Abstract
Herpes simplex virus type 1 (HSV)-specific CD8+ T cells provide immunosurveillance of trigeminal ganglion (TG) neurons that harbor latent HSV-1. In C57BL/6 mice the TG-resident CD8+ T cells are HSV-specific and maintain a 1:1 ratio of cells recognizing an immunodominant epitope on viral glycoprotein B (gB498–505-Tet+) and cells reactive to subdominant epitopes (gB-Tet−). The gB-Tet− CD8+ T cells maintain their frequency in TG by balancing a higher rate of proliferation with a correspondingly higher rate of apoptosis. The increased apoptosis is associated with higher expression of Programmed Death-1 (PD-1) on gB-Tet− CD8+ T cells, and the interaction with PD-1 ligand (PD-L1/B7H1). IFN-γ regulated expression of the PD-1 ligand (PD-L1/B7H1) on neurons bearing higher copies of latent viral genome. In latently infected TG of B7H1−/− mice, the number and frequency of PD-1+ gB-Tet− CD8+ T cells increases dramatically, but gB-Tet− CD8+ T cells remain largely non-functional, and do not provide increased protection from HSV-1 reactivation in ex vivo cultures of latently infected TG. Unlike observations in some chronic infection models, B7H1 blockade did not increase the function of exhausted gB-Tet− CD8 T cells in latently infected TG.
Introduction
Persistent exposure to cognate antigen during chronic viral infections can lead to CD8+ T cell functional exhaustion (1–3), which is associated with expression of several inhibitory receptors including the programmed death-1 (PD-1) receptor (4–9). CD8+ T cells transiently express PD-1 during initial antigenic exposure (10, 11), but constitutively express the receptor when they become exhausted (4, 5, 8). CD8+ T cell exhaustion ranges in severity from functional impairment to deletion, and the latter stages are associated with elevated PD-1 expression. There are two known ligands for PD-1; PD-ligand 2 (PD-L2) is expressed primarily on antigen presenting cells such as dendritic cells, while PD-L1 (B7-HI) is inducible on a variety of cell types (12).
Herpesviruses including herpes simplex virus type 1 (HSV-1) cause latent infections that lie somewhere between the extremes of acute and chronic infections. During HSV-1 latent infections the viral genome is primarily restricted to neurons of the sensory ganglia (trigeminal ganglion [TG] following infection of the oral facial region), where viral genes are largely transcriptionally repressed. The concept of a silent HSV-1 latent infection where HSV-1 can avoid immune elimination by hiding from the host immune system during latency has been challenged by molecular and immunologic studies suggesting low level expression of viral transcripts, and localization of activated CD8+ T cells to latently infected neurons. (13–19). The CD8+ T cells in latently infected mouse and human TG persistently express an activation phenotype, and mouse CD8+ T cells form apparent immunological synapses with latently infected neurons (15, 16), suggesting persistent or intermittent exposure to low levels of viral antigens that are expressed in latently infected neurons.
Whether or not persistent, low level antigenic exposure during latent viral infections leads to CD8+ T cell exhaustion remains controversial. A previous report by Allen et al (20) suggested that the HSV-1 latency associated transcripts (LATs) regulate exhaustion of TG-resident CD8+ T cells specific for an immunodominant epitope on HSV-1 glycoprotein B (gB498–505). However, the authors provided no direct evidence that the gB498–505-specific CD8+ T cells were functionally compromised. Moreover, several other studies demonstrated that the gB498–505-specific cells retain full functionality during latency, even when latency is interrupted by serial reactivation events (21, 22). Indeed, in examining the expression of the inhibitory receptor programmed death 1 (PD-1) that is associated with exhausted CD8+ T cells, Allen et al showed a very low frequency (7%) of PD-1 positive TG-resident immunodominant CD8+ T cells, and much higher expression on those that were not specific for the immunodominant epitope. It now appears that all CD8+ T cells in acutely and latently infected TG are HSV-specific, recognizing the immunodominant gB498–505 epitope and 18 subdominant epitopes on 11 HSV-1 proteins (23). Thus, these findings appear to be more consistent with the notion that it is the HSV-specific CD8+ T cells specific for subdominant epitopes that become preferentially exhausted in latently infected TG.
