Abstract
Rationale
Ziprasidone is an atypical antipsychotic recommended to be administered twice daily.
Objectives
The purpose of this study was to investigate whether occupancy of the dopamine D2/3 receptors by ziprasidone is maintained across a day employing a within subject design.
Methods
Positron Emission Tomography (PET) scans with [11C]-raclopride were performed in 12 patients with schizophrenia while treated with ziprasidone 60mg twice daily. Each patient completed [11C]-raclopride PET scans at 5, 13 and 23 hours after the last dose of ziprasidone. Dopamine D2/3 receptor occupancy was estimated with reference to binding potential data of 44 age- and sex-matched control subjects in the caudate, putamen and ventral striatum.
Results
Eleven scans were available at each time point, and the mean occupancies at 5-, 13- and 23-hour scans were 66%, 39% and 2% in the putamen; 62%, 35% and −6% in the caudate; and 68%, 47% and 11% in the ventral striatum, respectively. The time-course of receptor occupancy across the regions indicated an occupancy half-life of 8.3 hours. The serum level of ziprasidone associated with 50% D2/3 receptors occupancy was estimated to be 204nmol/L (84ng/ml). Prolactin levels were highest at 5-hour post-dose and none showed hyperprolactinemia at 23-hour scans.
Conclusions
The absence of ziprasidone striatal D2/3 receptor binding 23-hour after taking 60 mg under steady state conditions is consistent with its peripheral half-life. The results support our earlier report that ziprasidone 60 mg administered twice daily appears to be the minimal dose expected to achieve therapeutic central dopamine D2 receptor occupancy (i.e. 60%).
Keywords: [11C]-raclopride, dopamine D2 receptor, pharmacokinetics, Positron Emission Tomography, schizophrenia, ziprasidone
Introduction
Ziprasidone is an atypical antipsychotic shown to be effective in the treatment of schizophrenia and schizoaffective disorder (Daniel et al. 1999; Hirsch et al. 2002; Simpson et al. 2005; Stahl et al. 2010). Ziprasidone given 80–160 mg per day in divided dosage has been shown to be effective in treating positive, negative and affective symptoms with relatively low incidence of extrapyramidal side effects (EPS), weight gain, and metabolic side effects (Citrome 2009). Since ziprasidone has a relatively short peripheral half-life (6–7 hours), it is currently recommended to be taken in divided dosage – typically twice a day, with food (Caley and Cooper 2002).
Positron emission tomography (PET) studies have demonstrated a therapeutic window of striatal dopamine D2/3 receptor blockade by antipsychotics in vivo. Clinical response has been associated with 60–70% D2/3 receptor blockade and 80% or more has been associated with EPS (Farde et al. 1988; Kapur et al. 1995; Kapur et al. 2000a; Pilowsky et al. 1993). We have previously shown, using the radiotracer [11C]-raclopride, that ziprasidone (40–160mg/day) continued to occupy 56±18% of D2/3 receptors at expected plasma trough levels (12–16 hours post-dose) (Mamo et al. 2004). Recently, Vernaleken et al. (Vernaleken et al. 2008) have shown using the radiotracer [18F]-fallypride, that ziprasidone (80–160mg/day) continued to occupy 59% of D2/3 receptors in the putamen 7.4 hours post-dose. These results taken together, ziprasidone may continue to significantly occupy D2/3 receptors in the striatum at plasma trough levels.
However, the previous studies have employed a between-subjects design, and have not demonstrated continued D2/3 receptor occupancy beyond the predicted pharmacokinetics of ziprasidone within the same subjects with schizophrenia over time. A within-subjects design would more robustly determine whether there is a discrepancy between the time course of ziprasidone’s plasma concentration and D2/3 receptor occupancy in the striatum.
Using multiple [11C]-raclopride PET scans within the same subjects, we sought to characterize ziprasidone’s (60mg, twice a day) occupancy at the D2/3 receptors in relation to its peripheral pharmacokinetics over the 24 hours in patients with schizophrenia. Elucidating the relationship between ziprasidone’s central occupancy and peripheral pharmacokinetics will help inform optimal dosage frequency in the future; an important predictor of treatment adherence and relapse.
Methods
Participants
Adult patients aged 18–60 years old with a Structured Clinical Interview for DSM-IV (SCID-IV) diagnosis of schizophrenia or related psychoses were recruited from March 2007 to March 2010. All patients were found capable to participate in the study using the McArthur Competence Assessment Tool for Clinical Research (Appelbaum and Grisso, 2001). Exclusion criteria were: a history of intolerance or contraindication to ziprasidone, treatment with long-acting antipsychotics (within the past 6 months before entry), substance abuse or dependence (within the past 6 months), pregnancy, clinically significant abnormality in electrocardiography including a QTc interval of 450 msec or greater at the time of screening, co-treatment with medications known to affect QTc interval, annual radiation dose that would exceed 20mSv, a history of significant head trauma, unstable or uncontrolled medical conditions. The study was approved by the Research Ethics Board of the Centre for Addiction and Mental Health, Toronto, Canada, and all participants provided written informed consent. This trial has been registered in ClinicalTrials.gov (Identifier NCT00818298).
Treatment with ziprasidone
PET scanning was completed in eligible patients who were maintained on oral ziprasidone monotherapy at 120 mg/day in two equally divided doses for at least 1 week. Enrolled patients previously maintained on antipsychotics other than ziprasidone were switched to ziprasidone at a starting dosage of 20 mg twice a day, then titrated to 60mg twice a day. Patients previously maintained on olanzapine >5mg per day completed a one-week cross-titration to minimize the risk for withdrawal effects. Patients previously maintained on ziprasidone were kept on a dosage of 60 mg twice a day for at least 1 week prior to scanning. Study medication was dispensed in blister packs and adherence was monitored through pill count at each visit. All psychotropic agents other than ziprasidone were kept constant throughout the study and patients taking any psychotropics known to affect dopamine blockade other than ziprasidone were excluded from the study.
