Abstract
Fibromyalgia (FM) is characterized by widespread pain, as well as affective disturbance (e.g., depression). Given that emotional processes are known to modulate pain, a disruption of emotion and emotional modulation of pain and nociception may contribute to FM. The present study used a well-validated affective picture-viewing paradigm to study emotional processing and emotional modulation of pain and spinal nociception. Participants were 18 individuals with FM, 18 individuals with rheumatoid arthritis (RA), and 19 healthy pain-free controls (HC). Mutilation, neutral, and erotic pictures were presented in four blocks; two blocks assessed only physiological-emotional reactions (i.e., pleasure/arousal ratings, corrugator EMG, startle modulation, skin conductance) in the absence of pain and two blocks assessed emotional reactivity and emotional modulation of pain and the nociceptive flexion reflex (NFR, a physiological measure of spinal nociception) evoked by suprathreshold electric stimulations over the sural nerve. In general, mutilation pictures elicited displeasure, corrugator activity, subjective arousal, and sympathetic activation, whereas erotic pictures elicited pleasure, subjective arousal, and sympathetic activation. However, FM was associated with deficits in appetitive activation (e.g., reduced pleasure/arousal to erotica). Moreover, emotional modulation of pain was observed in HC and RA, but not FM, even though all three groups evidenced modulation of NFR. Additionally, NFR thresholds were not lower in the FM group, indicating a lack of spinal sensitization. Together, these results suggest that FM is associated with a disruption of supraspinal processes associated with positive affect and emotional modulation of pain, but not brain-to-spinal cord circuitry that modulates spinal nociceptive processes.
Keywords: emotion, affect, electric stimulation, descending pain modulation, chronic pain, startle reflex
1.0 Introduction
Fibromyalgia (FM) is characterized by widespread pain and hyperalgesia, which is believed to be a result of abnormal central nervous system (CNS) processing of nociception[32,41,55,77]. For example, in experimental pain studies, noxious stimuli elicit greater pain in FM than healthy pain-free controls (HC)[6,35,42,51,71,74] and imaging studies have found that FM patients have greater cortical and subcortical activation during noxious stimulation than HC[15,28]. Further, two studies have shown that lower stimulus intensities evoke the nociceptive flexion reflex (NFR; a spinally-mediated reflex activated by A-delta fiber activation that is used as an index of spinal nociception) in FM than HC[4,19].
It is still unclear what drives central sensitization, but animal studies suggest that it can be promoted by descending modulation from supraspinal structures (e.g., amygdala, periaqueductal grey [PAG], rostral ventromedial medulla [RVM])[26,49,54,57,79]. Consistent with this, several investigations have noted a relationship between abnormalities in descending modulation and clinical pain syndromes, including FM[36,41,76].
One supraspinal process that modulates pain is emotion[44,56,66,70,82]. Moreover, emotions also modulate the NFR[60,67–69], such that positive emotions inhibit pain/NFR, and negative emotions enhance pain/NFR. Because the NFR is a spinal reflex, these observations provide evidence that brain-to-spinal cord circuitry is engaged by emotional processes – a circuit likely to involve the amygdala, insula, PAG, and RVM[2,49,70]. Given how reliably emotion modulates pain and NFR in HC[45,60,67–70], emotion-induction procedures can be used to study the emotion-pain relationship, but also the integrity of modulatory mechanisms. Due to the fact that FM patients are prone to affective disturbance (e.g., anxiety, depression) and maladaptive cognitive-emotional coping, emotional processes may play a particularly important role in promoting pain in this group[3,14,27,72,75].
The present study used a well-validated picture-viewing paradigm to study emotional modulation of pain and NFR in FM[60,67–69]. Rheumatoid arthritis (RA) patients were included to control for history of chronic pain that could otherwise explain differences between FM and HC. Group differences in emotional processing of pictures were also studied in the absence of pain testing. Specifically, indices of emotional valence (i.e., pleasure/valence ratings, corrugator EMG, startle) and arousal (i.e., arousal ratings, skin conductance) were measured to comprehensively assess physiological-emotional reactivity to pictures[17,39,53]. The inclusion of startle was important, because it is: 1) a non-voluntary reflex (like NFR), 2) inhibited by positive emotions and enhanced by negative emotions (like pain/NFR), and 3) modulated by a descending circuit that includes the amygdala and PAG (like pain/NFR)[37,39].
It was predicted that, compared to RA and HC, FM will be associated with disrupted emotional processing[5,87] and disrupted emotional modulation of pain and NFR. An ancillary goal was to replicate prior observations that NFR threshold is lower in FM, suggesting tonic spinal sensitization.
2.0 Materials and Methods
2.1 General Overview of Procedures
This study used affective picture-viewing to evoke emotional reactions in FM, RA, and HC participants (Fig 1). Pictures were split up into 4 Blocks, with 2 Blocks assessing emotional processing in the absence of pain (which included the presentation of loud, abrupt noises to elicit startle) and 2 Blocks assessing emotional modulation of pain and NFR. The first Block always assessed emotional processing in the absence of pain (prior to any exposure to painful shocks), because the startle reflex can become sensitized by shock exposure [29]. Emotional reactions to pictures were assessed from startle eyeblink modulation (i.e., magnitude of orbicularis oculi EMG), corrugator EMG (i.e., frowning muscle), skin conductance (measure of sympathetic activation), and subjective ratings of valence (pleasure) and arousal. Ratings of the noises were made following each abrupt noise (i.e., startle probe) to keep procedures the same, given that pain ratings were made during pain/NFR Blocks. Next, NFR threshold was assessed in order to determine the electric stimulation intensity to use during pain/NFR Blocks (i.e., stimulation intensity = 120% NFR threshold) and to assess group differences in spinal sensitization. The next 3 Blocks alternated between modulation of pain/NFR, startle, and then pain/NFR. Corrugator EMG, skin conductance, valence ratings, and arousal ratings were also collected during emotional modulation of pain/NFR Blocks to assess reactivity to pictures in the presence of pain. All procedures were approved by the ethics review board at The University of Tulsa.
Figure 1.

Experimental procedures.
2.2 Participants
Participants were recruited from the community using fliers, radio/newspaper advertisements, and email announcements. Patients were also recruited from outpatient clinics, rheumatologist referrals, and FM/arthritis support groups. Mailed advertisements also targeted rheumatologists in the local area. Participants were excluded for: <18 years of age;, history of cardiac disorders, circulatory problems, or uncontrolled diabetes; body mass index of 35 or above (due to potential difficulties obtaining an NFR in individuals with high adiposity); use of antidepressant, anxiolytic, or high blood pressure medications (except as noted below); and/or recent psychological trauma. Healthy controls (HC) were also excluded for any history of chronic pain or neurological/neuromuscular disorders. FM participants were excluded if they had symptoms of a chronic pain condition unrelated to FM, including arthritis, sciatica, or injury (e.g., motor vehicle accident). RA patients were excluded for chronic pain conditions other than RA. FM and RA patients were required to have a formal diagnosis by a physician to be considered for the study, which was verified by medical chart review. Further, FM participants were examined by laboratory personnel (trained by a rheumatologist) and were only included if they met the 1990 American College of Rheumatology criteria of 11 out of 18 tender-points (assessed by digital algometer) and widespread pain for over 3 months [85]. Participants were asked to abstain from narcotic analgesics for 2 weeks prior to the experiment and non-narcotic analgesics (e.g., NSAID, acetaminophen) for 24 hours prior to the experiment. Low dose muscle relaxants and tricyclic antidepressants for the treatment of sleep problems were permitted [73]. Ultimately, recruitment of FM and RA patients who were not on any medications (e.g., analgesics, antidepressants, antihypertensives) proved difficult; thus, a few participants (4 FM, 4 RA) were allowed to participate as long as they were stabilized on their medications for at least 4 weeks and had not taken break-through or as-needed pain medications before the testing session (24 hours for OTC medications, 2 weeks for narcotic meds). Analyses were conducted with and without these individuals to determine whether medications confounded the results. Participants who completed the study received a $100 honorarium.
Effect size estimates for nociceptive outcomes based on our prior research were large and ranged from f = .43–.56. A power analysis with 2 within-subject degrees of freedom (3 picture contents), 2 between-group degrees of freedom (3 groups), α=.05, power=.80, and the lowest effect size (f = .43) suggested 19 per group. For the present study, a total of 55 participants were recruited (HC=19 [15 females], RA=18 [15 females], FM=18 [16 female]). Participant characteristics by group are presented in Table 1. All participants provided verbal and written informed consent. All participants were informed that they could withdraw from the study at any time.
Table 1.
