets for this assessment. BMI z-score (BMIz) based on the 2000 CDC Growth Chart standard was used as the measure of adiposity in analyses (36).
Covariates
Puberty
Participants were assigned to one of three pubertal stages (pre-pubertal, pubertal, post-pubertal) based on a validated algorithm using menarche and plasma estradiol concentration for girls, and plasma free testosterone concentration and stage of axillary hair development for boys (33).
Demographics
Date of birth (used to calculate age), parent-reported race/ethnicity, and gender were drawn from school district records. Household size, which was used as a covariate in all models assessing the effect of household income, was determined from information obtained from the baseline parent survey.
Data Analysis
First, bivariate analyses were conducted to assess relationships between SES measures and the biomarkers. As the biomarkers were not normally distributed, non-parametric tests were employed (Spearman’s correlations and Kruskal-Wallis). Second, multivariable regression models were developed to determine if SES gradients in each of the three biomarkers existed. Biomarkers were log-transformed for regression analyses to correct their positive skewness. The models were built to assess each SES measure (parent education, household income, and PSES) separately, adjusting for covariates, and then a combined model was constructed to determine independent effects. A combined model that included the three SES measures and adjusted for covariates was also run to determine independent associations adjusting for other domains of SES. We used generalized estimating equation (GEE) regression models in order to adjust for the shared environmental and genetic factors among siblings in the cohort (N=272 participants with at least one sibling in study sample). Third, for models that showed a significant association between an SES measure and inflammation biomarker, we tested whether adiposity mediated the relationship between SES and inflammation, by adding BMIz to the model to assess if the strength of the relationship between SES measure and the inflammatory biomarker would decrease or become nonexistent. If the association was attenuated, we confirmed mediation using a Sobel test (37). SES by race and by gender interactions were also explored and if found, plotted. Analyses were conducted using SPSS v15.0 and Sobel testing was performed in SAS (online Sobel test calculator: http://www.quantpsy.org/sobel/sobel.htm and bootstrapping (N=1000)).
RESULTS
Descriptive data on sociodemographic and physiologic measures are shown in Table 1. The sample was fairly evenly distributed by SES measures. Higher parent education was associated with higher household income (mean income: E1= $34,252, E2= $50,542, E3= $80,053, E4= $111,311, p< .001). PSES was also associated with the objective measures of SES. The correlation of PSES and household income was moderate (rho=0.33, p<.001) and mean PSES was higher at each level of parent education (mean PSES: E1=6.3, E2=6.4, E3=6.7, E4=7.2, p<.001).
TABLE 1.
Sociodemographic and Physiologic Descriptive Data (n = 941)
| n | % | ||||
|---|---|---|---|---|---|
| Objective SES measures | |||||
| Parent Education | |||||
| ≤ High School | 202 | 21.5 | |||
| Some College | 263 | 27.9 | |||
| College Graduate | 262 | 27.8 | |||
| Professional Degree | 214 | 22.7 | |||
| Household Income ($) | |||||
| <25,000 | 137 | 14.6 | |||
| 25–49,999 | 196 | 20.8 | |||
| 50–74,999 | 178 | 18.9 | |||
| 75–99,999 | 140 | 14.9 | |||
| ≥100,000 | 161 | 17.1 | |||
| Missing | 129 | 13.7 | |||
| Gender | |||||
| Female | 481 | 51.1 | |||
| Male | 460 | 48.9 | |||
| Race | |||||
| Black | 419 | 44.5 | |||
| White | 522 | 55.5 | |||
| Puberty Status | |||||
| Pre-pubertal or pubertal | 368 | 39.1 | |||
| Post-pubertal | 573 | 60.9 | |||
| Mean | SD | Median | Min | Max | |
| Adolescent Perceived SES* | 6.68 | 1.32 | 7 | 1 | 10 |
| Age | 14.99 | 1.58 | 14.80 | 12.20 | 19.34 |
| Weight (Kg) | 66.0 | 18.5 | 62.0 | 29.3 | 146.1 |
| Height (m) | 1.66 | 0.09 | 1.65 | 1.35 | 1.98 |
| BMI (kg/m2) | 23.9 | 5.9 | 22.3 | 15.3 | 53.7 |
| BMI z score | 0.74 | 1.00 | 0.72 | −2.42 | 2.98 |
| Proinflammatory Biomarkers | |||||
| IL-6 (pg/ml) (n = 852) | 1.27 | 1.94 | 0.72 | 0.16 | 26.91 |
| sTNFR2 (pg/ml) (n = 829) | 2344 | 674.8 | 2225 | 1111 | 8798 |
| Fibrinogen (mg/dl) (n = 932) | 285.5 | 58.5 | 275 | 130 | 580 |
BMI= Body Mass Index. SD = standard deviation; IL-6 = interleukin-6; sTNFR2 = soluble tumor necrosis factor receptor 2.