Assessments
The following scales were used to assess participants at baseline and at the time of each PET scan - Clinical Global Impression (CGI) (Guy W (1976). Clinical Global Impressions. In: ECDEU Assessment Manual for Psychopharmacology) and the Positive and Negative Syndrome Scale (PANSS).
PET image acquisition
[11C]-raclopride PET scans were completed at one of three time points following 60mg of oral ziprasidone: (Mean+SD) 287±12 minutes, 768±81 minutes, and 1402±46 minutes. Patients were taking ziprasidone twice daily, 60mg each in the morning and at night, and one night dose was skipped before the 23-h scan. Usually patients had 13-h scan in the morning after taking ziprasidone the night before, then again took the medication immediately after finishing the morning scan and scanned 5 hours late, although this schedule was slightly modified according to the availability of the PET camera. We refer to these as the 5-hour, 13-hour and 23-hour scans, respectively. We used a high-resolution neuro-PET camera system CPS-HRRT (Siemens Medical Imaging, Knoxville, TN, USA), which measures radioactivity in 207 brain sections with a thickness of 1.2mm each. The in-plane resolution of the scanner was 2.8mm full-width at half-maximum. Transmission scans were acquired for five minutes using a single photon point source, 137Cs (T=30.2yr, Eγ=662keV) and used to correct the emission scans for the attenuation of 511keV photons through tissue and head support. The emission data were acquired in list mode, thereby affording the highest possible temporal resolution. A custom-fitted thermoplastic mask (Tru-Scan Imaging, USA) was made for each subject and used with a head fixation system during PET scans in order to reduce any movement during the acquisition. No statistical differences were observed in injected dose, specific activity and mass of [11C]-raclopride among the PET scan sessions. A mean radioactivity dose of 9.91±0.71mCi with a specific activity of 2000±621mCi/mmol and mass of 1.92±0.72μg was injected as a bolus, followed by a flush with 2ml saline into an intravenous line placed in an antecubital vein. Scanning time was 60 minutes and 28 frames (5 one-minute, 20 two-minute, and 3 five-minute frames) were then defined.
MRI image acquisition
Patients were scanned with a 1.5T Signa scanner (General Electric Medical Systems, Milwaukee, WI) to acquire a proton density image (TE=17, TR=6000, FOV=22cm 2D, 256x256, slice thickness of 2mm, NEX=2). The MRI images were used for the analysis of the PET scans.
Image Analysis
The analysis of the PET images was performed as previously described (Graff-Guerrero et al. 2008). The quantitative estimate of binding was estimated using the Simplified Reference Tissue Method (SRTM) (Lammertsma et al. 1996) with the cerebellar cortex as the reference region and caudate, putamen and ventral striatum as regions of interest (ROIs). This method has been validated to reliably estimate binding potential nondisplaceable (BPND), which is the typical measurement from reference tissue methods, as it compares the concentration of radioligand in receptor-rich to receptor-free regions (Innis et al. 2007). BPND was estimated using the software PMOD v2.7 (PMOD Technologies Ltd., Zurich, Switzerland).
Receptor occupancy was defined as the percentage of reduction in the BPND from a baseline state to the treated state. In order to calculate receptor occupancy, a measure of baseline BPND in the antipsychotic-free (untreated) state was necessary. Since we were unable to obtain the baseline measures of receptor occupancy from the patients (when they were antipsychotic free) within our current design, we extrapolated the baseline BPND for each subject using our pooled data previously obtained from 44 age- and sex-matched healthy controls.
The occupancies were estimated with the following formula:
Nonlinear regression analysis was applied to correlate the plasma level of ziprasidone with receptor occupancy. The data was fitted to a rectangular hyperbola (one-site binding model) described by the equation: , where ED50 represents the serum level of ziprasidone resulting in 50% receptor occupancy and “X” corresponds to the ceiling receptor occupancy. In the model, X was fixed to 100 and ED50 >0. The ED50 was estimated for the ROIs combined. The time-course of receptor occupancy across the ROIs, indicating a central receptor occupancy half-life, was estimated by a 1-phase exponential decay equation:, where half-life is 0.6932/K and “X” corresponds to the ceiling receptor occupancy.
Serum ziprasidone and prolactin quantification
Serum ziprasidone levels were obtained immediately before tracer injection and before the end of the PET scan, 60 minutes apart, and the average value whenever available was used to estimate the ED50. They were determined using liquid chromatography with mass spectroscopy detection. Deuterated ziprasidone was used as the internal standard. Calibration curves were linear from 7.5 to 600 nmol/L, with 7.5 nmol/L being the limit of quantification and 3.0 nmol/L the limit of detection. The intra-assay coefficient of variation was less than 5%. Serum prolactin was measured immediately before tracer injection using the Beckman Coulter Access 2 instrument (reference range: male 3–13 ug/L; female 3–27 ug/L). The methodology utilizes paramagnetic particles in a chemiluminescent immunoassay. The reporting limit was 1 ug/L.
Statistical analysis
Wilcoxon signed rank test, Mann-Whitney’s U test, t-test and Spearman’s rank correlation coefficient were utilized as applicable. Statistical analyses were performed with SPSS (version 17.0, SPSS Inc, Chicago, Illinois) and a p-value of <0.05 was considered significant (two-tailed).