This notion was confirmed in a recent study demonstrating that immunodominant gB498–505-specific CD8+ T cells retain full functionality in latently infected TG, while the HSV-specific subdominant CD8+ T cells exhibit significantly reduced functionality (St Leger et al submitted for publication). Moreover, IL-10 was shown to selectively regulate the proliferation of the HSV-specific subdominant CD8+ T cells in latently infected TG leading to an increased number of functional cells, which is consistent with a state of partial exhaustion of these cells during latency. Here we confirm that most PD-1 expression is on TG-resident CD8+ T cells that do not recognize the immunodominant epitope, and further demonstrate that; i) increased PD-1 expression is associated with reduced functionality; ii) the PD-1 ligand (PD-L1/B7H1) is induced on neurons by IFN-γ in latently infected TG; iii) blocking PD-1/B7H1 interaction increases survival of gB-Tet-CD8+ T cells in latently infected TG leading to a dramatic increase in their numbers; but iv) the surviving cells are not functional and do not provide increased protection from HSV-1 reactivation from latency in ex vivo TG cultures.
Materials and Methods
Virus and infection
Wild-type HSV-1 strain RE was grown in Vero cells, and intact virions were isolated on Optiprep gradients according to the manufacturer’s instructions (Accurate Chemical and Scientific, Westbury, NY). Mice were anesthetized by intraperitoneal injection of 2.5mg ketamine hydrochloride and 0.25mg xylazine (Phoenix Scientific, San Marcos, CA) in 0.25ml HBSS (Bio Whittaker, Walkersville, MD). Mice received bilateral topical infection on scarified corneas with a dose of 1×105 PFU HSV-1.
Mice
Female wild-type (WT) C57BL/6 mice and IFN-γ receptor−/− (B6.129S7-Ifngr1tm1Agt/J) mice, 6–8 week old, were purchased from The Jackson Laboratory (Bar Harbor, ME). B7-H1−/− mice were provided by Dr. Lieping Chen at Yale University School of Medicine, New Haven, CT. All experimental animal procedures were reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) and the animals were handled in accordance with guidelines established by IACUC.
Generation of Bone Marrow Chimeras
Bone marrow chimeras were created by tail vein transfer of 1 × 106 bone marrow cells from 6-week-old WT C57BL/6 mice into 6-week-old lethally irradiated (2 × 500 rad treatments separated by 4 hrs rest) WT C57BL/6 recipients (WT→WT), IFN-γ receptor−/− recipients (WT→IFN-γR−/−), or B7-H1−/− recipients (WT→ B7-H1−/−). The resulting mice were housed under immune-compromised mouse conditions for 8 weeks following the transfer, and neomycin sulfate (2mg/ml) from Sigma-Aldrich (St. Louis, MO) was put in their drinking water for 2 weeks following the transfer. Bone marrow chimeras were fully reconstituted after 8 weeks.
Reagents
PE-conjugated H-2Kb tetramers conjugated to the gB498–505, RR1982–989, or RR1822–829 peptides were provided by the National Institute of Allergy and Infectious Diseases Tetramer Core Facility (Emory University Vaccine Center, Atlanta, GA). IFN-γ neutralizing antibody (XMG1.2) for in vivo neutralizations was purchased from BioXcell. Rat anti-mouse Pacific blue-conjugated anti-CD8α (clone 53–6.7), APC-conjugated anti-IFN-γ (clone XMG1.2), PerCP-conjugated anti-CD45 (clone 30–F11), and APC-conjugated anti-granzyme B (clone GB11) were purchased from BD Pharmingen. PE-conjugated anti-PD-L1 (clone MIH5) and APC-eFluor780-conjugated anti-Thy1.2 (clone 53–2.1) were purchased from eBioscience. A FITC-conjugated antibody specific for the neuronal nucleus marker (NeuN; clone A60) was purchased from Millipore (Billerica, MA). The PE-Cy7-conjugated anti-PD-1 (clone RMP1–30) was purchased from Biolegend (San Diego, CA). Appropriate isotype control Abs were purchased from BD Pharmingen, eBioscience, or Biolegend. For intracellular staining, Cytofix/Cytoperm and Permeabilization Buffer from BD Biosciences were used. All flow cytometry samples were collected on a FACSAria cytometer and analyzed by FACSDiva software (BD Biosciences). Graphpad Prism software (La Jolla, CA) was used for all statistics.
Flow cytometry gating strategies
For PE-conjugated gB498–505, RR1982–989, or RR1822–829 tetramer staining, negative controls (without PE-conjugated tetramer stain) were used to gate tetramer-positive cell populations. For all surface and intracellular staining, appropriate isotype control Abs were used to gate positive populations by FACSDiva software (BD biosciences).