BMI z-score was associated with all three proinflammatory biomarkers. This finding confirmed one of the associations needed for mediation by adiposity. The association was moderate for fibrinogen (rho=0.32) and IL-6 (rho=0.30), and weak for sTNFR2 (rho=0.14) (all p< 0.001). Proinflammatory biomarkers were also correlated with each other. IL-6 was moderately correlated with fibrinogen (rho= 0.44, p<.001) and sTNFR2 (rho= 0.32, p<.001). Fibrinogen and sTNFR2 were only weakly correlated (rho= 0.10, p=0.003). Proinflammatory biomarkers were not associated with pubertal status.
Table 2 presents the bivariate associations between SES measures and the three proinflammatory biomarkers. Objective SES gradients were found for IL-6 and fibrinogen, while no gradients were demonstrated for sTNFR2. PSES was not associated with any of the three biomarkers. Therefore, Table 3 presents results of the multivariable regression analyses only for household income and parent education. The associations of household income to both IL-6 and fibrinogen and parent education to fibrinogen became non-significant after adjustment for covariates. However, the association between parent education and IL-6 persisted. There appeared to be a ceiling effect in that the parent education categories of ≤ high school and some college had significantly higher IL-6, while the category of college graduate was not different from the reference category of professional degree. Parent education categories of ≤ high school and some college translated to 11% and 10% higher IL-6 levels compared to youth with parent(s) with a professional degree. Although parent education was not associated with sTNFR2 at the bivariate level, a similar relationship to that of parent education with IL-6 emerged in multivariable analyses: parent education categories of ≤ high school and some college corresponded with 3% and 2% higher sTNFR2 values compared to youth with parent(s) with a professional degree. There were no significant SES by race or SES by gender interactions demonstrated.
TABLE 2.
Bivariate Associations of Socioeconomic Status Measures with Biomarkers
| IL-6 (pg/ml) | sTNFR2 (pg/ml) | Fibrinogen (mg/dl) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | P | Median | IQR | P | Median | IQR | P | |
| Parent Education | <0.001 | 0.31 | 0.01 | ||||||
| ≤ High School | 0.83 | 0.92 | 2245 | 819 | 281 | 67 | |||
| Some College | 0.78 | 0.82 | 2277 | 750 | 286 | 70 | |||
| College Graduate | 0.70 | 0.64 | 2187 | 654 | 274 | 79 | |||
| Professional Degree | 0.65 | 0.52 | 2204 | 579 | 264 | 56 | |||
| Rho | Rho | Rho | |||||||
| Household Income ($10,000) | −0.09 | 0.01 | 0.02 | 0.63 | −0.07 | 0.04 | |||
| Adolescent Perceived SES | −0.03 | 0.32 | −0.02 | 0.54 | −0.04 | 0.21 | |||
IL-6 = interleukin-6; sTNFR2 = soluble tumor necrosis factor receptor 2; IQR = interquartile range; SES = socioeconomic status.
P values from Kruskal-Wallis test for Parent Education and Spearman correlations for Household income and Adolescent Perceived SES.
TABLE 3.