Participant Characteristics by Group
| HC (n=19) | RA (n=18) | FM (n=18) | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics (units) | M or n | SD or % | M or n | SD or % | M or n | SD or % | F / χ2 | comparisons |
| Age (years) | 46.68 | 14.14 | 44.67 | 14.32 | 49.44 | 9.62 | 0.62 | |
| Sex (% Female) | 15 | 79% | 15 | 83% | 16 | 89% | 0.67 | |
| Race (% Caucasian) | 17 | 89% | 14 | 78% | 16 | 89% | 1.27 | |
| Marital Status (% Married) | 9 | 47% | 7 | 39% | 8 | 44% | 0.28 | |
| Employed (% full or part-time) | 11 | 58% | 10 | 56% | 8 | 44% | 0.76 | |
| Years of Education | 15.79 | 2.80 | 14.67 | 2.11 | 15.13 | 4.27 | 0.60 | |
| Body Mass Index (kg /m2) | 23.63 | 3.79 | 26.26 | 4.00 | 25.16 | 4.88 | 1.80 | |
| Systolic Blood Pressure (mmHg) | 118.17 | 18.85 | 118.47 | 18.32 | 115.83 | 18.53 | 0.10 | |
| Diastolic Blood Pressure (mmHg) | 74.36 | 13.60 | 73.41 | 10.28 | 69.45 | 8.34 | 0.92 | |
| Positive Tender-points (# painful sites) | 4.83 | 3.94 | 10.78 | 5.07 | 16.89 | 1.71 | 45.10* | FM>RA>HC |
| Fibromyalgia Impact Questionnaire (0–100) | 14.02 | 13.02 | 36.22 | 21.94 | 55.02 | 18.57 | 23.72* | FM>RA>HC |
| Current Pain, MPQVAS (0–100) | 1.53 | 2.65 | 27.78 | 19.55 | 47.83 | 22.40 | 34.30* | FM>RA>HC |
| Psychological Distress, SCL-90 (0–4) | 1.41 | 0.53 | 1.44 | 0.61 | 1.91 | 0.48 | 4.97* | FM>RA, FM>HC |
| General Health Perception, SF-36 (0–100) | 86.05 | 11.25 | 55.00 | 23.95 | 44.44 | 24.96 | 20.02* | FM<HC, RA<HC |
| Pain Catastrophizing, PCS (0–52) | 12.53 | 9.40 | 15.72 | 12.31 | 22.11 | 13.07 | 3.22* | FM>HC |
Note:
p<.05;
comparisons noted in the last column are statistically significant at p<.05.
HC = healthy pain-free controls; RA= rheumatoid arthritis; FM = fibromyalgia.
2.3 Apparatus, Stimulus Parameters, and Physiological Signals
Stimulus presentation, self-report ratings, and physiological data collection were controlled by a PC with dual monitor capacity, A/D board (PCI-6036E; National Instruments, Austin, TX), and LabVIEW software (National Instruments). One computer monitor was used by the experimenter to monitor physiological signals, and a second monitor was used by the participant to complete electronic questionnaires and to make ratings of electric stimuli. Testing was completed in a sound-attenuated and electrically-shielded testing chamber. Participants were monitored from an adjacent control room via a video camera connected to a flat panel television. Participants wore sound-attenuating headphones (TDH-49, Telephonics, Farmingdale, New York) that allowed them to hear the experimenter's instructions and they could speak to the experimenter via the microphone on the video camera. The headphones were also used to present startle probe stimuli.
Acoustic startle noise bursts to assess startle were delivered by a Coulbourn Instruments audio signal generator (Part number A12-33, Whitehall, PA) and amplified by a 250 W amplifier (MPA-250A, Radio Shack, Fort Worth, TX) to 105 dB. Startle probes had a near instantaneous rise time and were 50 ms in duration. Electric stimuli to assess pain/NFR were generated by a Digitimer stimulator (DS5; Hertfordshire, England) and delivered using a bipolar surface stimulating electrode (Nicolet, Madison, WI; 30 mm inter-electrode distance) attached to the left leg over the retromalleolar pathway of the sural nerve. A computer controlled the timing and intensity of the stimulations, and the maximum stimulation intensity was set at 50 mA to ensure safety. Each electric stimulus was a train of 1 ms square wave pulses delivered at 250 Hz.
All physiological signals were amplified and filtered by a Grass Technologies (West Warwick, RI) Model 15LT amplifier (with AC Modules 15A54 and DC/AC Module 15A12). An adaptor (Grass, Model SCA1) was used to measure skin conductance response (SCR). Resting blood pressure was recorded using a Critikon Dinamap PRO 100 Monitor (Tampa, FL) four times at 3-min intervals before experimental testing began. A mechanical physical scale with attached height rod (Detecto, Webb City, MO) was used to assess weight and height in order to calculate body mass index (BMI). A Wagner Instruments Force Ten FDX Digital Force algometer with a 1.1 cm diameter tip (Greenwich, CT) was used to conduct the tender-point exam.
The NFR was assessed from biceps femoris electromyogram (EMG) recorded from two active Ag-AgCl electrodes placed 10 cm superior to the popliteal fossa. Biceps femoris EMG (for the measurement of the NFR) was amplified ×10,000 and bandpass filtered (10 Hz - 300 Hz) online. Corrugator EMG was used as a physiological measure of picture-evoked emotional valence [13,40] and was measured by two Ag/AgCl electrodes filled with conductive gel (EC60, Grass Technologies) affixed over the left corrugator supercilii muscle (pulls brow down during frown). Corrugator EMG was amplified ×20,000 and bandpass filtered (30 Hz - 1000 Hz) online. Startle eyeblink magnitude was used as a physiological measure of picture-evoked emotional valence [10,37] and measured by affixing two Ag-AgCl electrodes over the left orbicularis oculi muscle according to published guidelines [8]. Orbicularis oculi EMG was amplified ×20,000 and bandpass filtered (10 Hz - 1000 Hz) online. Skin conductance response (SCR) was used as a physiological measure of picture-evoked sympathetic arousal [10,40]. SCR was measured from two electrodes filled with isotonic paste (EC33, Grass Technologies) affixed to the volar surface of the index and middle fingers of the non-dominant hand after the participant's skin had been washed and dried. A ground electrode was placed over the lateral epicondyle of the femur.
All physiological signals were sampled at 1000 Hz. Before electrodes were applied (except SCR), the skin was cleaned with alcohol and exfoliated using an abrasive paste (Nuprep; Weaver and Company, Aurora, CO) to reduce impedances below 5kΩ. Electrodes were then attached with self-adhesive collars after conductive gel (EC60; Grass Technologies) was applied. NFR and SCR were recorded from 11 mm reusable disc electrodes (F-E9-40-5; Grass Technologies), whereas corrugator and orbicularis oculi EMG was recorded from 5 mm miniature electrodes (F-E9M-40-5; Grass Technologies).
2.4 Questionnaires
2.4.1 Background information
A custom-built demographics and health status questionnaire was used to obtain standard background information as well as information regarding health problems. The questionnaire asks about exclusionary criteria such as cardiovascular problems, neurological disorders, chronic pain, recent trauma, and medications. The Symptom Checklist-90-Revised (SCL-90-R) was used to measure psychological distress. The SCL-90-R is a reliable and valid questionnaire that consists of a list of 90 items asking about psychological problems. Respondents rated these items using a 5-point Likert scale (0=not at all, 4 = extremely). The Global Severity Index (GSI) of the SCL-90-R was used to measure overall psychological distress [18], with higher scores indicating greater distress. The Pain Catastrophizing Scale (PCS) was used to assess group differences in pain coping [78]. The PCS is a reliable and valid 13-item measure that assesses catastrophic thoughts (rumination, magnification, helplessness) associated with pain. Items were summed to compute a total pain catastrophizing score, with higher scores indicating greater catastrophizing. The general health scale from the Short-Form Health Survey (SF-36) was used to measure quality of life. This is a 5-item reliable and valid scale that ranges from 0 to 100 and measures the person's general perception of their health [46,84]. Higher scores reflect an individual's belief that his or her health is excellent. The visual analog scale(VAS) from the McGill Pain Questionnaire-short form (MPQ-SF) was used to measure current pain prior to the experiment [47]. The VAS ranges from 0–100 with anchors labeled as “no pain” and “worst possible pain,” respectively. The Fibromyalgia Impact Questionnaire (FIQ) was used to measure pain problems [7]. The FIQ is a 10-item self-report measure developed to assess fibromyalgia patient health status over the past week. The first item contains 11 questions that pertain to physical impairment and the other items ask about how they feel, days of work missed, work difficulty, pain, fatigue, tiredness, stiffness, anxiety, and depression. The possible range of scores on the FIQ is 0 – 100, with FM patients scoring around 50 on average [7], and scores over 70 indicating patients severely impacted by FM. FIQ instructions were altered slightly for the current study to refer to pain in general rather than only fibromyalgia pain (i.e., by removing the word “fibromyalgia”) so items could pertain to HC and RA as well as FM participants.
2.4.2 Subjective reactions to pictures
The Self Assessment Manikin (SAM) was used to assess emotional reactions to affective pictures [12]. The SAM is a questionnaire consisting of two sets of five pictographs measuring affective valence (unpleasant–pleasant) and arousal (calm–excited). A computerized version of the SAM was used. Participants responded by moving an indicator on or between any of the five pictographs and then submitted their answer by computer mouse. A rating between 1 and 9 was produced for each dimension (higher scores = greater pleasure or arousal).