Tissue preparation
Anesthetized mice were injected with 0.3 ml heparin (1000 U/ml) and euthanized by exsanguination. Excised TG were digested in 100 μl per TG of DMEM (Bio Whittaker) containing 10% FCS and 400 U/ml collagenase type I (Sigma-Aldrich) for 1 hour at 37°C, and dispersed into single-cell suspensions by trituration through a p-200 pipette tip.
Sorting neuronal subpopulations
Pooled TGs were harvested from HSV-1 latently infected C57BL/6 mice at 32 days post corneal infection (dpi) and dispersed into single cell suspensions as described above. TG cells were stained for surface markers (CD45, Thy1.2, and PD-L1) for 1 hour at 4°C, were fixed and permeabilized using cytofix/perm from BD bioscience and intracellularly stained for NeuN. The neurons (CD45−, Thy1.2+, NeuN+) were sorted into > 95% pure PD-L1+ and PD-L1− subpopulations using a FACSAria cytometer. Sorted cells were then used for quantification of HSV-1 gH DNA copies as described below.
Quantification of viral genome copies
The number of copies of HSV-1 genome in latently infected TG was determined by real-time PCR quantification of the HSV-1 glycoprotein H (gH) gene in DNA extracts of individual TG or neuronal subpopulations as previously described (24). Briefly, DNA was extracted from dispersed TG cells using DNeasy columns (Qiagen) according to manufacturers’ instructions, and quantified spectrophotometrically. The DNA was mixed with TaqMan Universal PCR Master Mix (Roche, Branburg, NJ) and an HSV-1 gH-specific primer-probe set, custom designed and synthesized by ABI Assays-by-Design service (applied Biosystems, Foster City, CA). Samples, standards, and controls were assayed in 96-well plates with an ABI Prism 7700 sequence detector. ABI Primer Express v1.5a software default settings were used for instrument control and data analysis. The gH sequences were: forward primer (5′–GACCACCAGAAAACCCTCTTT-3′), reverse primer (5′-ACGCTCTCGTCTAGATCAAAGC-3′), and probe (5′-[FAM]TCCGGACCATTTTC[NFQ]-3′). The number of copies of gH DNA in each sample was determined from a standard curve generated using known concentrations of gH-containing plasmid standards, and the number of copies of viral gH DNA/TG was calculated based on the total DNA extracted from each TG.
Measurement of CD8+ T cell proliferation and apoptosis
Latently infected mice received 2 daily intraperitoneal injections of BromodeoxyUridine (BrdU) (1mg/mouse) to measure CD8+ T cell proliferation. TG were excised 24 hrs after the second BrdU injection and dispersed cells were stained with anti-CD45, anti-CD8α, and tetramers for 1 h at room temperature. CD8+ T cells that divided over the 2-day period were quantified by flow cytometry using a BrdU proliferation assay kit (BD Pharmingen, Cat # 559619) according to manufacturers’ instructions. A Caspatag assay kit (Chemicon International, Cat # APT420) was used according to manufacturer’s instructions to quantify cells CD8+ T cells undergoing apoptosis.
Ex vivo stimulations
B6WT3 fibroblast targets were infected with HSV-1 (10 pfu/cell for 6 hours) or pulsed with peptides (gB498–505, RR1982–989, or RR1822–829) at a concentration of 0.8ug/ml for 45 min at 37°C/5% CO2. Cells from each TG were stimulated with 5×105 HSV-1-infected or peptide-pulsed fibroblasts in the presence of RPMI/10% FCS, and Golgi-Plug (BD Biosciences) for 6 h at 37°C/5% CO2. In some experiments, TG cells were pretreated with PE-conjugated gB(498–505) tetramers (2μg/ml) for 30 min at 37°C/5% CO2 prior to stimulation with HSV-1 infected B6WT3 cells. Following stimulation, cells were surface stained for CD8α, permeabilized and fixed with Cytofix/Cytoperm (BD Biosciences), and stained for intracellular IFN-γ and TNF-α.
