Abstract
The collagen phase plays an important role in mechanical behaviors of cortical bone. However, aging effects on the mechanical behavior of the collagen phase is still poorly understood. In this study, micro-tensile tests were performed on demineralized human cortical bone samples from young, middle-aged, and elderly donors and aging effects on the mechanical properties of the collagen phase in different orientations (i.e. longitudinal and transverse directions of bone) were examined. The results of this study indicated that the elastic modulus and ultimate strength of the demineralized bone specimens decreased with aging in both the longitudinal and transverse orientations. However, the failure strain exhibited no significant changes in both orientations regardless of aging. These results suggest that the stiffness and strength of the collagen phase in bone are deteriorated with aging in both longitudinal and transverse directions. However, the aging effect is not reflected in the failure strain of the collagen phase in both longitudinal and transverse orientations, implying that the maximum sustainable deformation of the collagen phase is independent of aging and orientation.
Keywords: Bone, collagen, orientation, aging, strength, failure strain
INTRODUCTION
Bone is a composite material consisting of collagen fibrils, mineral phase, and water [1]. Changes in each of the constituents may inflict significant effects on the fragility of bone. Among them, the mineral phase with greater elastic modulus (~100GPa) imparts rigidity to bone and makes bone behave anisotropically due to the anisotropic nature and uneven distribution of mineral crystals in different orientations [2]. In the past, the effect of the mineral phase in bone was mainly considered when studying the age-related degradation of bone mechanical properties, such as bone mass loss and the decreased bone mineral density [3, 4]. However, recent studies have found that the collagen phase may also play a significant role in the mechanical behavior of bone [5–10].
It has been reported that aging has significant effects on mechanical properties of the collagen phase in cortical bone [9]. Experimental evidence shows that the collagen phase maintains a preferentially organized arrangement of collagen fibrils in healthy bones, but somehow altered in unhealthy tissues [11, 12]. Also observed is that the ultimate strength of the collagen network in dentin varies with loading orientations [13]. Since human cortical bone undertakes different loading modes at distinct anatomic locations during daily activities, collagen fibrils at the locations may have different preferred orientations [14, 15]. However, it is still not clear whether the aging effect on the mechanical behavior of the collagen phase is orientation-dependent.
In this study, the mechanical behavior (i.e. the elastic modulus, ultimate strength, and failure strain) of demineralized human cortical bone samples was measured in different orientations using a micro-tensile test. We hypothesized that aging effects on the mechanical behavior of the collagen phase are reflected in both longitudinal and transverse orientations of bone.
MATERIALS AND METHODS
Specimen preparation
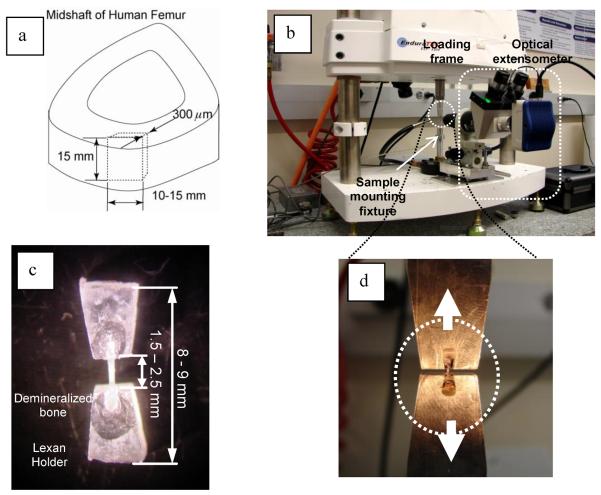
Middle shafts of fresh-frozen cadaveric femurs from fifteen male donors were procured from the National Disease Research Interchange (NDRI, Philadelphia, PA). These samples were divided into three age groups (N=5): young (20, 24, 25, 26 and 36 years old), middle aged (49, 51, 51, 52 and 55 years old), and elderly (72, 76, 76, 77 and 87 years old) groups. Briefly, bone slices with a thickness around 400μm were sectioned longitudinally from the same anatomic region (anterior region) of each femur using a low speed diamond saw (Buehler Isomet 2000 Precision Saw, Buehler, Lake Bluff, IL) (Fig.1). The slices were then lapped down to 300μm thick in sequential grits of grinding abrasives. Thereafter, all slices were demineralized by soaking them in a sodium citrate solution with formic acid for five (5) days with the solution changed once a day following the protocol reported elsewhere [16]. The completion of decalcification was verified by checking the concentration level of free calcium in the solution by neutralizing it with approximately 5ml of 0.5N NaOH and 1ml of 5% ammonium oxalate solution. Upon completion of decalcification, the samples were washed six times in a sonic water bath with distilled water to remove all traces of acid and soaked in PBS overnight. For ease of cut, all demineralized bone slices were partially dried for one hour at room temperature in a vacuum cascader with Drieriter granules. Strips about 500μm wide and 3.0mm long were dissected using a surgical scalpel under microscope in both the transverse and longitudinal orientations of bone. The dissected (collagen network) strips were secured in a pair of plastic (Lexan) holders using a cyanoacrylate glue for ease of later tensile tests (Fig. 1). All the specimens were soaked in PBS for at least one hour to ensure full hydration prior to mechanical tests. The areas of cross-sections of the specimens were measured under microcopy in reference with a standard micro-graduated slide.
