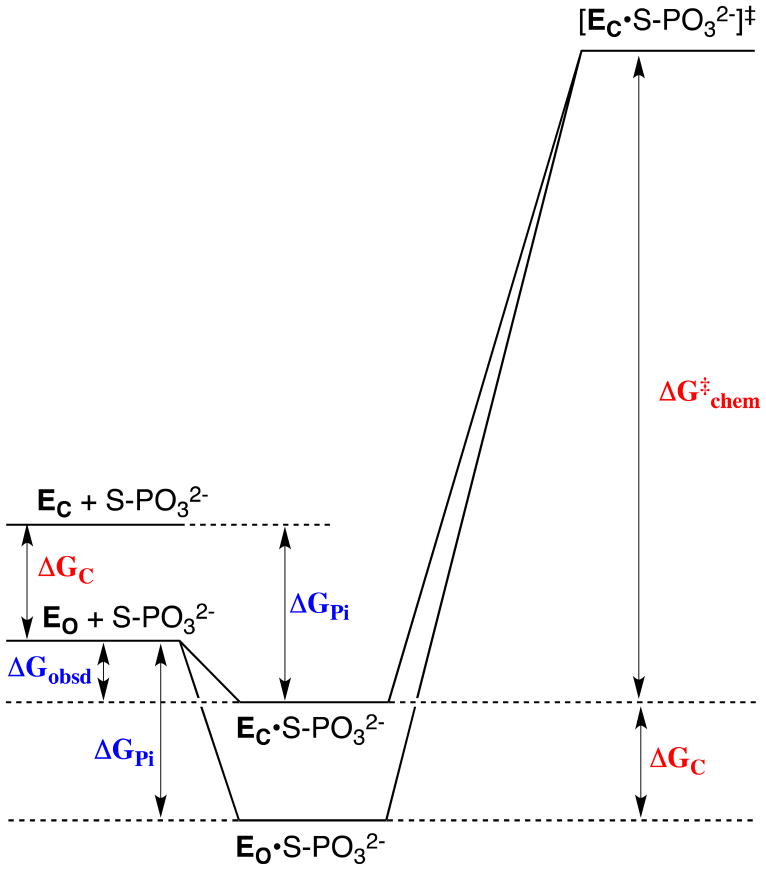
Figure 6.
Free energy profile for the reaction of a whole phosphorylated substrate S-PO32− catalyzed by an enzyme that exists predominantly in a stable inactive open form EO. For simplicity, the Figure shows only the binding energy of the phosphodianion portion of the substrate, ΔGPi. The relative small observed binding energy of the phosphodianion group (ΔGobsd) is the sum of the much larger total available binding energy of this group (ΔGPi) and the binding energy that is utilized to drive the unfavorable enzyme conformational change (ΔGC). Catalysis occurs at a higher energy active closed form EC, which is stabilized by interactions with the phosphodianion group of substrate at the EC•S-PO32− Michaelis complex (upper profile). In the case that the phosphodianion binding energy is not used to drive the unfavorable conformational change at the ground state Michaelis complex (lower profile), the activation barrier ΔG‡chem for turnover of the enzyme-bound substrate will increase by an amount ΔGC (see text).