Ex vivo TG cultures
HSV-1 latently infected TG cells were dispersed and cultured in 48-well tissue culture plates (0.2 TG/well) in culture medium (DMEM containing 10% FCS, 500 U/ml rIL-2 (R & D Systems, Minneapolis, MN), and 50 μm 2-mercaptoethanol (2-ME; Fisher Scientific, Fair Lawn, NJ) with or without anti-CD8α antibody (100μg/ml)) for up to 10 days. Supernatants were serially sampled and replaced with a similar volume of culture medium, and samples were assayed for the presence of infectious virus on Vero cell monolayers as previously described (25).
RESULTS
CD8+ T cells specific for subdominant HSV epitopes exhibit increased rates of proliferation and apoptosis in latently infected TG
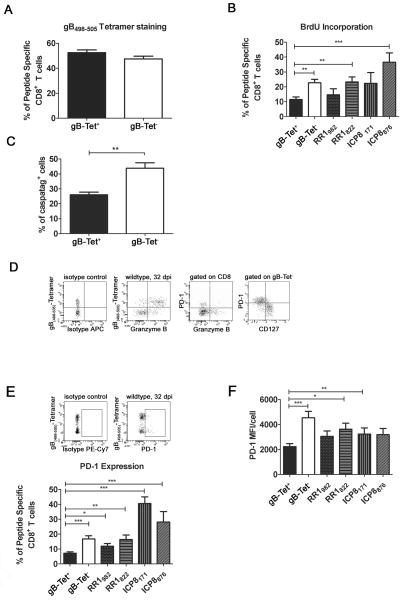
HSV-specific CD8+ T cells consistently maintain a 1:1 ratio of cells specific for an immunodomant gB498–505 epitope (here referred to as gB-Tet+ CD8) to those recognizing subdominant epitopes (here collectively referred to as gB-Tet− CD8) (Fig. 1A) (26), despite the fact that the latter population exhibits a significantly higher rate of cell division than gB-Tet+ CD8 in latently infected TG (Fig. 1B). The rate of proliferation varied among TG resident CD8+ T cells specific for individual subdominant epitopes, but all those analyzed except the RR1982-specific cells showed a significantly higher rate of proliferation than the gB-Tet+ CD8s. The gB-Tet− CD8s also exhibited a significantly higher rate of apoptosis than that seen in the gB-Tet+ CD8 population (Fig. 1C). Thus, the increased rate of cell division appears to be balanced by a corresponding increase in the rate of apoptosis among gB-Tet− CD8+ T cells, maintaining the 1:1 ratio of immunodominant to gB-Tet− cells. Also, a significantly lower percentage of gB-Tet− CD8 showed granzyme B expression, which had an inverse correlation with PD-1 expression (Fig. 1D) suggesting selective functional impairment in the PD-1+ cells. Furthermore, these PD-1+ gB-Tet− CD8 T cells seemed to be an effector cell population experiencing exhaustion as suggested by their lack of CD127 expression.
Figure 1. Differences between immunodominant gB(498–505)-specific CD8+ T cells and subdominant epitope-specific CD8+ T cells.
Mice latently infected with HSV-1 (30 dpi) received 2 intraperitoneal BrdU treatments (1mg/mouse) over a 2 day period and then trigeminal ganglia were excised and dispersed into single cell suspensions. Cells were stained with H-2Kb tetramers containing the immunodominant gB498–505 epitope (gB, A–F), or subdominant epitopes on HSV-1 ribonucleotide reductase 1 (RR1982–989, RR1822–829) and infected cell protein 8 (ICP8171–179, ICP8876–883) (B, E, F), with fluorescently tagged antibodies to CD45, CD8, PD-1, CD127 (D, E, F), and BrdU (B) or Granzyme B (GrzB) (D), or with a fluorescently labeled pan caspase inhibitor (caspatag) to identify apoptotic cells (C) and analyzed on a FACSAria using FACSDiva software. A, Bars represent the mean percentage of gB tetramer positive (gB-Tet+) and negative (gB-Tet−) CD8+ T cells/TG ± SEM (n = 10 mice). B, Bars represent the mean percentage of BrdU+ cells in HSV-1 epitope -specific CD8+ T cell populations ± SEM (n= 5 mice/group). C, Comparison of apoptosis within gB-Tet+ CD8 and gB-Tet− CD8+ cells (n = 5 mice). D, Representative dot plots showing gB tetramer and GrzB staining (far left & left), GrzBand PD-1 staining (right) and CD127 and PD-1 staining (far right) (n= 5 mice). E, Representative dot plots and a bar graph showing the mean percentage ± SEM of PD-1+ cells in HSV-1 epitope-specific CD8+ T cell populations (n= 5 mice). F, Mean PD-1 MFI for each HSV-1 epitope-specific CD8+ T cells (n= 5 mice). All data are from at least two independent experiments. * p ≤ 0.05, **p ≤0.01, and ***p ≤0.001
In latently infected TG PD-1 is preferentially up-regulated on gB-Tet− CD8 and the PD-1 ligand PD-L1/B7-H1 is up-regulated on neurons
The increased proliferation and decreased survival and function of TG-resident gB-Tet− CD8+ T cells suggested a state of partial exhaustion among these cells. Since CD8+ T cell functional exhaustion and deletion are associated with increased expression of PD-1, we hypothesized a higher level of PD-1 expression on gB-Tet− CD8 T cells relative to their gB-Tet+ CD8 counterparts in latently infected TG. Indeed, gB-Tet− CD8+ T cells exhibited a higher frequency of PD-1+ cells and a higher level of PD-1 expression/cell relative to their immunodominant gB-Tet+ CD8 counterparts in latently infected TG (Fig. 1E&F).