2.4.3 Pain ratings
To assess pain intensity in response to electric pain stimuli, participants used a computer-presented numerical rating scale (NRS) similar to that used in numerous prior studies [e.g., 20,23,24,64,65,67,80]. The pain NRS ranged from 0 to 100 with the following labels: 0 (no sensation), 50 (painful), and 100 (maximum tolerable). Participants used a computer mouse to slide an indicator along the scale to make ratings. A mouse button press was used to submit the rating and return the scale to zero before the next rating. This scale allowed participants to rate non-painful sensations, as well as painful sensations. The advantage of using such a scale is that it is possible to determine whether manipulations (e.g., emotional modulation) can cause a previously painful sensation to become non-painful (or vice versa).
2.4.4 Noise ratings
To keep procedures identical between startle modulation Blocks and pain/NFR modulation Blocks, participants were asked to rate their reactions to the acoustic startle stimuli using a computer-presented numerical rating scale (NRS) that was constructed to parallel the pain NRS. The noise NRS ranged from 0 to 100 with the following labels: 0 (no noise), 50 (loud), and 100 (maximum tolerable). A mouse button press was used to submit the rating and return the scale to zero before the next rating.
2.5 Emotion Evocation
2.5.1 Picture stimuli
Digital pictures from the International Affective Picture System (IAPS) [38] were used to evoke emotional reactions. Pictures were presented in 4 blocks of 18 pictures (6 mutilation, 6 neutral, 6 erotic), with 2 Blocks (Blocks 1 & 3) being used to assess emotional reactivity in the absence of pain and 2 Blocks (Blocks 2 & 4) being used to modulate pain/NFR (Fig 1). Mutilation and erotic picture contents were chosen because they have been shown to produce the most robust modulation of pain and NFR [60]. Pictures across the 4 Blocks were matched on normative ratings [16] of valence and arousal to ensure that they would produce similar emotional responses. Pictures within a Block were always the same, but the order within each Block was randomized across participants. IAPS stimuli numbers and normative ratings for the 4 Blocks were: Block 1 – mutilation (3010, 3030, 3069, 3102, 9253, 9405; MValence= 1.76, MArousal=6.52), neutral (7002, 7035, 7041, 7050, 7090, 7150; MValence= 4.96, MArousal=2.73), and erotica (4599, 4607, 4609, 4659, 4669, 4687; MValence= 6.76, MArousal=6.19); Block 2 - mutilation (3015, 3060, 3061, 3068, 3071, 3130; MValence= 1.82, MArousal=6.48), neutral (7009, 7020, 7038, 7080, 7170, 7950; MValence= 5.01, MArousal=2.67), and erotica (4611, 4650, 4660, 4672, 4676, 4695; MValence= 6.77, MArousal=6.21); Block 3 - mutilation (3000, 3053, 3062, 3101, 3120, 3150; MValence= 1.75, MArousal=6.50), neutral (6150, 7004, 7006, 7034, 7100, 7705; MValence= 4.99, MArousal=2.69), and erotica (4608, 4624, 4658, 4689, 4690, 4800; MValence= 6.78, MArousal=6.22); Block 4 – mutilation (3051, 3064, 3080, 3100, 3110, 3140; MValence= 1.74, MArousal=6.47), neutral (7000, 7175, 7211, 7217, 7233, 7235; MValence= 4.93, MArousal=2.73), and erotica (4623, 4643, 4652, 4666, 4670, 4694;MValence= 6.78, MArousal=6.22).
2.5.2 Emotional reactions
Emotional experience can be assessed using two continuous, but orthogonal, dimensions called valence and arousal [9,10]. Valence refers to the unpleasantness or pleasantness of the experience and generally indicates whether defensive or appetitive motivation is experienced, respectively. By contrast, arousal refers to the emotional activation or intensity that is evoked. To assess participants' emotional reactions to picture stimuli, five measures were employed. Self-reported valence and arousal were assessed using the SAM (described in Section 2.4.2). The corrugator muscle controls the eyebrow and pulls it down into a frown during unpleasant experiences and it relaxes during pleasant experiences. As a result, studies have shown that corrugator activity is inversely correlated with subjective reports of valence/pleasure[10,40]. Because corrugator activity is a facial display of emotion, it can be influenced by voluntary facial movements as well as individual differences in facial expressiveness[11]. The startle reflex is a whole body response to an abrupt, unexpected stimulus that helps an organism protect itself from a potential threat[30]. In humans, startle is quantified from the eyeblink response (via orbicularis oculi EMG) which occurs 21–120 milliseconds after the noise stimulus. Numerous studies have shown that startle magnitude is inhibited while viewing pleasant pictures and enhanced while viewing unpleasant pictures [30,37]; therefore, startle correlates with emotional valence. Because startle is a rapid response mediated by a simple neurocircuit[39] it is less likely to be influenced by voluntary control. Skin conductance is a method of assessing the electrical conductivity of the skin. Sweat glands, especially those on the palms of the hand and soles of the feet, are controlled exclusively by the sympathetic nervous system. As sweat glands open and release sweat, the skin more readily conducts electricity and skin conductance increases. As a result, skin conductance is a measure of sympathetic activation and correlates with subjective reports of arousal [10,40]. Taken together, corrugator EMG and startle magnitude were assessed as physiological correlates of valence, whereas SCR was assessed as a physiological correlate of arousal. That said, it is important to note that although measures of valence correlate with each other and measures of arousal correlate with each other, they can diverge [10,40]. Moreover, they are mediated by different supraspinal structures[43,52,86] For example, measures of subjective emotional experience are correlated with areas such as the hippocampus and orbitofrontal cortex, whereas startle modulation is associated with the amygdala and PAG[25,39,86]. Thus, these five measures provide unique indices of valence and arousal to comprehensively assess emotional experience.
2.6 Determination of Electric Stimulation Intensity: NFR Threshold Assessment
The suprathreshold stimulation intensity used during emotional picture viewing was 120% NFR threshold to ensure reliable reflexes. NFR threshold was assessed using 3 ascending-descending staircases of electric stimuli. The first ascending staircase started at 0 mA and increased in 2 mA steps until an NFR was detected. NFR was defined as a mean rectified biceps femoris EMG response in the 90–150 ms post-stimulus interval that exceeded the mean rectified biceps femoris EMG activity during the 60 ms pre-stimulus baseline interval by at least 1 standard deviation. This criterion was chosen because it increased sensitivity for detecting an NFR which reduced the burden on the chronic pain participants, but also retained adequate specificity [22,61]. After an NFR was obtained, the current was decreased in 1 mA steps until an NFR was no longer detected. The second and third ascending-descending staircases used 1 mA steps. The interval between electric stimulations varied randomly between 8–12 s to reduce predictability and reflex habituation. After each stimulus, participants rated their pain sensation using the pain NRS. The stimulus intensity (mA) of the 2 peaks and 2 troughs of the last two ascending-descending staircases were averaged and used to define NFR threshold.
2.7 Procedure
Interested participants were administered a brief phone screen to provide an overview of the study and to evaluate inclusion/exclusion criteria. Potentially eligible participants were invited to attend a laboratory session during which informed consent was obtained and then a comprehensive assessment of inclusion/exclusion criteria was conducted, including a tender-point exam. A release for medical records was obtained at that time so that diagnoses could be verified. Afterwards, participants were provided instructions on the NRS for rating pain (and noise) and the SAM for rating emotional pictures. Next, the participants were instrumented for physiological recording and then seated in a comfortable reclining chair (PC-6 Perfect Chair, Human Touch, Long Beach, CA) that kept their knee angle at approximately 160 degrees. Before testing began, participants filled out background questionnaires and then there was a 5-min acclimation period during which participants were asked to sit quietly and relax. Block 1 assessed startle modulation because exposure to electrical stimulations can sensitize the startle reflex [29]. To assess emotional reactivity in the absence of pain, affectively-charged pictures were presented in a random order, with the limitation that no more than 2 pictures of the same category could be shown consecutively. Each picture was shown for 6 s and inter-picture intervals varied randomly from 12–22 s. In each Block, startle probes (50 ms duration, 105 dB intensity) were delivered during 9 pictures (3 per content). Probes were also delivered during 4 randomly determined inter-picture intervals to minimize the predictability of their delivery. Thus, a total of 13 probes were delivered during each Block. Each probe started 3 to 5 s after picture onset and 11 to 21 s after inter-picture interval onset in order to reduce predictability. After the presentation of each picture, participants rated their emotional response on the SAM. A noise NRS was presented after each startle probe. If the probe occurred during an interval, the noise NRS was presented immediately after the probe. To ensure that a picture or probe was not delivered during a rating period, the computer automatically paused the experiment during presentation of the rating scale until the participant submitted their ratings by computer mouse. After Block 1, NFR threshold was assessed by procedures described previously. Block 2 assessed emotional modulation of pain and NFR. These procedures were identical to those used to assess emotional reactivity in the absence of pain, except that electric stimulations set at 120% NFR threshold were delivered instead of startle probes, and pain NRSs were administered rather than noise NRSs. Before Block 3, there was a 5-min mandatory break period during which participants were asked to sit quietly and relax. Blocks 3 and 4 used procedures identical to Blocks 1 and 2, respectively. To help minimize participants stress and burden, optional breaks (e.g., 1–5 min) were offered in between Blocks. After all procedures were completed, the participant was provided their honorarium. These data were collected between 2008 and 2012 and this is the first paper to come from this study.