Figure 1.
Preparation of demineralized bone specimens (a) and the setup for the tensile test (b). The specimens were glued onto a pair of plastic (Lexan) holders (c) and mounted on the loading fixtures (d) in the mechanical test machine.
Mechanical testing
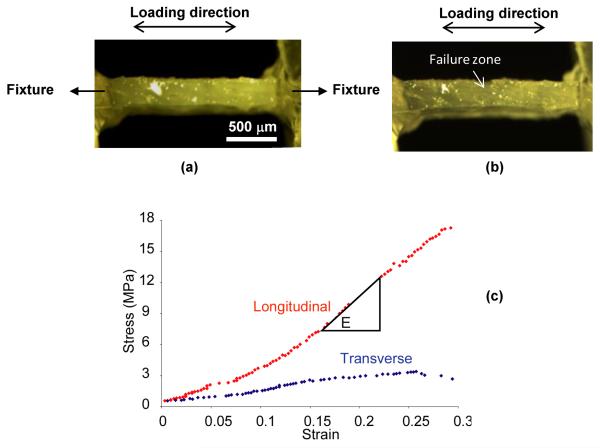
The tensile specimens with the plastic holders were attached to a loading fixture in a bench-top mechanical testing system (currently Bose ElectroForce@ 3330) (Fig. 1). All specimens were first preloaded to 4.0 grams in tension, wiped off the water on the surface, and then loaded to failure in tension at a constant loading rate of 0.01mm/s recommended by the previous studies [17]. Since the collagen phase is viscoelastic in nature, the consistent loading rate can help avoid potential effects of viscoelasticity on the measurements. The image (1280×960 Pixels) of the side view of the specimens during loading were recorded at a rate of one image per second using a digital microscopic photo system (a monocular microscope with a Motic 3000 CCD camera). Representative images of the specimen pre and post failure are shown in Fig. 2. Strain was calculated using a custom Matlab script that automatically captures the elongation of the distance (in pixels) between two reference spots along the gage length of the specimen on an image-by-image basis. Stress was calculated by dividing the applied force by the cross-sectional area of the specimens measured prior to load. Representative loading strain-stress curves for the transverse and longitudinal specimens are presented in Fig. 2. Elastic modulus was determined as the slope of the linear part of the strain-stress curve measured in a strain range of 0.05. The ultimate strength (σu) of each specimen was determined as the maximum stress and the failure strain (εf) as the corresponding maximum strain at the failure of the specimen.
Figure 2.
Representative images of the longitudinal specimen (a) pre and (b) at failure and representative loading curves of demineralized bone specimens from the Middle aged group in both longitudinal and transverse axes (c).
Statistical analysis
Two-way analysis of variance (ANOVA) (JMP 5.1, SAS institute Inc., Cary, NC) was used to determine the effects of age and orientation on the ultimate strength and failure strain of the demineralized bone (collagen network) specimens. Simple t-tests were performed to detect the difference between the groups. The significance level for all tests was p < 0.05.
RESULTS
The descriptive statistics of the mechanical properties of the mineralized bone samples in different age groups and loading orientations are shown in Table 1. Two-way ANOVA analyses indicated that both aging and orientation imposed significant effects on the elastic modulus and ultimate strength, but had little influences on the failure strain of the demineralized bone specimens (Table 2).
Table 1.
Mechanical properties of the collagen network (N=5)
| Age groups | Etrans (MPa) | EIong (MPa) | ε ftranse | ε flong | σutranse (MPa) | σulong (MPa) |
|---|---|---|---|---|---|---|
| Young | 21.5±11.8 | 90.3±35.5 | 0.25±0.05 | 0.34±0.12 | 4.48±1.58 | 19.9±2.58 |
| Middle-aged | 12.1±7.49 | 89.3±43.5 | 0.33±0.17 | 0.26±0.13 | 2.91±0.39 | 15.4±1.22 |
| Elderly | 7.71±2.88 | 46.5±21.2 | 0.26±0.14 | 0.33±0.12 | 2.31±0.41 | 13.2±2.44 |
Etrans, elastic modulus along the transverse direction; Elong, elastic modulus along the longitudinal direction; εftranse, failure strain loaded along the transverse direction; εflong, failure strain loaded along the longitudinal direction; σutranse, ultimate strength loaded along the transverse direction; σulong, ultimate strength loaded along the longitudinal direction.