Association of Objective Socioeconomic Status Measures with Inflammatory Biomarkers in Multivariable Regression Models and Sobel Testing for Mediation by BMI z score
| IL-6 | sTNFR2 | Fibrinogen | ||||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | ||||||
| SES | SES + BMIz | SES | SES + BMIz | SES | SES + BMIz | |||
| Parent Educationa | ||||||||
| ≤ High School |
.112 (.044, .181) |
.089 (.023, .155) |
.031 (.008, .053) |
.025 (.003, .047) |
.017 (.0008, .033) |
.009 (−.007, .024) |
||
| Some College |
.096 (.032, .160) |
.063 (.0004, .126) |
.022 (.0008, .043) |
.014 (−.007, .035) |
.016 (.001, .032) |
.006 (−.009, .020) |
||
| College Graduate | .017 (−.045, .078) |
.007 (−.052, .067) |
.0009 (−.018, .020) |
−.001 (−.021, .018) |
.008 (−.007, .024) |
.004 (−.010, .019) |
||
| Overall P | <.001 | .01 | .01 | .07 | .13 | .74 | ||
| BMIz |
.080 (.057, .102) |
.018 (.010, .026) |
.027 (.021, .032) |
|||||
| Sobel testing |
Z statistic |
P |
Z statistic |
P | ||||
| 3.41 | <.001 | 2.98 | .003 | |||||
| Household Income ($10,000) | −.005 (−.010, .0002) |
−.003 (−.008, .002) |
−.001 (−.003, .0005) |
−.0008 (−.003, .0009) |
−.0005 (−.002, .0008) |
−.0001 (−.001, .001) |
||
IL-6 = interleukin-6; sTNFR2 = soluble tumor necrosis factor receptor 2; CI = confidence interval; SES = socioeconomic status; BMIz = body mass index z-score
Reference category is professional degree.
NOTE: Biomarkers are log-transformed. Models adjust for age, race, gender, and pubertal status. Income models also adjust for number of people in the household.
Mediation testing of these models showed that, with addition of BMIz to the model, the parent education-sTNFR2 relationship became non-significant suggesting that adiposity explained the parent education-sTNFR2 association. Addition of BMIz to the IL-6 model caused attenuation of the parent education-IL-6 relationship, suggesting that adiposity partially mediated this relationship. A Sobel test confirmed BMIz’s role as a partial mediator (p<.001). In the combined models which included all three SES measures, the same pattern was present (data available on request). The associations between parent education and IL-6 and sTNFR2 remained robust, but became attenuated when BMIz was added to the models.
While no main effect for PSES was noted in bivariate or multivariable models, testing for interactions suggested that race moderated an association of PSES to sTNFR2 (p=.02) and IL-6 (p=.06). No significant interactions were noted for gender. The race interactions are presented in Figure 1. To explore the suggested effect modification, we performed additional stratified analyses. While perceived SES was inversely associated with IL-6 (P=.04) and marginally associated with sTNFR2 (P=.09) in white youth, PSES was not associated with either IL-6 or sTNFR2 among black youth. The large difference in sTNFR2 among black and white youth at low PSES can be attributed to the main effect of race in the models with blacks having lower levels of sTNFR2.
Figure 1.
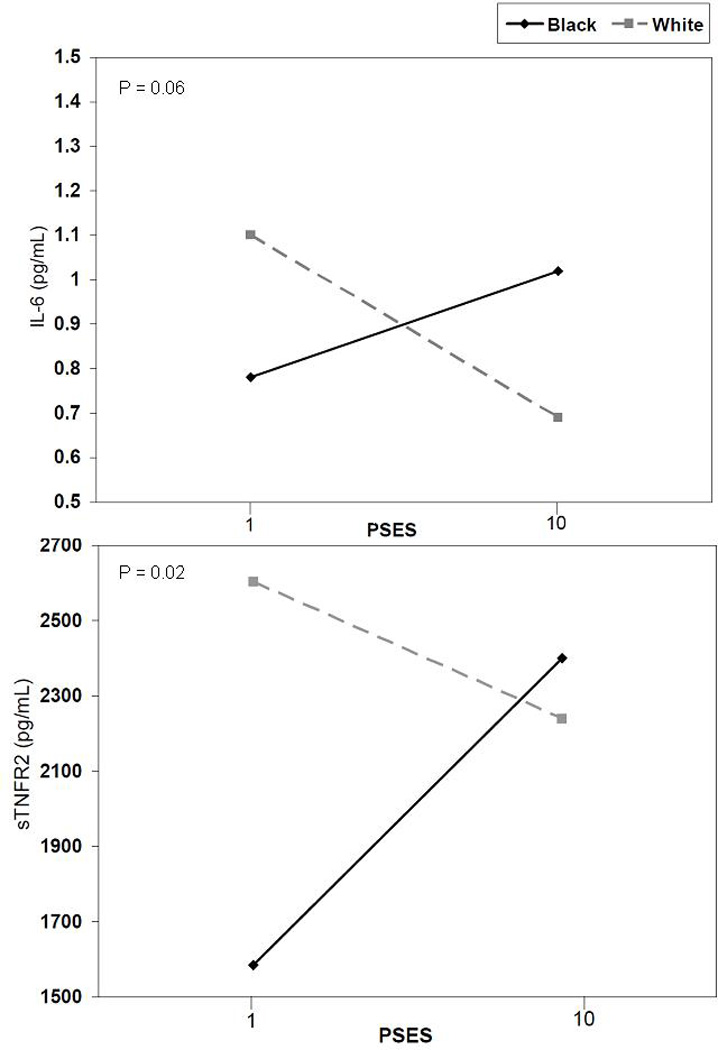
PSES by race interactions. IL-6 = interleukin-6; sTNFR2 = soluble tumor necrosis factor receptor 2; PSES = perceived socioeconomic status.