2.8 Physiological Data Processing
All physiological signals were scored offline and inspected for errors. Further, to avoid stimulus artifact from electric stimulations and startle probes, picture-evoked corrugator EMG and SCR were only calculated in response to pictures during which a stimulation/probe was not delivered. Corrugator responding was calculated by subtracting the mean rectified EMG (in V) in the 1 s prior to picture onset from the mean rectified EMG during the 6 s of picture presentation. SCR was calculated by subtracting the mean skin conductance (in S) in the 1 s prior to picture onset from the peak skin conductance that occurred in the 2 – 6 s interval after picture onset. Startle magnitude was scored by subtracting the mean rectified orbicularis oculi EMG of the 60 ms prior to startle probe onset from the maximum rectified and integrated (8 ms time constant) EMG response in the 21 – 120 ms following startle probe onset. NFR magnitude was calculated from a d-score (d = [mean rectified EMG during 90–150 ms post-stimulus interval – mean rectified EMG during 60 ms prestimulus interval] / [pooled standard deviation of EMG from the baseline and 90–150 ms intervals]). Research has shown that calculating NFR magnitude from a d-score produces a stronger correlation with pain ratings than other methods of scoring NFR magnitude and improves the distributional qualities of the NFR (i.e., distribution is normal in shape) [62,63].
2.9 Data Analysis
Dependent variables assessed during emotional reactivity in the absence of pain task were: valence ratings, corrugator EMG, startle magnitude, arousal ratings, SCR, and noise intensity ratings. Dependent variables assessed during emotional modulation of pain/NFR were: valence ratings, corrugator EMG, arousal ratings, SCR, pain ratings, and NFR magnitude. Valence/arousal ratings, corrugator EMG, and SCR were reactions to pictures, whereas noise ratings, startle magnitudes, pain ratings, and NFR magnitudes were reactions to probes/stimulations. Independent variables were: Group (HC, RA, FM) and Picture Content (Mut, Neu, Ero). In addition, a continuous predictor called “Order” was entered that coded for the order in which stimulations, probes, or pictures occurred (e.g., stims 1 – 9 in each Block). This variable controlled for any habituation or sensitization effects within a Block that are unrelated to emotional modulation. Controlling for order improves statistical power and improves the validity of the statistical models by removing potential habituation/sensitization confounding [59].
Outcomes assessed during emotional reactivity and pain modulation were analyzed separately and data were kept in “long form” so that the SPSS 17.0 MIXED procedure could be used to conduct mixed effects modeling of the data. Each Block contained 18 pictures and electric stimulations/startle probes were delivered during 9 pictures in each Block; therefore, each participant contributed 36 responses (2 Blocks × 18 pictures = 36) for analysis of emotional reactivity to pictures and 18 responses (2 Blocks × 9 stims or probes = 18) for analysis of reactions to stimulations/probes. The MIXED procedure in SPSS 17.0 was used to increase power and also because cases with missing data are not excluded [31]. Subject ID was used as the grouping variable to designate the Level 2 units (i.e., to account for non-independence of observations given that each participants contributed multiple rows of data). Level 1 units were responses to pictures (valence and arousal ratings) or stimulations/probes (trials described above). The variance-covariance structure of the repeated measures within each Block was modeled using an autocorrelation matrix (AR1). All models included a random intercept to allow outcomes to vary across individuals (Level 2 units). The SPSS MIXED procedure uses Satterthwaite estimation for the denominator degrees of freedom (df) which produces non-integer values that vary from analysis to analysis (even if the number of observations are the same across analyses). For ease of reporting, these dfs were rounded to the nearest integer. All analyses were conducted on data at the trial-by-trial level (rather than averaging by picture content) in order to take full advantage of all variance in the data and to maximize power. Although the analytic approach appears to be complex, it is important to note that the results are interpreted as if 3(Group) × 3(Picture Content) ANOVAs were conducted. Follow-up mean comparisons to significant F-tests were conducted using Fisher's LSD tests. Significance was set at p < 0.05 (two tailed).
3.0 Results
3.1 Participant Characteristics
Participant characteristics by group are reported in Table 1. Of the 55 participants recruited, 3 reached pain tolerance before an NFR threshold was achieved and chose to discontinue the experiment (1 HC, 1 RA, 1 FM). Additionally, an NFR could not be obtained on 5 FM participants, because they went to the 50 mA maximum without ever achieving a reflex. The first 3 of these were dismissed from the study given that emotional modulation of NFR could not be tested. However, because this issue turned out to be prevalent in the FM group, the last 2 FM participants were allowed to continue the study and the stimulus intensity during picture viewing was set at 120% pain threshold (rather than 120% NFR threshold), which allowed a test of emotional modulation of pain, but not NFR. Pain threshold was defined as the first stimulus in the first ascending series of electric stimuli during NFR threshold testing that was rated ≥ 50 on the pain NRS. Thus, there were 55 participants (19 HC, 18 RA, 18 FM) with at least partial data available for emotional reactivity analyses in the absence of pain, there were 53 participants with data available for the analysis of NFR threshold (19 HC, 17 RA, 17 FM; 50 mA was entered as the NFR threshold for those that reached the 50 mA max intensity), and there were 49 participants with data available in the emotional modulation of pain analyses (18 HC, 17, RA, 14 FM [12 FM included in emotional modulation of NFR analyses]).
To compare groups on background variables, 1-way ANOVAs and chi-squared analyses were used (Table 1). Groups did not differ in age, distribution of sex, race, marital status, employment status, years of education, BMI, or blood pressure. As expected, there were group differences on the number of positive tender-point sites, with FM having more positive sites than RA and RA having more positive sites than HC (ps < .05). Moreover, there were group differences on the Fibromyalgia Impact Questionnaire (FIQ), current pain (MPQ-VAS), psychological distress (SCL-90), general perceptions of health (SF-36), and pain catastrophizing. FM patients had higher FIQ scores and current pain than RA and HC, and RA was higher on the FIQ and current pain than HC (ps < .05). FM had higher psychological distress than RA and HC (ps < .05), but RA and HC did not differ. Health perceptions were lower in RA and FM compared to HC (ps < .05), but the two pain groups did not differ from one another. Finally, FM patients catastrophized more than HC (p < .05), but RA did not differ from HC or FM.
3.2 Emotional Reactivity in the Absence of Pain
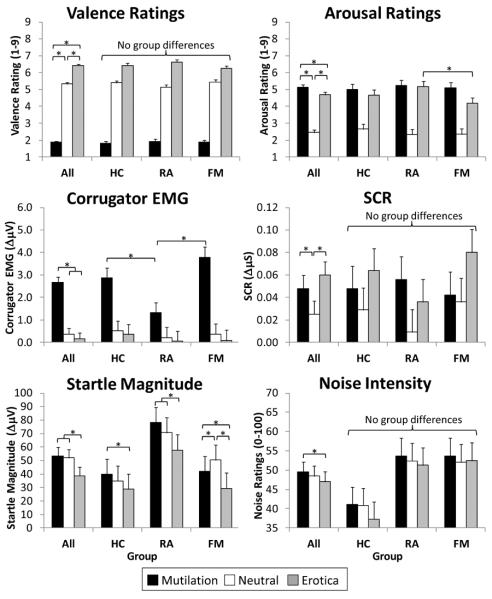
Table 2 presents the inferential statistics for mixed effects ANOVAs of startle modulation outcomes, whereas Figure 2 depicts means, SEMs, and results of follow up tests. There was a significant main effect of picture content for all outcomes (main effects depicted by the “All” group in Figure 2). These results indicate that, in general, mutilation pictures increased displeasure (reduced valence) ratings, corrugator EMG, arousal ratings, and SCR, whereas erotic pictures increased pleasure (valence) ratings, arousal ratings, and SCR, and inhibited startle. Additionally, mutilation evoked slightly more subjective arousal than erotica. Interestingly, there was emotional modulation of noise ratings such that intensity ratings were highest during mutilation and lowest during erotic.
Table 2.