Since CD8+ T cells interact closely with neurons in infected TG (15, 16) we asked if TG neurons constitutively express the PD-1 ligand, PD-L1/B7H1 or are induced to express the ligand following HSV-1 infection. The frequency of PD-L1/B7H1 positive neurons was very low (<5%) in non-infected TG, but increased significantly following infection reaching expression on nearly 70% of neurons by 7 dpi (Fig 2A–C). The frequency of PD-L1/B7H1 positive neurons decreased from acute (8 dpi) to latent (>30 dpi) infection, but remained significantly higher than that observed in non-infected TG (Fig. 2C&D).
Figure 2. PD-L1/B7H1 expression on neurons in the trigeminal ganglia following HSV-1 infection.
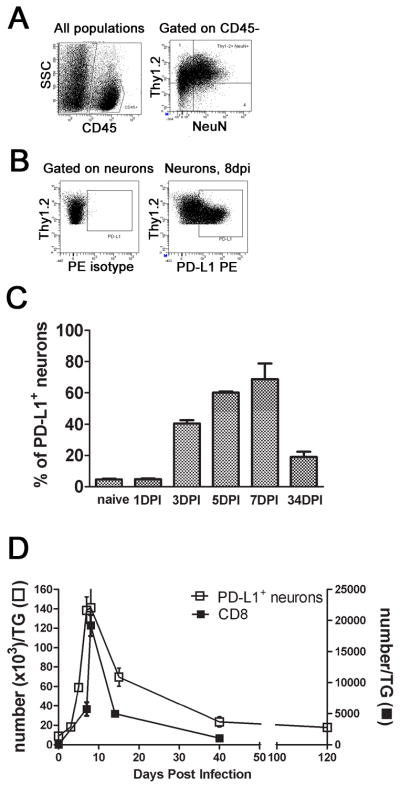
HSV-1 infected TG were excised at various times after infection and dispersed neurons were stained with antibodies against CD45, Thy1.2, PD-L1, and intracellular NeuN. A and B, Representative dot plots showing gating strategies for quantification of PD-L1+ neurons at 8 dpi. C, Bars represent the mean percentage ± SEM of PD-L1+ cellswithin the CD45− Thy1.2+ NeuN+ neuron population (n= 5 mice/time). D, Representative graph shows the average ± SEM number of CD8+ T cells or PD-L1+ neurons in the TG at various times following infection (n = 3–7 mice/time). Three independent experiments produced similar results.