Results
Sample characteristics
Fourteen ambulatory patients meeting eligibility criteria were enrolled in the study. Antipsychotics prior to enrolling in the study were olanzapine (n=6), ziprasidone (n=4), quetiapine (n=2), and risperidone (n=2). Two subjects (one on olanzapine and one on risperidone) showed clinical deterioration during the switching/cross-titration and were withdrawn from the study before completing the PET scans.
Twelve subjects (6 female) with a diagnosis of schizophrenia underwent the PET scanning procedures. One subject did not complete the 13-hr scan due to clinical deterioration. In another subject, the 5-hr scan data was not analyzable due to an interruption of the scan, and the 23-hr scan data was not analyzable due to technical problems. Excluding these three scans, there were 11 scans per time point. Participants were on average 37.4 (±12.8) years of age and had a mean illness duration of 14.5 (±12.7) years. The study was not powered to address the clinical efficacy of ziprasidone and the results are presented for descriptive purposes only. The average total PANSS score for the 12 patients was 53.5 (±13.7) at baseline while the CGI-severity was 2.9 (±1.2), reflecting an overall mild severity. From baseline to the time of the respective PET scans, no significant differences were noted in any of the PANSS sub-scales. Similarly, CGI-change scores did not reveal a pattern of clinical deterioration during the scanning interval. At the time of the 5-/13-/and 23-hr scans, CGI-change scores compared to baseline were 3 (minimally better) for 2/3/1 patients, 4 (no change) for 7/6/8, and 5 (minimally worse) for 2/2/2, respectively.
Serum ziprasidone and prolactin levels
Figure 1 shows ziprasidone levels both before and after each PET scan. For 1 patient, post-scan level was not available and pre-scan level (at 23 hours post-dose) was utilized for analysis. Pre-scan ziprasidone levels were higher both at 5 hours versus 13 hours (Z=−2.578, p=0.010), and higher at 13 hours versus 23 hours (Z=−2.934, p=0.003). Pre-scan levels were significantly higher than post-scans at 13 hours in all cases. At 5 hours, pre-scan levels were higher than post-scan levels in 10 of 12 subjects but the difference did not reach statistical significance. At 23 hours, pre- and post-scan levels did not differ significantly.
Figure 1.
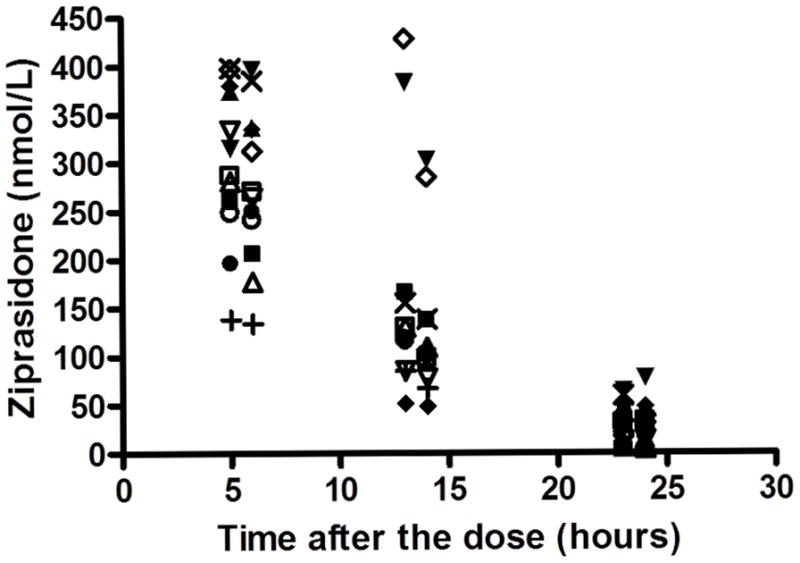
Serum ziprasidone level before and after each PET scan. Each symbol represents the same individual.
Prolactin levels were higher than the reference range in 7 patients at 5-hour (mean±SD: 23.5±13.6 ug/L, n=12), 2 at 13-hour (9.6 ±−9.4 ug/L, n=11) and 0 at 23-hour (5.1±3.7 ug/L, n=12). Prolactin levels were higher at 5 hours than 13 hours (Z=−2.934, p=0.003), and higher at 13 hours than 23 hours (Z=−1.994, p=0.046).
PET data
Radiochemical characteristics of raclopride at 5-, 13-, and 23-hour postziprasidone scans were as follows: injected dose 10.1 ± 0.69, 9.75 ± 0.82, and 9.90 ± 0.65 mCi; specific activity 2230 ± 573, 1792 ± 699, and 1979 ± 557 mCi/mmol; injected mass 1.68 ± 0.49, 2.18 ± 0.90, and 1.90 ± 0.69 μg, with no significant differences in the values across the scans. As is shown in Table 1 and Figure 3, ziprasidone produced a clinically relevant D2/3 receptor occupancy 5 hours after administration in the ROIs, which decreased at 13 hours and was equivalent to the baseline level of controls in 23 hours. There were no significant differences between the left and the right ROIs.
Table 1.