Results of mixed effect models predicting responses during startle modulation testing.
| Valence Ratings | Corrugator EMG | Startle Magnitude | Arousal Ratings | SCR | Noise Intensity Ratings | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANOVA Effects | df | F | df | F | df | F | df | F | df | F | df | F |
| Group | 2, 53 | 0.04 | 2, 53 | 1.59 | 2, 55 | 2.79# | 2, 55 | 0.38 | 2, 55 | 0.34 | 2, 55 | 2.78# |
| Content | 2, 1479 | 1962.95* | 2, 832 | 55.67* | 2, 697 | 23.69* | 2, 1436 | 412.65* | 2,748 | 4.49* | 2, 637 | 5.98* |
| Group × Content | 4, 1477 | 3.68* | 4, 831 | 4.46* | 4, 696 | 3.03* | 4, 1434 | 6.05* | 4, 747 | 0.99 | 4, 637 | 1.24 |
| Order | 1, 853 | 0.18 | 1, 662 | 0.77 | 1, 480 | 28.60* | 1, 843 | 0.45 | 1, 589 | 10.98* | 1, 767 | 16.28* |
Note:
p<.05,
p<.10
Figure 2.
Emotional valence (valence ratings, corrugator EMG, startle) and arousal (arousal ratings, skin conductance response [SCR]) reactions to pictures in the absence of pain testing. Emotional modulation of noise ratings is depicted in the bottom right graph. HC=pain-free healthy controls, RA= participants with rheumatoid arthritis, FM=participants with fibromyalgia. *p<.05
There was also a significant Group × Picture Content interaction for corrugator EMG, startle magnitude, and arousal ratings, indicating that some measures of emotional processing were disrupted in FM. Most importantly, FM showed an altered pattern of startle modulation, such that both erotica and mutilation inhibited the reflex relative to neutral pictures, a pattern that was not observed in RA and HC. Moreover, compared to RA, FM participants experienced less arousal in response to erotica and greater corrugator activity in response to mutilation. Although there was also a significant Group × Picture Content interaction for valence ratings, none of the simple effects tests were significant. The only emotional processing difference noted for RA was that they had smaller corrugator responses to mutilation than HC.
The significant order effect noted for startle, SCR, and noise ratings, indicated that startle magnitudes and SCRs habituated over time (unstandardized slopes: Bstartle = −1.44, BSCR = −.003), whereas noise ratings sensitized (B = 0.55). None of the main effects of group were significant, although there was a marginal group effect for startle (p = .07) and noise ratings (p = .07) suggesting that RA had larger startle reflexes than HC and HC had lower noise ratings that RA and FM (Fig 2).
There were only a few minor changes in the conclusions when participants on meds were excluded: (1) FM had lower valence ratings in response to erotica than HC (p < .05), (2) the group main effect for startle was significant (p = .008) indicating that RA had larger startle reflexes than HC and FM, (3) there was no difference between FM and RA in arousal ratings of erotica as noted in the total group, and (4) there was a significant Group × Picture Content interaction for noise ratings indicating that emotional modulation of noise ratings (Ero<Neu, Ero<Mut) was noted in the HC group, but not FM or RA.
3.3 NFR Threshold
Figure 3 depicts these results. A 1-way ANOVA found that there were significant group differences in NFR threshold, F(2, 53) = 6.10, p = .004. Surprisingly, FM participants had higher NFR thresholds than RA and HC (ps < .05). However, if FM participants who reached the 50 mA max were excluded from analysis (n = 5), there were no group differences, F(2, 45) < 1, p = .45, even though the means trended in the same direction (MHC = 17.18 mA, MRA = 15.10 mA, MFM = 19.86 mA, ps > .05). In either case, it appears that FM participants were not tonically sensitized at the spinal level. Moreover, this indicates that groups did not statistically differ in the intensity of stimulation used during emotional modulation of pain testing. Conclusions were unchanged if participants on medications were excluded.
Figure 3.
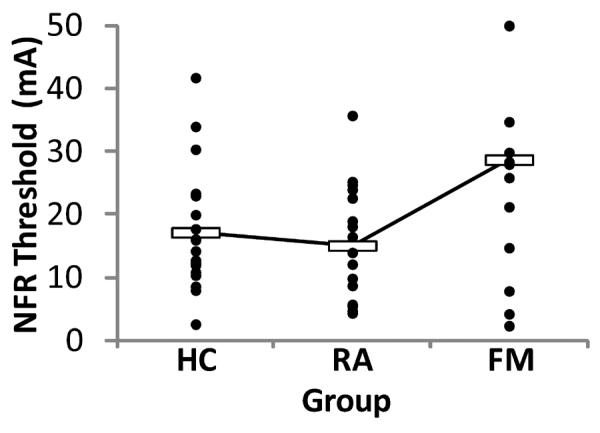
Group differences in nociceptive flexion reflex (NFR) threshold. Filled circles depict raw data for each group, whereas unfilled boxes represent group means. NFR threshold was significantly higher in participants with fibromyalgia (FM) compared to pain-free healthy controls (HC) and participants with rheumatoid arthritis (RA) (ps < .05). If those participants with FM who reached the 50 mA max were removed (n=5), there were no group differences. Together, these data indicate that the FM group does not have tonic spinal sensitization.
3.4 Outcomes during Emotional Modulation of Pain and NFR
Table 3 presents the inferential statistics for mixed effects ANOVAs of pain and NFR modulation outcomes. Figure 4 depicts means, SEMs, and mean contrasts for emotional reactivity variables and Figure 5 depicts means, SEMs, and mean contrasts for pain outcomes.
Table 3.
Results of mixed effect models predicting responses during pain modulation testing.
| Emotion Outcomes | Pain-Related Outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Valence Ratings | Corrugator EMG | Arousal Ratings | SCR | Pain Intensity Ratings | NFR Magnitude | |||||||
| ANOVA Effects | df | F | df | F | df | F | df | F | df | F | df | F |
| Group | 2, 49 | 2.03 | 2, 49 | 2.05 | 2, 49 | 0.46 | 2, 49 | 0.08 | 2, 49 | 1.88 | 2, 47 | 1.32 |
| Content | 2, 1410 | 1938.48* | 2, 747 | 67.28* | 2, 1295 | 303.51* | 2, 559 | 0.66 | 2, 587 | 14.28* | 2, 673 | 8.26* |
| Group × Content | 4, 1408 | 5.41* | 4, 746 | 6.98* | 4, 1292 | 8.42* | 4, 557 | 1.50 | 4, 586 | 3.55* | 4, 671 | 0.71 |
| Order | 1, 820 | 3.50# | 1, 569 | 6.23* | 1, 778 | 0.24 | 1, 397 | 3.05# | 1, 583 | 0.36 | 1, 425 | 1.04 |
Note:
p<.05,
p<.10
Figure 4.
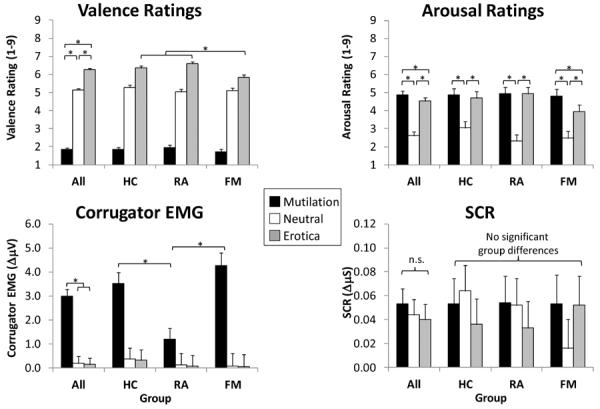
Emotional valence (valence ratings, corrugator EMG) and arousal (arousal ratings, skin conductance response [SCR]) reactions to pictures during pain testing. HC=pain-free healthy controls, RA= participants with rheumatoid arthritis, FM=participants with fibromyalgia. *p<.05
Figure 5.
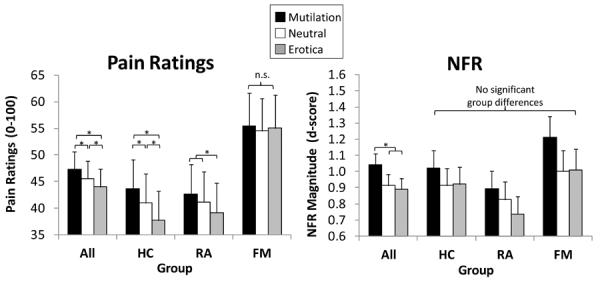
Emotional modulation of pain (left graph) and nociceptive flexion reflex (NFR; right graph) in healthy controls (HC), participants with rheumatoid arthritis (RA), and participants with fibromyalgia (FM). Emotional modulation of pain was evident in HC and RA, but not FM. By contrast, all groups demonstrated emotional modulation of spinal nociception (NFR).
3.4.1. Emotional reactions
There was a significant main effect of picture content for valence ratings, corrugator EMG, and arousal ratings, but not SCR (main effects depicted by the “All” group in Fig 4). These results indicate that, in general, mutilation pictures increased displeasure (reduced valence) ratings, corrugator EMG, and arousal ratings, whereas erotic pictures increased pleasure (valence) ratings and arousal ratings. SCR was not higher during mutilation or erotic pictures in the pain testing blocks (as we have observed elsewhere [67]).