DISCUSSION
Using a biracial, school-based sample of adolescents, this study demonstrated relationships between objective and subjective SES measures and two proinflammatory biomarkers associated with CVD risk. We found SES gradients, particularly for parent education, for both IL-6 and TNF-α. We analyzed parent education as a categorical variable rather than as a continuous variable. The latter assumes that the effect of moving between each categorical level is identical, whereas our method allowed us to demonstrate that the effects of parent education were strongest for those from families with lower levels of parent education (less than college graduate). We did not find SES effects for fibrinogen. The lack of an association with fibrinogen may be attributed to its downstream role in inflammatory pathways and its physiological role as a hypercoagulant, which is not as closely linked with adiposity as the other two biomarkers. These biomarkers, although well studied in adults, have not been the focus of much research in younger cohorts. The gradients we demonstrated confirm and strengthen the findings of prior studies which seek to elucidate the links between social position and cardiovascular health. For example, our work suggests that the higher level of parent education in the household influences IL-6 in adolescence, which corresponds with a prior study’s finding of an association between maternal education and IL-6 levels in youth (26). We used a household level measure of parent education, rather than education related to a specific parent to match the household level measure of income employed herein. The comparability of maternal education, paternal education, and highest level of parent education has also been shown in relation to adolescent obesity and depression in a prior study (38).
How social factors become embodied to create health disparities is a key question. This study examined one possible pathway: adiposity. Low SES can account for approximately one-third of the obesity risk among US adolescents (38). Today, more than one-third of youth in the U.S. are overweight or obese (39). We found that adiposity partially mediated the parent education gradient in IL-6 and completely explained the gradient for TNF-alpha. These findings reinforce the importance of obesity prevention and treatment early in the life course. Of note, adiposity did not completely explain the parent education gradient in IL-6. This suggests that there are other mechanisms linking lower parent education to inflammation. Education has been linked with greater coping skills and resourcefulness, which may affect parenting and, in turn, the child/adolescent’s ability to self regulate and cope, which may lead to changes in regulatory pathways such as inflammation (40, 41). Household income, which was not significantly associated with inflammatory biomarkers in this study, is a more variable factor than parent education. Because household income can vary greatly across years, the single measurement of household income this study relies on likely does not capture the cumulative effect of that SES marker, while the cumulative effect of parent education, which is static for most children and youth, is more readily captured through a single assessment. Further, household income may affect health through different pathways and by influencing different outcomes than parent education.
This is the first study to assess both objective and subjective measures of SES in regard to inflammation. Despite its strong associations with CVD risks in adulthood (6), PSES was associated only with TNF-α among white adolescents in the study and perhaps IL-6. No associations were found for black adolescents. There are a number of possible explanations for this effect modification by race. Prior research suggests that in-group comparisons may be more important than out-group comparisons for black youth and may be one reason SES is less salient for black youth (35). The lack of a PSES gradient for black youth is also consistent with the adult literature that has shown that PSES is not as strongly related to health in blacks as it is for whites (42).
There may also be developmental explanations. Adolescents’ perceptions of social stratification are not yet fully formed (35) and because they are still in development, they may not provide a sustained influence on physiological functioning. In adults, PSES is more strongly associated with psychological health (i.e. depression, self-rated health) than physiological health (i.e. angina, diabetes) (6). This pattern is likely echoed earlier in the life course, with PSES more relevant to psychological than physiological measures in adolescence.