Dopamine D2 receptor occupancy by ziprasidone (n=11 for each scan)
| 5 hours post- dose scan | 13 hours post- dose scan | 23 hours post- dose scan | Differences across time-points (Wilcoxon signed rank test) | ||
|---|---|---|---|---|---|
| Putamen | Mean ± SD (%) | 65.6 ± 9.9 | 38.5 ± 25.8 | 1.99 ± 30.2 | 5h vs 13h: Z = −2.191, p = 0.028; 13h vs 23h: Z = −2.701, p = 0.007 |
| Median (%) | 67.0 | 31.0 | −9.97 | ||
| Range (%) | 9.4–78.6 | −4.41–76.8 | −37.6 −66.2 | ||
| Caudate | Mean ± SD (%) | 62.4 ± 10.0 | 34.9 ± 24.6 | −5.72 ± 31.3 | 5h vs 13h: Z = −2.497, p = 0.013; 13h vs 23h: Z = −2.701, p = 0.007 |
| Median (%) | 64.5 | 30.7 | −14.0 | ||
| Range (%) | 41.9 −77.9 | −0.449 −75.8 | −61.4–49.2 % | ||
| Ventral striatum | Mean ± SD (%) | 68.1 ± 7.7 | 46.8 ± 20.9 | 11.2 ± 29.8– | 5h vs 13h: Z = −2.497, p = 0.013; 13h vs 23h: Z = −2.497, p = 0.013 |
| Median (%) | 69.7 | 43.0 | 2.50 | ||
| Range (%) | 56.0–80.1 | 11.0–79.4 | −26.9–61.5 |
Figure 3.
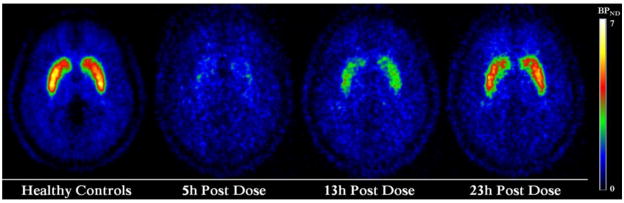
[11C]-raclopride binding potential non-displaceable maps. Images correspond to averaged [11C]-raclopride binding potential maps of healthy controls (n=44) and patients with schizophrenia after 5-hour (n=11), 13-hour (n=11) and 23-hour (n=11) of the last dose of ziprasidone (60 mg). The binding potential maps are presented with an absolute threshold between 0 and 7 and in axial projection illustrating the dorsal striatum regions.
Figure 2 shows serum ziprasidone levels versus D2/3 occupancy across the ROIs. There was a significant positive correlation in the putamen (n=33, rho=0.802, p=0.000), caudate (n=33, rho=0.846, p=0.000) and ventral striatum (n=33, rho=0.789, p=0.000). The ED50 across the ROIs was estimated to be 204nmol/L (84ng/ml; 95% confidence interval: 177–231nmol/L; R2=0.68). The corresponding value was 174 nmol/L for putamen, 205 nmol/L for caudate and 190 nmol/L for ventral striatum. The time-course of receptor occupancy across ROIs indicated an occupancy half-life of 8.3 hours (95% confidence interval: 4.6–39 hours; R2=0.56).
Figure 2.
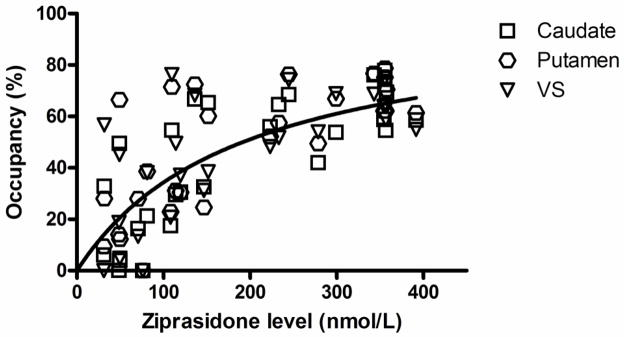
Relationship between ziprasidone serum levels and D2 receptor occupancy in regions of interest. The line corresponds to the fitting to one-site binding model across the regions of interest (ED50 = 204 nmol/L, R2 = 0.68). The occupancies in negative values were excluded from the graph and no considered to estimate the ED50.
Discussion
To our knowledge, there have been 5 published PET studies evaluating central dopamine occupancy by ziprasidone (Bench et al. 1996; Bench et al. 1993; Lammertsma et al. 1996; Mamo et al. 2004; Vernaleken et al. 2008). However, this is the first study to assess ziprasidone’s central binding at 3 time points using a within-subject design. Our results indicate that ziprasidone, as dosed in this study, has a mean occupancy of 66% in the putamen 5 hours after administration, which then declines to 39% at 13 hours and 2% at 23 hours, corresponding to an estimated central half-life of ziprasidone of 8.3 hours. Thus, the time-course of the drug’s central binding mirrors its known peripheral pharmacokinetics (peripheral half-life of 6–7 hours).
The serum ziprasidone levels we observed 13 hours post-dose in this study (150±103 nmol/L; median: 114 nmol/L) are consistent with data reported at trough in a larger therapeutic drug monitoring sample (median: 125 nmol/L for 120 mg/day) (Cherma et al. 2008). In contrast, the occupancy at 13 hours (39±26%) was slightly lower than that reported in previous PET studies (Mamo et al. 2004; Vernaleken et al. 2008), as well as a single photon emission tomography study that found D2/3 receptor occupancy of 60% at 12 hours post-dose (Corripio et al. 2005). We observed no appreciable drug binding 23 hours after the last dose of 60 mg, consistent with an earlier report in healthy subjects using a single dose of 40 mg (Bench et al. 1996).
Our results contrast with those reported by Vernaleken et al. (Vernaleken et al. 2008), possibly attributable to differences in study design and methodology. Specifically, Vernaleken et al. (Vernaleken et al. 2008) did not employ a within subject design, but instead compared occupancy in 7 subjects 7.4 hours after ziprasidone administration and in 8 other subjects 21.4 hours after ziprasidone administration. By employing a within-subject design, our study presumably better controls for the large inter-individual variability in the pharmacokinetics and dopamine D2/3 receptor occupancy of ziprasidone over time. Notably, Vernaleken et al. also used higher doses in their study (range: 120–200 mg/day; mean±SD 153±34 mg/day), versus a fixed dose of 60mg BID in our study.