There was also a significant Group × Picture Content interaction for valence ratings, corrugator EMG, and arousal ratings indicating that some measures of emotional processing were disrupted in FM. Specifically, (1) FM participants experienced less pleasure (valence) during erotica than RA and HC, (2) FM had greater corrugator activity in response to mutilation than RA, and (3) FM experienced less arousal to erotica than mutilation (unlike the other two groups). The significant order effect noted for corrugator EMG indicated that its activity habituated over time (unstandardized slope: B = −0.05). There were no substantive changes in the conclusions when participants on meds were excluded, except that the difference between FM and RA on corrugator activity was no longer significant.
3.4.2. Pain outcomes
There was a significant main effect of picture content for pain and NFR (main effects depicted by the “All” group in Fig 5). These results indicate that, in general, pain was highest during mutilation, intermediate during neutral, and lowest during erotica (all ps < .05), whereas NFR was higher during mutilation than neutral and erotica (ps < .05). However, there was a significant Group × Picture Content interaction for pain ratings that indicated emotional modulation of pain was disrupted in FM. Specifically, pain ratings did not differ by picture content in the FM group (Fig 5), but there was emotional modulation of pain in HC and RA. There were no substantive changes in these conclusions when participants on meds were excluded.
3.5 Exploratory Analyses: Are FM-related Differences due to Increased Psychological Distress?
To determine whether group differences in psychological distress (as measured by the Global Severity Index of SCL-90) mediates the group differences in emotional reactivity or emotional modulation of pain, the analyses were conducted again with psychological distress entered as a covariate. To minimize the number of these post-hoc analyses, only pain ratings and the emotional reactions with a significant Group × Picture Content interaction measured in the absence of pain were analyzed (i.e., valence ratings, corrugator EMG, startle, arousal ratings). Psychological distress was not a significant covariate in any analyses and all results were identical to those previously reported.
4.0 Discussion
4.1 Emotional Processing in FM
Consistent with prior studies[10], mutilation pictures evoked displeasure, corrugator activity, subjective arousal, and sympathetic activation (SCR), whereas erotic pictures evoked pleasure, subjective arousal, and sympathetic activation. This was true in the presence or absence of pain, except that SCR was not evoked by mutilation or erotica during pain testing, ostensibly due to concurrent pain-evoked sympathetic activation[67].
As hypothesized, there was evidence that emotional processing was disrupted in FM. In the absence of experimental pain, FM participants experienced less arousal to erotica and more corrugator activity to mutilation compared to RA (Fig 2). In the presence of experimental pain, FM participants experienced less pleasure and subjective arousal to erotica compared to HC and RA, and greater corrugator activity in response to mutilation compared to RA (Fig 4). Together, this suggests blunted appetitive responding in FM that is worsened by pain because lower valence ratings to erotica were only noted during pain testing. These observations may reflect a general deficit in appetitive responding in FM as noted by others[21,81,87]. However, because sexual victimization is prevalent in FM[83], it is also possible that the deficits were specific to erotica (not appetitive stimuli in general). Unfortunately, this issue cannot be resolved by the current study because non-sexual appetitive stimuli were not presented as a comparison and history of sexual victimization was not assessed.
Interestingly, startle was inhibited by erotica in all groups, including FM. Given that startle is inhibited by appetitive motivation[10], then the disruption of appetitive responding in FM may be restricted to neural structures associated with the conscious experience of positive affect. Emotional processing (both appetitive and defensive) involves a number of supraspinal structures[43,52,86], with some (e.g., hippocampus, orbitofrontal cortex) correlating with conscious emotional experience, and others (e.g., amygdala) correlating with the non-conscious detection and processing of emotional stimuli[25,86]. Given that startle modulation is mediated by the amygdala[39,86], our results suggest that amygdala responding to erotica was intact in FM. Therefore, disrupted hippocampus and/or orbitofrontal responsivity may have mediated the blunted appetitive responding in FM.
Mutilation (i.e., pictures of injured humans) evoked reactions consistent with defensive motivation in all groups: displeasure, arousal, and corrugator activity. However, mutilation-evoked corrugator activity was higher in FM than RA, perhaps suggesting a greater tendency to communicate negative affect via facial expressions by FM participants. Additionally, mutilation evoked startle inhibition in FM, but not in HC and RA, suggesting appetitive activation in this group[10]. While it is unclear what caused this atypical responding, it could reflect a greater tendency for the FM group to empathize with the injured subjects thus evoking an approach/appetitive motivation. If true, this appears to be a non-conscious process, because appetitive motivation was not apparent in the FM participants' subjective ratings of mutilation.
Taken together, some emotional processing differences were noted in FM, mostly related to deficits in appetitive responding. These findings are somewhat at odds with our prior study that found heightened defensive responding in FM, but not deficits in appetitive responding[5]. However, this is likely due to sampling differences, because disease severity in the current sample was higher (FIQcurrent=55 vs. FIQprior=15.5) and closer to the average FM patient than our previous study[7].
4.2 Emotional Modulation of Pain and Nociception in FM
As hypothesized, FM was associated with disrupted emotional modulation of pain. Pain was lower during pleasant compared to unpleasant pictures in HC and RA, a finding that has been found in several studies of HC[33,58,60,67,69,70]. However, pain was not modulated by pictures in FM (Fig 5). Interestingly, this difference remained even after controlling for SCL-90 scores, indicating that the deficit in pain modulation is not fully mediated by heightened psychological distress (e.g., anxiety, depression) in FM. Moreover, group differences in pain modulation were noted even though there were no group differences in NFR modulation. Therefore, descending modulation of NFR was intact in FM, a notion consistent with a recent study that showed a brief cognitive-behavioral intervention increased NFR thresholds in FM[1]. While preliminary, our data suggest that FM is associated with a dysfunction of circuits that modulate pain, not circuits that modulate spinal nociception.
Indeed, two separate modulatory mechanisms are engaged by emotional picture-viewing. This idea originally stemmed from a study that manipulated whether painful stimuli during pictures were unpredictable or predictable[68]. When unpredictable, pictures modulated pain and NFR in parallel[67,68]. However, when they were predictable, pictures modulated pain, but not NFR [68]. This suggested at least two mechanisms are engaged by emotional pictures: 1) brain-to-spinal cord circuitry that modulates NFR and 2) supraspinal (brain-only) circuitry that modulates pain perception. These two circuits were identified by an fMRI study that used emotional pictures to modulate pain and NFR[70]. They found that modulation of NFR involves the dorsolateral prefrontal cortex, parahippocampal gyrus, thalamus, amygdala, and brainstem nuclei, whereas pain modulation was associated with activity in the orbitofrontal cortex, subgenual cingulate cortex, cuneus, and insula. Thus, when taken together with the emotion processing data, it appears that amygdala function and emotional modulation of spinal nociception are intact in FM. By contrast, emotional modulation of pain and positive affect is disrupted, a problem that may stem from dysfunctions of supraspinal processing (e.g., insula, orbitofrontal cortex)[34,48].
4.3 Spinal Sensitization in FM
FM participants were clearly hyperalgesic on the tender-point exam (Table 1), but they did not have reduced NFR thresholds as would be expected if there was spinal sensitization. In fact, the FM group had higher NFR thresholds than RA and HC when FM participants who reached the 50 mA max (but no NFR, suggesting their NFR threshold was even higher) were included in the analyses (Fig 3). Moreover, results were the same when those on medications were excluded. So, it appears that FM is not associated with sensitization of spinal nociceptive processes.
This is inconsistent with two prior studies that found NFR thresholds in FM were lower than in HC[4,19]. In the largest study (NFM=85, NHC=40), the median NFR threshold for FM was approximately 22 mA[19]. Interestingly, this threshold is similar to what we observed in the present FM sample (Fig 3). However, another study reported even higher thresholds in FM (~35 mA), but they did not have a pain-free comparison group[1]. The first study's HC group had a median threshold near 35 mA[19], which is higher than published threshold norms[50] and our own studies of NFR in HC[22,61]. Therefore, it is not clear whether that study's HC sample was representative. In the only other study that compared FM (n=22) and HC (n=25), Banic et al. found that median thresholds were ~7 mA in FM, compared to ~13.5 mA in HC[4]. When considered together, it is unclear what may have led to discrepancies across studies. One possibility is procedural difference (e.g., supine vs. sitting testing position, criterion for reflex). Alternatively, our FM group may have been unusual in that their NFR modulation was intact, which reduced spinal sensitization.
4.4 Emotional Modulation of Noise Ratings
To our knowledge, this is the first study to demonstrate that emotion can modulate ratings of startle probes: noise intensity ratings were higher during mutilation than erotica. This finding was unexpected because noise ratings were only made to keep the procedures parallel across startle and pain blocks. Nonetheless, it is consistent with a study by Kenntner-Mabiala and Pauli[33] that found emotional pictures modulated ratings of non-painful, but aversive, electric stimuli in HC.