Table 2.
ANOVA analysis on the effects of aging and orientation (N=5)
| Factor | E (MPa) | εf (μm/μm) | σu (MPa) |
|---|---|---|---|
| Age | S | NS | S |
| Orientation | S | NS | S |
S: significant difference (p<0.05); NS: not significant (p>0.05). E, elastic modulus along the transverse direction; εf, failure strain loaded along the transverse direction; σu, ultimate strength loaded along the longitudinal direction.
The multiple comparisons indicated that the elastic modulus (E) of the collagen network decreased with age in both longitudinal and transverse directions. The significant difference in E was observed between young and elderly groups in both orientations, whereas such a difference was significant only in the longitudinal direction between the middle-aged and elderly group (Tables 1 and 3).
Table 3.
p- values of multiple comparisons between groups
| Etrans (MPa) | Elong (MPa) | ε ftranse | ε flong | σutranse (MPa) | σulong (MPa) | |
|---|---|---|---|---|---|---|
| Young vs. Middle-aged | 0.17 | 0.98 | 0.35 | 0.32 | <0.05 | <0.05 |
| Young vs. Elderly | <0.05 | <0.05 | 0.90 | 0.85 | <0.05 | <0.05 |
| Middle-aged vs. Elderly | 0.26 | <0.05 | 0.49 | 0.39 | <0.05 | 0.10 |
The ultimate strength (σu) of the collagen phase decreased with age in both longitudinal and transverse directions (Tables 1 and 3). In the longitudinal direction, significant differences were observed only between young age groups and the other two age groups (middle aged and elderly), whereas no significant difference was found between the middle aged and elderly groups. In the transverse direction, however, significant differences were present among all the age groups.
The failure strain (εf) of the collagen network showed no significant trend of changes with age in both transverse and longitudinal orientations. In addition, εf didn't exhibit significant differences between the two loading directions (Tables 1 and 3).
DISCUSSION
The results of this study indicate that aging may impose significant effects on the mechanical integrity of the collagen network (demineralized bone) in both longitudinal and transverse orientations. Such effects are mainly reflected in its elastic modulus and tensile strength, but not in the failure strain of the collagen network.
The mechanical properties of demineralized bone specimens have been reported in the literature, showing a wide range of variations in the elastic modulus (from 275±94MPa to 860±113MPa), ultimate stress (from 15±4.2MPa to 61.5±13.1MPa), and strain-to-failure (from 8.4 ±1.6% to 12.3±0.5%) [18, 19]. Comparing with the values reported in the literature, the elastic modulus obtained in this study is much smaller; the ultimate stress is within the range, whereas the failure strain is much higher (Table 1). Such discrepancies are most likely due to the differences in the test conditions between the studies, such as type of bone, anatomic locations, loading rate, and specimen shape/size. In addition, similar to the results of this study, a previous study also reported that there was no significant orientation-dependence in the failure strain between the demineralized bovine bone specimens [20]. Moreover, by testing individual collagen fibrils, it was shown that the elastic modulus yield and failure strain of fibrils were 0.86±0.45GPa, 22±14%, and as high as 100%, respectively [21].
Similar age-related decreases in the strength and stiffness of the collagen phase in the longitudinal direction of bone have been observed in other studies [9, 22], whereas this study indicate that age-related decreases in these properties also exist in the transverse direction. Such changes are considered to be attributable to multiple factors. For instance, age-related increases in collagen porosity may directly lead to decreases in the stiffness and strength of the collagen phase. In addition, the fibril concentration in bone was reported to decrease significantly from middle aged to elderly donors [14]. In this case, the reduced concentration of collagen fibrils may make the collagen phase more compliant and weaker. Moreover, ex vivo studies on human bone indicated that age-related increases in the amount of advanced glycation end products (AGEs) in bone matrix were associated with decreases in the stiffness and strength of the collagen phase [10, 23]. However, some in vitro studies exhibited that the stiffness of collagen phase treated with ribose or other glucose solutions increased due to the non enzymatic crosslinks induced through the glycation processes [24–26]. Thus, it would be unlikely that non enzymatic crosslinks are dominant in the age-related changes in the stiffness and strength of the collagen phase of human cortical bone.