We found the serum level of ziprasidone resulting in 50% receptor occupancy was estimated to be 84 ng/ml, which was higher than the previously reported values of 39–64 ng/ml (depending on the brain region) by Vernaleken et al. (2008) and 33 ng/ml by Mamo et al. (2004). It has been pointed out that a range of 50–200 ng/ml for ziprasidone may be effective (Hiemke et al. 2011) but it has also been identified that heterogeneity in levels is substantial with hits medication, both centrally (Gründer et al. 2011) and peripherally (Cherma et al. 2008). While this point is highly relevant in trying to translate the results of PET data to peripheral therapeutic drug monitoring (TDM) that is more easily applicable, and efforts are ongoing to better model pharmacokinetics of the medication (Wessels et al. 2011) and to predict central occupancy levels using peripheral drug levels (Uchida et al. 2011), it has been reported that blood levels of ziprasidone failed to well filter out responders, with no correlations observed between improvements or side effects and doses or serum levels (Vogel et al. 2009). As such TDM of ziprasidone may currently be of limited use in the real-world clinical practice although more studies are necessary.
Our results bear closer resemblance to those with quetiapine and clozapine for which no occupancy has been shown one day after the last dose (Kapur et al. 2000b; Kapur et al. 1999; Mamo et al. 2008; Suhara et al. 2002); they also stand in a sharp contrast to the longer central than peripheral pharmacokinetics reported with olanzapine and risperidone (Tauscher et al. 2002) as well as aripiprazole (Grunder et al. 2008). However, ziprasidone, which has a dissociation constant (K) between 2.7nM and to 6 nM, exhibits in vitro more than 20-fold higher affinity for the dopamine D2 receptors in comparison to clozapine (K=76 to 180 nM) and quetiapine (K=140 to 680 nM) (Seeman 2011). The rapid central decrease in D2/3 receptor occupancy we observed with ziprasidone may entirely be associated with a short central half-life that mirrors the peripheral half-life. This is in contrast to the combination of loose binding, fast dissociation and short central half-life described with quetiapine (Kapur et al. 2000b) and clozapine (Suhara et al. 2002). In addition, the short central occupancy half-life may contribute to its relatively low incidence of EPS at clinical doses (Citrome 2009). The time-course of striatal occupancy also was mirrored by a similar rate of decline in plasma prolactin levels, lending further support to our main finding. Further a marked discrepancy in occupancy values between single and multiple dose administration has been observed with ziprasidone (Vernaleken et al. 2008). Potential upregulation in D2 receptors upon chronic administration of ziprasidone (Heard et al. 2008) might account for somewhat higher binding potentials after 23 hours in patients than controls and worsening that was noted in some patients as well. Taken together, more work is necessary to better characterize this unique antipsychotic medication that binds tightly to dopamine receptors.
A major strength of this study is its within-subject design using [11C]-raclopride, a radiotracer with well-established test-retest stability (Uchida et al. 2009), and any residual effects from the previous antipsychotis are unlikely to affect dopamine blockade with ziprasidone under a steady state in a significant manner. Limitations of this study include a relatively small number of participants, precluding analysis of differences between responders and non-responders. Similarly, observed gender differences warrant replication in view of the small sample. The absence of baseline (untreated) PET data in subjects with schizophrenia is another potential limitation shared with most PET studies examining antipsychotic medications in patients with schizophrenia. However, having a “baseline” scan in the absence of any antipsychotics would be difficult and can be ethically problematic. Further, exposing subjects to many PET scans is not without risks and our facility regulates that a volunteer can have only up to 4 PET scans per year to protect safety. In addition, the magnitude of the error due to using a baseline obtained from healthy controls is estimated to be low: 0–4% for patients with an 80% occupancy and 0–9% for those with a 50% occupancy (Farde et al. 1992; Kapur et al. 1996), although a small elevation in receptor availability is still possible (Howes et al. 2012) and caution is warranted in extrapolating data from healthy controls. Our interest was confined to striatal dopamine D2/3 receptors using [11C]-raclopride. While ziprasidone may show some preferential extrastriatal binding (Vernaleken et al. 2008), the affinity of [11C]-raclopride for dopamine D2/3 receptors precludes accurate measurements of binding in extrastriatal regions. Furthermore, ziprasidone acts on multiple receptors and transporters (Stahl and Shayegan 2003) including serotonergic 5-HT2A receptors for which central kinetics is reported to be longer (Fischman et al. 1996), as well as 5-HT1A receptors (Frankle et al. 2011). Finally, our study adopted a fixed-dose design that might not reflect its dosing in clinical situations (Citrome et al. 2009).
We found that 13 hours after the last dose of ziprasidone, the dopamine D2/3 occupancy was well below the suggested 60% therapeutic threshold for PET studies with [11C]-raclopride. This time is expected to reflect the trough occupancy when ziprasidone is taken twice a day. Some antipsychotics such as clozapine and quetiapine have been shown to be clinically effective notwithstanding the fact that they are associated with transient and/or loose binding at D2/3 receptors (Kapur et al. 2000b; Mamo et al. 2008; Nordstrom et al. 1995; Pickar et al. 1996). This raises the possibility that ensuring 24-hour continuous D2/3 blockade above 60%, as predicted by the drugs’ pharmacokinetics, may not be a prerequisite to maintain wellness in schizophrenia. Nevertheless, our results suggest that in order to maintain continuous dopamine D2/3 receptor blockade, ziprasidone should be administered in its currently recommended divided dosage. In published studies, and in this study, some patients remain stable when they receive ziprasidone twice a day. However, more work is necessary to understand how the central occupancy of antipsychotics like ziprasidone relates to their clinical efficacy and tolerability. Well-designed clinical studies are necessary to quantify the risks and benefits of once daily versus twice-daily dosing of ziprasidone.