There are several limitations to this study. The study is a cross-sectional study, so we cannot determine causality. Second, this study only examines non-Hispanic black and white youth, so findings cannot be generalized to other race/ethnicities. Third, this study did not control for smoking or other health-related behaviors. However, controlling for these behaviors could be viewed as over-adjusting, as they may lie along the causal chain between SES and inflammation. Fourth, we used BMIz which has its limitations as an adiposity measure, but is highly correlated with waist circumference (43), widely used, and was the most feasible measure for this large school-based study. Fifth, we performed meditational testing based on the generally accepted hypothesis that adiposity leads to increased inflammation. However, this is a cross sectional study and the relationships between inflammation and adiposity are complex and reciprocal. The mediational tests should be interpreted with these limitations in mind. Finally, it is possible that some participants had preclinical infections, which could raise their proinflammatory cytokine levels. This possibility was felt to be minimal as participants were not scheduled for study visits if there was a recent or current acute illness as part of the study design.
This study has significant strengths which balance these limitations. The study sample had nearly equal representation of black and white youth. We used multiple SES measures including both objective and subjective measures to assess social positions’ effects on inflammation. The objective SES measures were reported by a parent, not the adolescent. Finally, we included multiple well measured proinflammatory biomarkers, including the understudied TNF-α, and we had measured rather than self reported height and weight data.
CONCLUSIONS
SES, broadly measured, is inversely associated with proinflammatory biomarkers in non-Hispanic black and white adolescents. Further research is needed to determine whether these relationships are causal and to ascertain the mechanisms underlying these gradients in health. While our findings indicate that adiposity may be one such mechanism, other factors are also implicated as the relationship between SES and IL-6 was only partially mediated by adiposity. Finally, our study suggests that high PSES is associated with lower inflammation for white, but not black youth. Further studies are needed to corroborate this preliminary finding and elucidate mechanisms underlying these social inequalities in health.
Acknowledgments
We thank Susan Malspeis for statistical support.
Funding: This research was supported by NIH grants HD041527 (EG) and DK59183.
Glossary
- BMIz
Body mass index (z-score)
- CRP
C-reactive protein
- CVD
cardiovascular disease
- IL-6
interleukin-6
- PSES
perceived socioeconomic status
- SES
socioeconomic status
- sTNFR2
soluble tumor necrosis factor receptor 2
- TNF-α
tumor necrosis factor-alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Neither author has a conflict of interest to report.
REFERENCES
- 1.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57:1–134. [PubMed] [Google Scholar]
- 2.Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. Deaths: Preliminary Data for 2009. Natl Vital Stat Rep. 2011;59:1–68. [PubMed] [Google Scholar]
- 3.World Health Organization. [December 16, 2011];The top 10 causes of death. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/index.html.
- 4.Clark AM, DesMeules M, Luo W, Duncan AS, Wielgosz A. Socioeconomic status and cardiovascular disease: risks and implications for care. Nat Rev Cardiol. 2009;6:712–722. doi: 10.1038/nrcardio.2009.163. [DOI] [PubMed] [Google Scholar]
- 5.Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health. 2005;5:7. doi: 10.1186/1471-2458-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh-Manoux A, Adler NE, Marmot MG. Subjective social status: its determinants and its association with measures of ill-health in the Whitehall II study. Soc Sci Med. 2003;56:1321–1333. doi: 10.1016/s0277-9536(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 7.Steptoe A, Marmot M. The role of psychobiological pathways in socio-economic inequalities in cardiovascular disease risk. Eur Heart J. 2002;23:13–25. doi: 10.1053/euhj.2001.2611. [DOI] [PubMed] [Google Scholar]