An intriguing finding of this study is that the failure strain of the collagen network (demineralized bone) is not age-dependent (p>0.05) in both longitudinal and transverse directions, showing that the failure strain of the collagen phase in bone is relatively consistent irrespective of aging and orientations. The underlying mechanism is not clear due to the limited scope of this study. To address this issue, further investigations are needed.
Moreover, it is noteworthy that age-related changes in the mechanical integrity of the collagen phase may not be reflected in the mechanical behavior of intact bone. For instance, some studies have shown that the elastic modulus of human cortical bone is not significantly affected by aging [9, 27]. Since the stiffness of bone is mainly determined by the mineral phase, age-related changes in the elastic modulus of the collagen network may not inflict marked influence on the overall elastic modulus of bone. In addition, consistent with the previous study [9] the results of this study show that aging has a limited effect on the failure strain of the collagen network. However, the ultimate strain of intact bone has been reported to decrease significantly with age [27], suggesting that the age-related effect on the failure strain of the collagen phase is not directly related to the intact bone behavior. It is not surprising because bone usually fails at much smaller strain (3–4%) in tension than the failure strain (~30%) for the collagen phase.
There are several limitations of this study. First, the sample size is relatively small (N=5), thus leading to a limited power to distinguish smaller differences shown in the ultimate strength between the middle-aged and elderly bone specimens. Next, the stress calculated in this study is an apparent one which does not take into account effects of tissue porosity and other microstructure changes. Moreover, no effects by gender and anatomic location are considered in this study. Nonetheless, this approach can be used in the future to address these issues.
In summary, by testing the demineralized bone specimens it was found that the elastic modulus and strength of the collagen phase were age and orientation-dependent, whereas its failure strain was relatively consistent irrespective of age and orientation.
ACKNOWLEDGEMENT
This study was partially supported by a NIH/NIAMS grant (1R01AR055955), a NSFC grant (11002004) and a grant from the Ph.D. Programs Foundation of Ministry of Education of China (200900001120106). The authors are grateful of Mr. Siyuan Ding for his assistance in preparing the test specimens.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Katz JL. Hard tissue as a composite material. I. Bounds on the elastic behavior. J Biomech. 1971;4:455–73. doi: 10.1016/0021-9290(71)90064-9. [DOI] [PubMed] [Google Scholar]
- [2].Hasegawa K, Turner CH, Burr DB. Contribution of collagen and mineral to the elastic anisotropy of bone. Calcified Tissue Int. 1994;55:381–6. doi: 10.1007/BF00299319. [DOI] [PubMed] [Google Scholar]
- [3].Currey JD, Brear K, Zioupos P. The effects of ageing and changes in mineral content in degrading the toughness of human femora. J Biomech. 1996;29:257–60. doi: 10.1016/0021-9290(95)00048-8. [DOI] [PubMed] [Google Scholar]
- [4].Kotha SP, Walsh WR, Pan Y, Guzelsu N. Varying the mechanical properties of bone tissue by changing the amount of its structurally effective bone mineral content. Biomed Mater Eng. 1998;8:321–34. [PubMed] [Google Scholar]
- [5].Boskey AL, Wright TM, Blank RD. Collagen and bone strength. J Bone Miner Res. 1999;14:330–5. doi: 10.1359/jbmr.1999.14.3.330. [DOI] [PubMed] [Google Scholar]
- [6].Burr DB. The contribution of the organic matrix to bone's material properties. Bone. 2002;31:8–11. doi: 10.1016/s8756-3282(02)00815-3. [DOI] [PubMed] [Google Scholar]
- [7].Burstein AH, Zika JM, Heiple KG, Klein L. Contribution of collagen and mineral to the elastic-plastic properties of bone. J Bone Joint Surg. 1975;57-A:956–961. [PubMed] [Google Scholar]
- [8].Skedros JG, Sorenson SM, Takano Y, Turner CH. Dissociation of mineral and collagen orientations may differentially adapt compact bone for regional loading environments: results from acoustic velocity measurements in deer calcanei. Bone. 2006;39:143–51. doi: 10.1016/j.bone.2005.12.007. [DOI] [PubMed] [Google Scholar]