This study was the first to examine within-subject changes in ziprasidone’s occupancy at the dopamine D2/3 receptors over 24-hours using [11C]-raclopride. Our findings lead us to support that there is not discrepancy between the time course of ziprasidone’s plasma concentration and dopamine D2/3 receptor occupancy in the brain.
Acknowledgments
The authors gratefully acknowledge Alan Wilson, PhD, for supervising the radiochemical syntheses and Sylvain Houle, MD, PhD; Armando Garcia, BSc; Wanna Mar MSc (all are from the Centre for Addiction and Mental Health, Toronto, Ontario, Canada) for their assistance. They report no financial or other relationships with other organization, including pharmaceutical companies.
Footnotes
Previous Presentation:
Part of this work was presented at the Society of Biological Psychiatry 66th Annual Scientific Convention, in San Francisco May 12–14, 2011
Clinical Trials Registration: 24-Hour Time Course of Striatal Dopamine D2 Receptor Occupancy of Ziprasidone: A PET Study, www.clinicaltrials.gov/ct2/show/NCT00818298, NCT00818298
Conflicts of interest:
This study was supported by an investigator initiated research grant from Pfizer Canada Inc. Dr. Suzuki has received manuscript or speaker’s fees from Dainippon Sumitomo Pharma, Eli Lilly, Astellas, Meiji Seika Pharma and Novartis Pharma. Dr. Graff-Guerrerro currently receives research support from the following external funding agencies: Canadian Institutes of Health Research (CIHR), the US National Institute of Health (NIH), Ontario Mental Health Foundation, Mexico ICyTDF and CONACyT. Dr. Uchida has received grants from Pfizer and Dainippon-Sumitomo Pharma, and speaker’s honoraria from Otsuka Pharmaceutical, Eli Lilly, Novartis Pharma, Shionogi, GlaxoSmithKline, Yoshitomi Yakuhin, Dainippon-Sumitomo Pharma, and Janssen Pharmaceutical within the past 2 years. Dr. Gary Remington, in the last 3 years, has received research support (Principal Investigator) from the following external funding agencies: CIHR, Research Hospital Fund - Canada Foundation for Innovation GUF- CFI), Schizophrenia Society of Ontario (SSO), and the Canadian Diabetes Association (CDA). He has also received support from Novartis Canada Medicure Inc., and Neurocrine Bioscience. He has received consultant fees from Laboratorios Farmacduticos ROVI, Novartis, and Roche, as well as speaker’s fees from Novartis. He holds no commercial investments in any pharmaceutical company. Mr. Caravaggio and Ms. Borlido have nothing to disclose. Dr. Pollock receives research support from the US NIH, CIHR, American Psychiatric Association and the Foundation of the Centre for Addiction and Mental Health. Dr Mulsant currently receives research support from the CIHR, the US NIH, Bristol-Myers Squibb (medications for a NIH-funded clinical trial), and Pfizer (medications for a NIH-funded clinical trial). He directly owns stocks of General Electric (less than $5, 000). Within the past five years he has also received some grant support from Eli Lilly (medications for a NIH-funded clinical trial) and Janssen and some travel support from Roche. Dr. DeLuca has nothing to disclose. Dr. Ismail received support as consultant for Sunovion, BMS, Pfizer and Lundbeck. He also received research support form Pfizer and has honoraria from Otsuka, Lundbeck and Pfizer. Dr. Mamo currently receives research support from the following external funding agencies: CIHR, the US NIH, Ontario Ministry of Health and Long Term Care. He has also received grants or consultant fees from the Bristol-Myers Squibb and Pfizer and has received speaker’s honoraria from AstraZeneca in the past 3 years.