- 8.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood) 2002;21:60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- 9.Stringhini S, Dugravot A, Shipley M, Goldberg M, Zins M, Kivimaki M, Marmot M, Sabia S, Singh-Manoux A. Health behaviours, socioeconomic status, and mortality: further analyses of the British Whitehall II and the French GAZEL prospective cohorts. PLoS Med. 2011;8:e1000419. doi: 10.1371/journal.pmed.1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 12.von Kanel R, Mills PJ, Fainman C, Dimsdale JE. Effects of psychological stress and psychiatric disorders on blood coagulation and fibrinolysis: a biobehavioral pathway to coronary artery disease? Psychosom Med. 2001;63:531–544. doi: 10.1097/00006842-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 14.Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, Folsom AR, Wu K, Benderly M, Goldbourt U, Willeit J, Kiechl S, Yarnell JW, Sweetnam PM, Elwood PC, Cushman M, Psaty BM, Tracy RP, Tybjaerg-Hansen A, Haverkate F, de Maat MP, Fowkes FG, Lee AJ, Smith FB, Salomaa V, Harald K, Rasi R, Vahtera E, Jousilahti P, Pekkanen J, D'Agostino R, Kannel WB, Wilson PW, Tofler G, Arocha-Pinango CL, Rodriguez-Larralde A, Nagy E, Mijares M, Espinosa R, Rodriquez-Roa E, Ryder E, Diez-Ewald MP, Campos G, Fernandez V, Torres E, Marchioli R, Valagussa F, Rosengren A, Wilhelmsen L, Lappas G, Eriksson H, Cremer P, Nagel D, Curb JD, Rodriguez B, Yano K, Salonen JT, Nyyssonen K, Tuomainen TP, Hedblad B, Lind P, Loewel H, Koenig W, Meade TW, Cooper JA, De Stavola B, Knottenbelt C, Miller GJ, Cooper JA, Bauer KA, Rosenberg RD, Sato S, Kitamura A, Naito Y, Palosuo T, Ducimetiere P, Amouyel P, Arveiler D, Evans AE, Ferrieres J, Juhan-Vague I, Bingham A, Schulte H, Assmann G, Cantin B, Lamarche B, Despres JP, Dagenais GR, Tunstall-Pedoe H, Woodward M, Ben-Shlomo Y, Davey Smith G, Palmieri V, Yeh JL, Rudnicka A, Ridker P, Rodeghiero F, Tosetto A, Shepherd J, Ford I, Robertson M, Brunner E, Shipley M, Feskens EJ, Kromhout D, Dickinson A, Ireland B, Juzwishin K, Kaptoge S, Lewington S, Memon A, Sarwar N, Walker M, Wheeler J, White I, Wood A. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–2011. doi: 10.1161/01.cir.97.20.2007. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 17.Loucks EB, Pilote L, Lynch JW, Richard H, Almeida ND, Benjamin EJ, Murabito JM. Life course socioeconomic position is associated with inflammatory markers: the Framingham Offspring Study. Soc Sci Med. 2010;71:187–195. doi: 10.1016/j.socscimed.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunner E, Davey Smith G, Marmot M, Canner R, Beksinska M, O'Brien J. Childhood social circumstances and psychosocial and behavioural factors as determinants of plasma fibrinogen. Lancet. 1996;347:1008–1013. doi: 10.1016/s0140-6736(96)90147-6. [DOI] [PubMed] [Google Scholar]
- 19.Folsom AR, Qamhieh HT, Flack JM, Hilner JE, Liu K, Howard BV, Tracy RP. Plasma fibrinogen: levels and correlates in young adults. The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1993;138:1023–1036. doi: 10.1093/oxfordjournals.aje.a116821. [DOI] [PubMed] [Google Scholar]
- 20.Steinvil A, Shirom A, Melamed S, Toker S, Justo D, Saar N, Shapira I, Berliner S, Rogowski O. Relation of educational level to inflammation-sensitive biomarker level. Am J Cardiol. 2008;102:1034–1039. doi: 10.1016/j.amjcard.2008.05.055. [DOI] [PubMed] [Google Scholar]
- 21.Friedman EM, Herd P. Income, education, and inflammation: differential associations in a national probability sample (The MIDUS study) Psychosom Med. 2010;72:290–300. doi: 10.1097/PSY.0b013e3181cfe4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gimeno D, Ferrie JE, Elovainio M, Pulkki-Raback L, Keltikangas-Jarvinen L, Eklund C, Hurme M, Lehtimaki T, Marniemi J, Viikari JS, Raitakari OT, Kivimaki M. When do social inequalities in C-reactive protein start? A life course perspective from conception to adulthood in the Cardiovascular Risk in Young Finns Study. Int J Epidemiol. 2008;37:290–298. doi: 10.1093/ije/dym244. [DOI] [PubMed] [Google Scholar]