4.5 Study Limitations
This study had a number of strengths including measurement of both physiologic and subjective outcomes, well-validated paradigms, and powerful mixed-effects statistical models. Despite this, a few limitations should be noted. First, the sample sizes for all groups were relatively small, especially in the FM group because a few participants were unable to get an NFR. Second, we were unable to recruit enough FM and RA participants who were free of medications. However, removal of medicated individuals from the analyses did not substantively change the conclusions. And finally, only a few emotional contents were used to assess emotional processing and emotional modulation of pain/NFR. These were chosen because they produce the largest modulatory effects in HC, but it is possible that use of other contents would have produced a wider range of emotional responses and perhaps a more complete representation of group differences.
4.6 Summary
This study found that FM was associated with disrupted appetitive processing. Specifically, measures of subjective positive affect (i.e., valence and arousal ratings) were dampened in response to erotica. However, this deficit may be restricted to conscious processes, because viewing erotica inhibited the non-conscious startle reflex. Interestingly, mutilation pictures may have also evoked a paradoxical appetitive motivation in FM, because mutilation inhibited startle in FM only. Emotional modulation of pain was observed in HC and RA, but not FM. This deficit in pain modulation was not due to a dysfunction of circuitry that modulates spinal nociception, because emotional modulation of NFR was observed in all groups. Taken together, these results suggest that a disruption of supraspinal processing of emotion and pain may contribute to FM.
Acknowledgements
The project described was supported by Grant Number 5R03AR054571 from the National Institute of Arthritis And Musculoskeletal And Skin Diseases (NIAMS/NIH) awarded to Jamie L. Rhudy. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAMS or the NIH. The authors would like to thank Ashley Vincent and Satin Martin with help in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors have no conflicts of interest to report.
5.0 References
- [1].Ang DC, Chakr R, Mazzuca S, France CR, Steiner J, Stump T. Cognitive-behavioral therapy attenuates nociceptive responding in patients with fibromyalgia: a pilot study. Arthritis Care Res. 2010;62:618–623. doi: 10.1002/acr.20119. [DOI] [PubMed] [Google Scholar]
- [2].Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- [3].Ardic F, Toraman F. Psychological Dimensions of Pain in Patients with Rheumatoid Arthritis, Fibromyalgia Syndrome, and Chronic Low Back Pain. Journal of Musculoskeletal Pain. 2002;10:19. [Google Scholar]
- [4].Banic B, Petersen-Felix S, Andersen OK, Radanov BP, Villiger PM, Arendt-Nielsen L, Curatolo M. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain. 2004;107:7–15. doi: 10.1016/j.pain.2003.05.001. [DOI] [PubMed] [Google Scholar]
- [5].Bartley EJ, Rhudy JL, Williams AE. Experimental assessment of affective processing in fibromyalgia. J Pain. 2009;10:1151–1160. doi: 10.1016/j.jpain.2009.04.008. [DOI] [PubMed] [Google Scholar]
- [6].Bendtsen L, Norregaard J, Jensen R, Olesen J. Evidence of qualitatively altered nociception in patients with fibromyalgia. Arthritis Rheum. 1997;40:98. doi: 10.1002/art.1780400114. [DOI] [PubMed] [Google Scholar]
- [7].Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol. 2005;23:S154. [PubMed] [Google Scholar]
- [8].Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiol. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- [9].Bradley MM. Emotion and motivation. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. Vol. Cambridge University Press; New York: 2000. pp. 602–642. [Google Scholar]
- [10].Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- [11].Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001;1:300–319. [PubMed] [Google Scholar]
- [12].Bradley MM, Lang PJ. Measuring emotion: The Self-Assessment Manikin and the semantic differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- [13].Bradley MM, Lang PJ. Measuring emotion: Behavior, feeling, and physiology. In: Lane RD, Nadel L, editors. Cognitive neuroscience of emotion. Vol. Oxford University Press; 2000. pp. 242–276. [Google Scholar]
- [14].Celiker R, Borman P. Fibromyalgia versus rheumatoid arthritis: A comparison of psychological disturbance and life satisfaction. Journal of Musculoskeletal Pain. 2001;9:35. [Google Scholar]
- [15].Cook DB, Lange G, Ciccone DS, Liu WC, Steffener J, Natelson BH. Functional imaging of pain in patients with primary fibromyalgia. The Journal Of Rheumatology. 2004;31:364–378. [PubMed] [Google Scholar]
- [16].CSEA The International Affective Picture System: Digitized photographs. 1999 [Google Scholar]
- [17].Davis M. The neurophysiological basis of acoustic startle modulation: Research on fear motivation and sensory gating. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and orienting: Sensory and motivational processes. Vol. Lawrence Erlbaum Associates, Publishers; Mahwah, NJ: 1997. pp. 69–96. [Google Scholar]
- [18].Derogatis LR. Symptom Checklist 90-R: Administration, scoring, and procedures manual. Minneapolis: National Computer Systems. 1994 [Google Scholar]
- [19].Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48:1420. doi: 10.1002/art.10893. [DOI] [PubMed] [Google Scholar]
- [20].Edwards L, Ring C, McIntyre D, Carroll D. Modulation of the human nociceptive flexion reflex across the cardiac cycle. Psychophysiol. 2001;38:712–718. [PubMed] [Google Scholar]
- [21].Finan PH, Zautra AJ, Davis MC. Daily affect relations in fibromyalgia patients reveal positive affective disturbance. Psychosom Med. 2009;71:474–482. doi: 10.1097/PSY.0b013e31819e0a8b. [DOI] [PubMed] [Google Scholar]
- [22].France CR, Rhudy JL, McGlone S. Using normalized EMG to define the Nociceptive Flexion Reflex (NFR) threshold: Further evaluation of standardized scoring criteria. Pain. 2009;145:211–218. doi: 10.1016/j.pain.2009.06.022. [DOI] [PubMed] [Google Scholar]
- [23].France CR, Suchowiecki S. Assessing supraspinal modulation of pain perception in individuals at risk for hypertension. Psychophysiol. 2001;38:107–113. [PubMed] [Google Scholar]
- [24].French DJ, France CR, France JL, Arnott LF. The influence of acute anxiety on assessment of nociceptive flexion reflex thresholds in healthy young adults. Pain. 2005;114:358–363. doi: 10.1016/j.pain.2004.12.034. [DOI] [PubMed] [Google Scholar]
- [25].Garrett AS, Maddock RJ. Separating subjective emotion from the perception of emotion-inducing stimuli: an fMRI study. Neuroimage. 2006;33:263. doi: 10.1016/j.neuroimage.2006.05.024. [DOI] [PubMed] [Google Scholar]
- [26].Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev. 2004;27:729–737. doi: 10.1016/j.neubiorev.2003.11.008. [DOI] [PubMed] [Google Scholar]
- [27].Geisser ME, Casey KL, Brucksch CB, Ribbens CM, Appleton BB, Crofford LJ. Perception of noxious and innocuous heat stimulation among healthy women and women with fibromyalgia: Association with mood, somatic focus, and catastrophizing. Pain. 2003;102:243–250. doi: 10.1016/S0304-3959(02)00417-7. [DOI] [PubMed] [Google Scholar]
- [28].Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- [29].Greenwald MK, Bradley MM, Cuthbert BN, Lang PJ. Startle potentiation: Shock sensitization, aversive learning, and affective picture modulation. Behav Neurosci. 1998;112:1069–1079. doi: 10.1037//0735-7044.112.5.1069. [DOI] [PubMed] [Google Scholar]
- [30].Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- [31].Hox JJ. Multilevel Analysis: Techniques and Applications. Lawrence Erlbaum Associates; Mahwah, NJ: 2010. [Google Scholar]
- [32].Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114:295. doi: 10.1016/j.pain.2004.12.032. [DOI] [PubMed] [Google Scholar]
- [33].Kenntner-Mabiala R, Pauli P. Affective modulation of brain potentials to painful and nonpainful stimuli. Psychophysiol. 2005;42:559–567. doi: 10.1111/j.1469-8986.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- [34].Kim SH, Chang Y, Kim JH, Song HJ, Seo J, Han SW, Nam EJ, Choi TY, Lee SJ, Kim SK. Insular cortex is a trait marker for pain processing in fibromyalgia syndrome--blood oxygenation level-dependent functional magnetic resonance imaging study in Korea. Clin Exp Rheumatol. 2011;29:S19–S27. [PubMed] [Google Scholar]
- [35].Kosek E, Ekholm J, Hansson P. Sensory dysfunction in fibromyalgia patients with implications for pathogenic mechanisms. Pain. 1996;68:375. doi: 10.1016/s0304-3959(96)03188-0. [DOI] [PubMed] [Google Scholar]
- [36].Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70:41. doi: 10.1016/s0304-3959(96)03295-2. [DOI] [PubMed] [Google Scholar]
- [37].Lang PJ. The emotion probe: Studies of motivation and attention. Am Psychol. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- [38].Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Instruction manual and affective ratings. Technical Report A-4. 1999 [Google Scholar]