References
- Bench C, Lammerstma A, Grasby P, Dolan R, Frackowiak R, Warrington S, Boyce M, Gunn K, Brannick L. The time course of binding to striatal dopamine D2 receptors by the neuroleptic ziprasidone (CP-88,059-01) determined by positron emission tomography. Psychopharmacology. 1996;124:141–147. doi: 10.1007/BF02245614. [DOI] [PubMed] [Google Scholar]
- Bench C, Lammertsma A, Dolan R, Grasby P, Warrington S, Gunn K, Cuddigan M, Turton D, Osman S, Frackowiak R. Dose dependent occupancy of central dopamine D2 receptors by the novel neuroleptic CP-88,059-01: a study using positron emission tomography and11C-raclopride. Psychopharmacology. 1993;112:308–314. doi: 10.1007/BF02244926. [DOI] [PubMed] [Google Scholar]
- Caley CF, Cooper CK. Ziprasidone: the fifth atypical antipsychotic. Ann Pharmacother. 2002;36:839–51. doi: 10.1345/aph.1A053. [DOI] [PubMed] [Google Scholar]
- Cherma MD, Reis M, Hagg S, Ahlner J, Bengtsson F. Therapeutic drug monitoring of ziprasidone in a clinical treatment setting. Ther Drug Monit. 2008;30:682–8. doi: 10.1097/FTD.0b013e31818ac8ba. [DOI] [PubMed] [Google Scholar]
- Citrome L. Using oral ziprasidone effectively: the food effect and dose-response. Advances in Therapy. 2009;26:739–748. doi: 10.1007/s12325-009-0055-0. [DOI] [PubMed] [Google Scholar]
- Citrome L, Reist C, Palmer L, Montejano LB, Lenhart G, Cuffel B, Harnett J, Sanders KN. Impact of real-world ziprasidone dosing on treatment discontinuation rates in patients with schizophrenia or bipolar disorder. Schizophr Res. 2009;115:115–20. doi: 10.1016/j.schres.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Corripio I, Catafau AM, Perez V, Puigdemont D, Mena E, Aguilar Y, Carrió I, Álvarez E. Striatal dopaminergic D2 receptor occupancy and clinical efficacy in psychosis exacerbation: a 123I-IBZM study with ziprasidone and haloperidol. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29:91–96. doi: 10.1016/j.pnpbp.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Daniel DG, Zimbroff DL, Potkin SG, Reeves KR, Harrigan EP, Lakshminarayanan M. Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacology. 1999;20:491–505. doi: 10.1016/S0893-133X(98)00090-6. [DOI] [PubMed] [Google Scholar]
- Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–44. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- Farde L, Wiesel F-A, Halldin C, Sedvall G. Central D2-Dopamine Receptor Occupancy in Schizophrenic Patients Treated With Antipsychotic Drugs. Arch Gen Psychiatry. 1988;45:71–76. doi: 10.1001/archpsyc.1988.01800250087012. [DOI] [PubMed] [Google Scholar]
- Fischman AJ, Bonab AA, Babich JW, Alpert NM, Rauch SL, Elmaleh DR, Shoup TM, Williams SA, Rubin RH. Positron emission tomographic analysis of central 5-hydroxytryptamine2 receptor occupancy in healthy volunteers treated with the novel antipsychotic agent, ziprasidone. J Pharmacol Exp Ther. 1996;279:939–47. [PubMed] [Google Scholar]
- Frankle WG, Lombardo I, Kegeles LS, Slifstein M, Martin JH, Huang Y, Hwang DR, Reich E, Cangiano C, Gil R, Abi-Dargham A, Laruelle M. Measurement of the serotonin 1A receptor availability in patients with schizophrenia during treatment with the antipsychotic medication ziprasidone. J Psychopharmacol. 2011;25:734–43. doi: 10.1177/0269881110388329. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, Vitcu I, Seeman P, Wilson AA, Kapur S. Brain region binding of the D(2/3) agonist [(11)C]-(+)-PHNO and the D(2/3) antagonist [(11)C]raclopride in healthy humans. Hum Brain Mapp. 2008;29:400–10. doi: 10.1002/hbm.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunder G, Fellows C, Janouschek H, Veselinovic T, Boy C, Brocheler A, Kirschbaum KM, Hellmann S, Spreckelmeyer KM, Hiemke C, Rosch F, Schaefer WM, Vernaleken I. Brain and plasma pharmacokinetics of aripiprazole in patients with schizophrenia: an [18F]fallypride PET study. Am J Psychiatry. 2008;165:988–95. doi: 10.1176/appi.ajp.2008.07101574. [DOI] [PubMed] [Google Scholar]
- Gründer G, Hiemke C, Paulzen M, Veselinovic T, Vernaleken I. Therapeutic plasma concentrations of antidepressants and antipsychotics: lessons from PET imaging. Pharmacopsychiatry. 2011;44:236–48. doi: 10.1055/s-0031-1286282. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology rDPNA-NIoMHR, MD. 1976. Clinical Global Impressions; pp. 218–222. [Google Scholar]
- Heard K, Krier S, Zahniser NR. Administration of ziprasidone for 10 days increases cocaine toxicity in mice. Hum Exp Toxicol. 2008;27:499–503. doi: 10.1177/0960327108095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemke C, Baumann P, Bergemann N, Conca A, Dietmaier O, Egberts K, Fric M, Gerlach M, Greiner C, Gründer G, Haen E, Havemann-Reinecke U, Jaquenoud Sirot E, Kirchherr H, Laux G, Lutz UC, Messer T, Müller MJ, Pfuhlmann B, Rambeck B, Riederer P, Schoppek B, Stingl J, Uhr M, Ulrich S, Waschgler R, Zernig G. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44:195–235. doi: 10.1055/s-0031-1286287. [DOI] [PubMed] [Google Scholar]