It was unclear if PD-L1/B7H1 expression was directly or indirectly regulated by HSV-1 infection. The latter possibility was suggested by the fact that more neurons expressed PD-L1/B7H1 at 7 dpi than would be expected to be HSV-1 infected (Fig. 2C), and by the close association between CD8+ T cell infiltration and neuronal PD-L1/B7H1 expression in the TG (Fig. 2D). Since TG-resident CD8+ T cells produce IFN-γ when stimulated by HSV antigens (26) and PD-L1/B7H1 expression on oligodendroglia is selectively up-regulated by IFN-γ (27), we hypothesized that PD-L1/B7H1 expression on neurons is indirectly regulated by IFN-γ in infected TG. Indeed we found that PD-L1/B7H1 expression on TG neurons at 8 dpi was significantly, but not completely reduced when mice were treated with IFN-γ neutralizing antibody at 4 and 6 dpi (Fig. 3A). To investigate the role of IFN-γ in regulating neuronal PD-L1/B7H1 expression in a cleaner system, bone marrow chimeras were created by transferring wild type (WT) bone marrow into irradiated WT or IFN-γ receptor knockout (IFN-γR−/−) mice. The WT recipients of WT bone marrow showed the expected up-regulation of PD-L1/B7H1 expression on TG neurons at 8 dpi and 32 dpi (Fig. 3B). In contrast, the IFN-γR−/− recipients of WT bone marrow that were incapable of expressing IFN-γR on neurons showed no up-regulation of neuronal PD-L1/B7H1 expression. The IFN-γR+ and IFN-γR− neurons had similar viral loads at 8 and 32 dpi (data not shown), demonstrating that neuronal PD-L1/B7H1 expression in HSV-1 infected TG is regulated by IFN-γ and not directly by the virus.
Figure 3. IFN-γ regulates PD-L1 expression on trigeminal ganglion neurons.
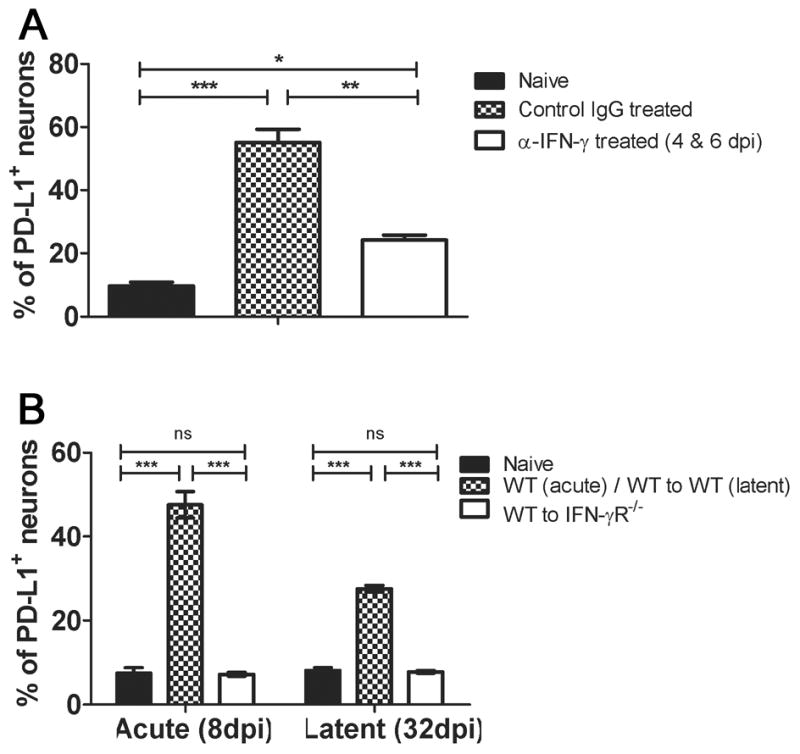
A, Mice received intraperitoneal injections (1 mg/ mouse) of either anti-IFN-γ or control antibody at 4 and 6 dpi, TG were excised at 7 dpi and dispersed cells stained for PD-L1/B7H1, CD45, Thy1.2, and NeuN. Bar graph shows the mean ± SEM percentage of PD-L1/B7H1 positive PD-L1+ CD45− Thy1.2+ NeuN+ neurons. B, TG were obtained from non-infected (naïve) mice or from bone marrow chimeric mice that expressed IFN-γR on all cells (WT to WT) or only on bone marrow-derived cells (WT to IFN-γR−/−), and dispersed TG were stained as in A and analyzed by flow cytometry. The bar graph shows the mean ± SEM percentage of PD-L1/B7H1 positive neurons. Data are representative of two independent experiments (n = 5 mice (10 TG)/group/experiment). *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001
Based on the assumption that latently infected TG neurons would be more likely to be exposed to IFN-γ we proposed that PD-L1/B7H1 positive neurons would contain a higher load of latent virus than their non-infected counterparts. In fact, when latently infected TG neurons were FACS sorted into PD-L1 positive and negative subpopulations, the PD-L1 positive neurons were found to contain more copies of latent HSV-1 genomes per 100 cells than the PD-L1 negative population (Fig. 4). However, two points merit mention. First, PD-L1+ neurons contained only 30 copies of viral genome per 100 cells. Assuming 1 copy of viral genome/infected neuron (quite possibly an underestimate) our data suggest a maximum of 30% of PD-L1+ neurons harbor latent virus. Second, since PD-L1+ neurons represent approximately 30% of total TG neurons during latency, the distribution of total latent virus is approximately equivalent in PD-L1+ and PD-L1− neurons.