- [39].Lang PJ, Davis M. Emotion, motivation, and the brain: Reflex foundations in animal and human research. Prog Brain Res. 2006;156:3–29. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- [40].Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiol. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- [41].Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. The Clinical Journal Of Pain. 1997;13:189. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- [42].Lautenbacher S, Rollman GB, McCain GA. Multi-method assessment of experimental and clinical pain in patients with fibromyalgia. Pain. 1994;59:45. doi: 10.1016/0304-3959(94)90046-9. [DOI] [PubMed] [Google Scholar]
- [43].LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- [44].Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, Schubiner H, Keefe FJ. Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol. 2011;67:942–968. doi: 10.1002/jclp.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].McGlone ST, Rhudy JL, France CR. Emotional modulation of pain in normotensive individuals with and without a parental history of hypertension. Psychosomatic Medicine. 2009;71:A98. [Google Scholar]
- [46].McHorney CA, Ware JE, Lu JFR, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Medical Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- [47].Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- [48].Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 2012;64:2398–2403. doi: 10.1002/art.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. The Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- [50].Neziri AY, Andersen OK, Petersen-Felix S, Radanov B, Dickenson AH, Scaramozzino P, Arendt-Nielsen L, Curatolo M. The nociceptive withdrawal reflex: normative values of thresholds and reflex receptive fields. European Journal Of Pain (London, England) 2010;14:134–141. doi: 10.1016/j.ejpain.2009.04.010. [DOI] [PubMed] [Google Scholar]
- [51].Okifuji A, Turk DC, Marcus DA. Comparison of generalized and localized hyperalgesia in patients with recurrent headache and fibromyalgia. Psychosom Med. 1999;61:771. doi: 10.1097/00006842-199911000-00009. [DOI] [PubMed] [Google Scholar]
- [52].Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- [53].Pissiota A, Frans O, Michelgard A, Appel L, Langstrom B, Flaten MA, Fredrikson M. Amygdala and anterior cingulate cortex activation during affective startle modulation: a PET study of fear. The European Journal Of Neuroscience. 2003;18:1325–1331. doi: 10.1046/j.1460-9568.2003.02855.x. [DOI] [PubMed] [Google Scholar]
- [54].Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- [55].Price DD, Staud R. Neurobiology of fibromyalgia syndrome. The Journal of Rheumatology. 2005;75:22–28. [PubMed] [Google Scholar]
- [56].Rainville P, Bao QVH, Chretien P. Pain-related emotions modulate experimental pain perception and autonomic responses. Pain. 2005;118:306. doi: 10.1016/j.pain.2005.08.022. [DOI] [PubMed] [Google Scholar]
- [57].Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- [58].Rhudy JL, Bartley EJ. The effect of the menstrual cycle on affective modulation of pain and nociception in healthy women. Pain. 2010;149:365–372. doi: 10.1016/j.pain.2010.02.041. [DOI] [PubMed] [Google Scholar]
- [59].Rhudy JL, Bartley EJ, Williams AE. Habituation, sensitization, and emotional valence modulation of pain responses. Pain. 2010;148:320–327. doi: 10.1016/j.pain.2009.11.018. [DOI] [PubMed] [Google Scholar]
- [60].Rhudy JL, Bartley EJ, Williams AE, McCabe KM, Chandler MC, Russell JL, Kerr KL. Are there sex differences in affective modulation of spinal nociception and pain? The Journal of Pain. 2010;11:1429–1441. doi: 10.1016/j.jpain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- [61].Rhudy JL, France CR. Defining the nociceptive flexion reflex (NFR) threshold in human participants: A comparison of different scoring criteria. Pain. 2007;128:244–253. doi: 10.1016/j.pain.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rhudy JL, France CR, Bartley EJ, McCabe KM, Williams AE. Psychophysiological responses to pain: Further validation of the nociceptive flexion reflex (NFR) as a measure of nociception using multilevel modeling. Psychophysiol. 2009;46:939–948. doi: 10.1111/j.1469-8986.2009.00835.x. [DOI] [PubMed] [Google Scholar]
- [63].Rhudy JL, Green BA, Arnau RC, France CR. Taxometric analysis of biceps femoris EMG following electrocutaneous stimulation over the sural nerve: Determining the latent structure of the nociceptive flexion reflex (NFR) International Journal of Psychophysiology. 2008;69:18–26. doi: 10.1016/j.ijpsycho.2008.02.006. [DOI] [PubMed] [Google Scholar]
- [64].Rhudy JL, Martin SL, Terry EL, DelVentura JL, Kerr KL, Palit S. Using multilevel growth curve modeling to examine emotional modulation of temporal summation of pain (TS-pain) and the nociceptive flexion reflex (TS-NFR) Pain. 2012;153:2274–2282. doi: 10.1016/j.pain.2012.07.030. [DOI] [PubMed] [Google Scholar]
- [65].Rhudy JL, Martin SL, Terry EL, France CR, Bartley EJ, DelVentura JL, Kerr KL. Pain catastrophizing is related to temporal summation of pain, but not temporal summation of the nociceptive flexion reflex. Pain. 2011;152:794–801. doi: 10.1016/j.pain.2010.12.041. [DOI] [PubMed] [Google Scholar]
- [66].Rhudy JL, Meagher MW. The role of emotion in pain modulation. Curr Opin Psychiatry. 2001;14:241–245. [Google Scholar]
- [67].Rhudy JL, Williams AE, McCabe K, Nguyen MA, Rambo P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiol. 2005;42:579–587. doi: 10.1111/j.1469-8986.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- [68].Rhudy JL, Williams AE, McCabe KM, Rambo PL, Russell JL. Emotional modulation of spinal nociception and pain: The impact of predictable noxious stimulation. Pain. 2006;126:221–233. doi: 10.1016/j.pain.2006.06.027. [DOI] [PubMed] [Google Scholar]
- [69].Rhudy JL, Williams AE, McCabe KM, Russell JL, Maynard LJ. Emotional control of nociceptive reactions (ECON): Do affective valence and arousal play a role? Pain. 2008;136:250–261. doi: 10.1016/j.pain.2007.06.031. [DOI] [PubMed] [Google Scholar]
- [70].Roy M, Piche M, Chen J-I, Peretz I, Rainville P. Cerebral and spinal modulation of pain by emotions. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20900–20905. doi: 10.1073/pnas.0904706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Staud R. Abnormal Pain Processing in Patients with Fibromyalgia Syndrome. Journal of Chronic Fatigue Syndrome. 2004;12:71–77. [Google Scholar]
- [72].Staud R. Predictors of clinical pain intensity in patients with fibromyalgia syndrome. Current rheumatology reports. 2004;6:281–286. doi: 10.1007/s11926-004-0036-x. [DOI] [PubMed] [Google Scholar]
- [73].Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain. 2005;118:176–184. doi: 10.1016/j.pain.2005.08.007. [DOI] [PubMed] [Google Scholar]
- [74].Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. The Journal Of Pain: Official Journal Of The American Pain Society. 2007;8:893–901. doi: 10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Staud R, Robinson ME, Vierck CJ, Jr, Cannon RC, Mauderli AP, Price DD. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003;105:215–222. doi: 10.1016/s0304-3959(03)00208-2. [DOI] [PubMed] [Google Scholar]
- [76].Staud R, Robinson ME, Vierck CJ, Jr, Price DD. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain. 2003;101:167–174. doi: 10.1016/s0304-3959(02)00325-1. [DOI] [PubMed] [Google Scholar]
- [77].Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- [78].Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- [79].Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25:613. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- [80].Terry EL, France CR, Bartley EJ, DelVentura JL, Kerr KL, Vincent AL, Rhudy JL. Standardizing procedures to study sensitization of human spinal nociceptive processes: Comparing parameters for temporal summation of the nociceptive flexion reflex (TS-NFR) Int J Psychophysiol. 2011;81:263–274. doi: 10.1016/j.ijpsycho.2011.06.021. [DOI] [PubMed] [Google Scholar]
- [81].van Middendorp H, Lumley MA, Jacobs JWG, van Doornen LJP, Bijlsma JWJ, Geenen R. Emotions and emotional approach and avoidance strategies in fibromyalgia. J Psychosom Res. 2008;64:159–167. doi: 10.1016/j.jpsychores.2007.08.009. [DOI] [PubMed] [Google Scholar]
- [82].Villemure C, Bushnell MC. Cognitive modulation of pain: How do attention and emotion influence pain processing? Pain. 2002;95:195–199. doi: 10.1016/S0304-3959(02)00007-6. [DOI] [PubMed] [Google Scholar]
- [83].Walker EA, Keegan D, Gardner G, Sullivan M, Bernstein D, Katon WJ. Psychosocial factors in fibromyalgia compared with rheumatoid arthritis: II. Sexual, physical, and emotional abuse and neglect. Psychosom Med. 1997;59:572–577. doi: 10.1097/00006842-199711000-00003. [DOI] [PubMed] [Google Scholar]
- [84].Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- [85].Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- [86].Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- [87].Zautra AJ, Fasman R, Reich JW, Harakas P, Johnson LM, Olmsted ME, Davis MC. Fibromyalgia: Evidence for Deficits in Positive Affect Regulation. Psychosom Med. 2005;67:147. doi: 10.1097/01.psy.0000146328.52009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]