- Hirsch SR, Kissling W, Bauml J, Power A, O’Connor R. A 28-week comparison of ziprasidone and haloperidol in outpatients with stable schizophrenia. J Clin Psychiatry. 2002;63:516–23. doi: 10.4088/jcp.v63n0609. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–86. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G, Zipursky RB, Wilson AA, Houle S. The D2 dopamine receptor occupancy of risperidone and its relationship to extrapyramidal symptoms: a PET study. Life Sci. 1995;57:PL103–7. doi: 10.1016/0024-3205(95)02037-j. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000a;157:514–20. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Jones C, Shammi CS, Remington G, Seeman P. A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiatry. 2000b;57:553–9. doi: 10.1001/archpsyc.57.6.553. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky RB, Jones C, Remington GJ, Wilson AA, DaSilva J, Houle S. The D2 receptor occupancy profile of loxapine determined using PET. Neuropsychopharmacology. 1996;15:562–6. doi: 10.1016/S0893-133X(96)00100-5. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286–93. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, Frackowiak RSJ. Comparison of Methods for Analysis of Clinical [11C]Raclopride Studies. J Cereb Blood Flow Metab. 1996;16:42–52. doi: 10.1097/00004647-199601000-00005. [DOI] [PubMed] [Google Scholar]
- Mamo D, Kapur S, Shammi CM, Papatheodorou G, Mann S, Therrien F, Remington G. A PET study of dopamine D2 and serotonin 5-HT2 receptor occupancy in patients with schizophrenia treated with therapeutic doses of ziprasidone. Am J Psychiatry. 2004;161:818–25. doi: 10.1176/appi.ajp.161.5.818. [DOI] [PubMed] [Google Scholar]
- Mamo DC, Uchida H, Vitcu I, Barsoum P, Gendron A, Goldstein J, Kapur S. Quetiapine extended-release versus immediate-release formulation: a positron emission tomography study. J Clin Psychiatry. 2008;69:81–6. doi: 10.4088/jcp.v69n0111. [DOI] [PubMed] [Google Scholar]
- Nordstrom AL, Farde L, Nyberg S, Karlsson P, Halldin C, Sedvall G. D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am J Psychiatry. 1995;152:1444–9. doi: 10.1176/ajp.152.10.1444. [DOI] [PubMed] [Google Scholar]
- Pickar D, Su TP, Weinberger DR, Coppola R, Malhotra AK, Knable MB, Lee KS, Gorey J, Bartko JJ, Breier A, Hsiao J. Individual variation in D2 dopamine receptor occupancy in clozapine-treated patients. Am J Psychiatry. 1996;153:1571–8. doi: 10.1176/ajp.153.12.1571. [DOI] [PubMed] [Google Scholar]
- Pilowsky LS, Costa DC, Ell PJ, Murray RM, Verhoeff NP, Kerwin RW. Antipsychotic medication, D2 dopamine receptor blockade and clinical response: a 123I IBZM SPET (single photon emission tomography) study. Psychol Med. 1993;23:791–7. doi: 10.1017/s0033291700025575. [DOI] [PubMed] [Google Scholar]
- Seeman P. All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2(high) receptors. CNS Neurosci Ther. 2011;17:118–32. doi: 10.1111/j.1755-5949.2010.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GM, Weiden P, Pigott T, Murray S, Siu CO, Romano SJ. Six-month, blinded, multicenter continuation study of ziprasidone versus olanzapine in schizophrenia. Am J Psychiatry. 2005;162:1535–8. doi: 10.1176/appi.ajp.162.8.1535. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Malla A, Newcomer JW, Potkin SG, Weiden PJ, Harvey PD, Loebel A, Watsky E, Siu CO, Romano S. A post hoc analysis of negative symptoms and psychosocial function in patients with schizophrenia: a 40-week randomized, double-blind study of ziprasidone versus haloperidol followed by a 3-year double-blind extension trial. J Clin Psychopharmacol. 2010;30:425–30. doi: 10.1097/JCP.0b013e3181e69042. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Shayegan DK. The psychopharmacology of ziprasidone: receptor-binding properties and real-world psychiatric practice. J Clin Psychiatry. 2003;64(Suppl 19):6–12. [PubMed] [Google Scholar]
- Suhara T, Okauchi T, Sudo Y, Takano A, Kawabe K, Maeda J, Kapur S. Clozapine can induce high dopamine D(2) receptor occupancy in vivo. Psychopharmacology (Berl) 2002;160:107–12. doi: 10.1007/s00213-001-0967-0. [DOI] [PubMed] [Google Scholar]
- Tauscher J, Jones C, Remington G, Zipursky RB, Kapur S. Significant dissociation of brain and plasma kinetics with antipsychotics. Mol Psychiatry. 2002;7:317–21. doi: 10.1038/sj.mp.4001009. [DOI] [PubMed] [Google Scholar]
- Uchida H, Graff-Guerrero A, Mulsant BH, Pollock BG, Mamo DC. Long-term stability of measuring D(2) receptors in schizophrenia patients treated with antipsychotics. Schizophr Res. 2009;109:130–3. doi: 10.1016/j.schres.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Uchida H, Takeuchi H, Graff-Guerrero A, Suzuki T, Watanabe K, Mamo DC. Predicting dopamine D2 receptor occupancy from plasma levels of antipsychotic drugs: a systematic review and pooled analysis. J Clin Psychopharmacol. 2011:318–25. doi: 10.1097/JCP.0b013e318218d339. [DOI] [PubMed] [Google Scholar]
- Vernaleken I, Fellows C, Janouschek H, Brocheler A, Veselinovic T, Landvogt C, Boy C, Buchholz HG, Spreckelmeyer K, Bartenstein P, Cumming P, Hiemke C, Rosch F, Schafer W, Wong DF, Grunder G. Striatal and extrastriatal D2/D3-receptor-binding properties of ziprasidone: a positron emission tomography study with [18F]Fallypride and [11C]raclopride (D2/D3-receptor occupancy of ziprasidone) J Clin Psychopharmacol. 2008;28:608–17. doi: 10.1097/JCP.0b013e31818ba2f6. [DOI] [PubMed] [Google Scholar]
- Vogel F, Gansmüller R, Leiblein T, Dietmaier O, Wassmuth H, Gründer G, Hiemke C. The use of ziprasidone in clinical practice: analysis of pharmacokinetic and pharmacodynamic aspects from data of a drug monitoring survey. Eur Psychiatry. 2009;24:143–8. doi: 10.1016/j.eurpsy.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Wessels AM, Bies RR, Pollock BG, Schneider LS, Lieberman JA, Stroup S, Li CH, Coley K, Kirshner MM, Marder SR. Population pharmacokinetic modeling of ziprasidone in patients with schizophrenia from the CATIE study. J Clin Pharmacol. 2011;51:1587–91. doi: 10.1177/0091270010387604. [DOI] [PubMed] [Google Scholar]