- 23.Dowd JB, Zajacova A, Aiello AE. Predictors of inflammation in U.S. children aged 3–16 years. Am J Prev Med. 2010;39:314–320. doi: 10.1016/j.amepre.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murasko JE. Male-female differences in the association between socioeconomic status and atherosclerotic risk in adolescents. Soc Sci Med. 2008;67:1889–1897. doi: 10.1016/j.socscimed.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Cook DG, Mendall MA, Whincup PH, Carey IM, Ballam L, Morris JE, Miller GJ, Strachan DP. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis. 2000;149:139–150. doi: 10.1016/s0021-9150(99)00312-3. [DOI] [PubMed] [Google Scholar]
- 26.Howe LD, Galobardes B, Sattar N, Hingorani AD, Deanfield J, Ness AR, Davey-Smith G, Lawlor DA. Are there socioeconomic inequalities in cardiovascular risk factors in childhood, and are they mediated by adiposity? Findings from a prospective cohort study. Int J Obes (Lond) 2010;34:1149–1159. doi: 10.1038/ijo.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook DG, Whincup PH, Miller G, Carey IM, Adshead FJ, Papacosta O, Walker M, Howarth D. Fibrinogen and factor VII levels are related to adiposity but not to fetal growth or social class in children aged 10–11 years. Am J Epidemiol. 1999;150:727–736. doi: 10.1093/oxfordjournals.aje.a010075. [DOI] [PubMed] [Google Scholar]
- 28.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 29.Ford ES, Giles WH, Myers GL, Rifai N, Ridker PM, Mannino DM. C-reactive protein concentration distribution among US children and young adults: findings from the National Health and Nutrition Examination Survey, 1999–2000. Clin Chem. 2003;49:1353–1357. doi: 10.1373/49.8.1353. [DOI] [PubMed] [Google Scholar]
- 30.Goodman E, Adler NE, Daniels SR, Morrison JA, Slap GB, Dolan LM. Impact of objective and subjective social status on obesity in a biracial cohort of adolescents. Obes Res. 2003;11:1018–1026. doi: 10.1038/oby.2003.140. [DOI] [PubMed] [Google Scholar]
- 31.Skinner AC, Steiner MJ, Henderson FW, Perrin EM. Multiple markers of inflammation and weight status: cross-sectional analyses throughout childhood. Pediatrics. 2010;125:e801–e809. doi: 10.1542/peds.2009-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beale F. Double-jeopardy: to be black and female. In: Cade T, editor. The Black Woman. New York, NY: Signet; 1970. pp. 90–110. [Google Scholar]
- 33.Dolan LM, Bean J, D'Alessio D, Cohen RM, Morrison JA, Goodman E, Daniels SR. Frequency of abnormal carbohydrate metabolism and diabetes in a population-based screening of adolescents. J Pediatr. 2005;146:751–758. doi: 10.1016/j.jpeds.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 34.Goodman E, Adler NE, Kawachi I, Frazier AL, Huang B, Colditz GA. Adolescents' perceptions of social status: development and evaluation of a new indicator. Pediatrics. 2001;108:E31. doi: 10.1542/peds.108.2.e31. [DOI] [PubMed] [Google Scholar]
- 35.Goodman E, Huang B, Schafer-Kalkhoff T, Adler NE. Perceived socioeconomic status: a new type of identity that influences adolescents' self-rated health. J Adolesc Health. 2007;41:479–487. doi: 10.1016/j.jadohealth.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Growth Charts. [December 16, 2011];2010 Available from: http://www.cdc.gov/growthcharts/cdc_charts.htm.
- 37.Sobel M. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol. 1982;13:290–312. [Google Scholar]
- 38.Goodman E, Slap GB, Huang B. The public health impact of socioeconomic status on adolescent depression and obesity. Am J Public Health. 2003;93:1844–1850. doi: 10.2105/ajph.93.11.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. Childhood Obesity Facts. [March 13, 2012];2011 Available from: http://www.cdc.gov/healthyyouth/obesity/facts.htm.
- 40.Kristenson M, Eriksen HR, Sluiter JK, Starke D, Ursin H. Psychobiological mechanisms of socioeconomic differences in health. Soc Sci Med. 2004;58:1511–1522. doi: 10.1016/S0277-9536(03)00353-8. [DOI] [PubMed] [Google Scholar]
- 41.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 42.Adler N, Singh-Manoux A, Schwartz J, Stewart J, Matthews K, Marmot MG. Social status and health: a comparison of British civil servants in Whitehall-II with European- and African-Americans in CARDIA. Soc Sci Med. 2008;66:1034–1045. doi: 10.1016/j.socscimed.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 43.Goodman E, McEwen BS, Huang B, Dolan LM, Adler NE. Social inequalities in biomarkers of cardiovascular risk in adolescence. Psychosom Med. 2005;67:9–15. doi: 10.1097/01.psy.0000149254.36133.1a. [DOI] [PubMed] [Google Scholar]