- [9].Wang X, Shen X, Li X, Mauli Agrawal C. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- [10].Zioupos P, Currey JD, Hamer AJ. The role of collagen in the declining mechanical properties of aging human cortical bone. J Biomed Mater Res. 1999;45:108–16. doi: 10.1002/(sici)1097-4636(199905)45:2<108::aid-jbm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [11].Fountos G, Kounadi E, Tzaphlidou M, Yasumura S, Glaros D. The effects of inflammation-mediated osteoporosis (IMO) on the skeletal Ca/P ratio and on the structure of rabbit bone and skin collegen. Appl Radiat Isotopes. 1998;49:657–659. doi: 10.1016/s0969-8043(97)00086-9. [DOI] [PubMed] [Google Scholar]
- [12].Tzaphlidou M, Kafantari H. Influence of nutritional factors on bone collagen fibrils in ovariectomized rats. Bone. 2000;27:635–638. doi: 10.1016/s8756-3282(00)00382-3. [DOI] [PubMed] [Google Scholar]
- [13].Miguez PA, Pereira PNR, Atsawasuwan P, Yamauchi M. Collagen Cross-linking and Ultimate Tensile Strength in Dentin. J Dent Res. 2004;83:807–810. doi: 10.1177/154405910408301014. [DOI] [PubMed] [Google Scholar]
- [14].Goldman HM, Bromage TG, Thomas CD, Clement JG. Preferred collagen fiber orientation in the human mid-shaft femur. Anat Rec A Discov Mol Cell Evol Biol. 2003;272:434–45. doi: 10.1002/ar.a.10055. [DOI] [PubMed] [Google Scholar]
- [15].Portigliatti Barbos M, Bianco P, Ascenzi A, Boyde A. Collagen orientation in compact bone: II. Distribution of lamellae in the whole of the human femoral shaft with reference to its mechanical properties. Metab Bone Dis Relat Res. 1984;5:309–15. doi: 10.1016/0221-8747(84)90018-3. [DOI] [PubMed] [Google Scholar]
- [16].Yeni Y, Schaffler M, Gibson G, Fyhrie D. Prestress due to dimensional changes caused by demineralization: A potential mechanism for microcracking in bone. Ann Biomed Eng. 2002;30:1–9. doi: 10.1114/1.1451078. [DOI] [PubMed] [Google Scholar]
- [17].Paul SJ, Leach M, Rueggeberg FA, Pashley DH. Effect of water content on the physical properties of model dentine primer and bonding resins. J Dent. 1999;27:209–14. doi: 10.1016/s0300-5712(98)00042-6. [DOI] [PubMed] [Google Scholar]
- [18].Bowman SM, Zeind J, Gibson LJ, Hayes WC, McMahon TA. The tensile behavior of demineralized bovine cortical bone. J Biomech. 1996;29:1497–501. doi: 10.1016/0021-9290(96)84546-5. [DOI] [PubMed] [Google Scholar]
- [19].Catanese J, 3rd, Iverson EP, Ng RK, Keaveny TM. Heterogeneity of the mechanical properties of demineralized bone. J Biomech. 1999;32:1365–9. doi: 10.1016/s0021-9290(99)00128-1. [DOI] [PubMed] [Google Scholar]
- [20].Trebacz H, Zdunek A, Dys W, Gieroba T, Wlizlo E. Effects of nonenzymatic glycation on mechanical properties of demineralized bone matrix under compression. J Appl Biomater Biomech. 2011;9:144–9. doi: 10.5301/JABB.2011.8568. [DOI] [PubMed] [Google Scholar]
- [21].Shen ZL, Dodge MR, Kahn H, Ballarini R, Eppell SJ. Stress-strain experiments on individual collagen fibrils. Biophys J. 2008;95:3956–63. doi: 10.1529/biophysj.107.124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Danielsen CC, Andreassen TT, Mosekilde L. Mechanical properties of collagen from decalcified rat femur in relation to age and in vitro maturation. Calcif Tissue Int. 1986;39:69–73. doi: 10.1007/BF02553293. [DOI] [PubMed] [Google Scholar]
- [23].Wang X, Bank RA, TeKoppele JM, Agrawal CM. The role of collagen in determining bone mechanical properties. J Orthop Res. 2001;19:1021–6. doi: 10.1016/S0736-0266(01)00047-X. [DOI] [PubMed] [Google Scholar]
- [24].Tang SY, Vashishth D. Non-enzymatic glycation alters microdamage formation in human cancellous bone. Bone. 2010;46:148–54. doi: 10.1016/j.bone.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007;40:1144–51. doi: 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vashishth D. Collagen glycation and its role in fracture properties of bone. J Musculoskelet Neuronal Interact. 2005;5:316. [PubMed] [Google Scholar]
- [27].McCalden RW, McGeough JA, Barker MB, Court-Brown CM. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg Am. 1993;75:1193–205. doi: 10.2106/00004623-199308000-00009. [DOI] [PubMed] [Google Scholar]