Figure 4. Elevated HSV-1 genome copy number in PD-L1+ neurons.
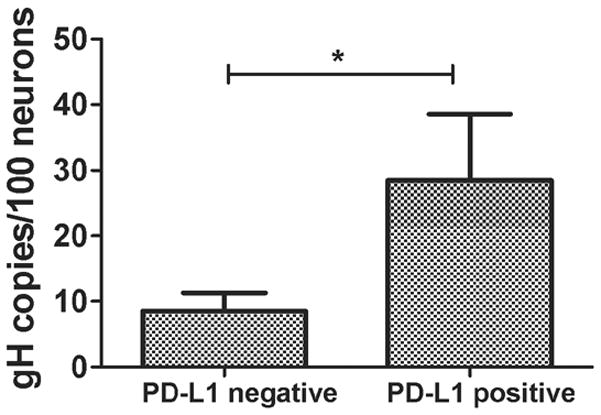
Both TG were excised from 3 mice at 32 dpi, pooled and dispersed cells stained for CD45, Thy1.2, PD-L1 and intracellular NeuN. The neurons (CD45−, Thy1.2+, NeuN+) were sorted into > 95% pure PD-L1+ and PD-L1− subpopulations using a FACSAria cytometer. DNA extracts from sorted cells were then analyzed for viral genome copy number by quantitative PCR. Bars represent the mean ± SEM copies of HSV-1 genome/100 cells. Data are pooled from 3 independent experiments with 6 pooled TG/experiment. *p ≤ 0.05
PD-1/B7H1 interaction regulates the frequency of gB-Tet− CD8+ T cells in latently infected TG
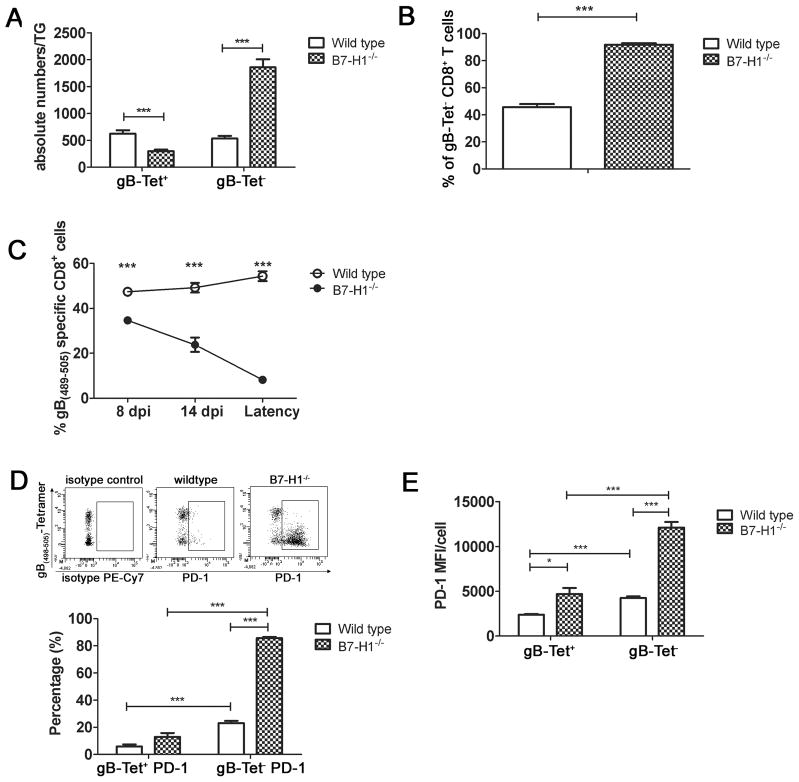
Since gB-Tet− CD8+ T cells in latently infected TG express more PD-1 and undergo apoptosis at a higher rate, we asked if PD-1 interaction with PD-L1/B7H1 regulates the frequency of gB-Tet− CD8+ T cells within latently infected TG. As demonstrated above, latently infected TG of wild type mice contain equivalent numbers of immunodominant gB-Tet+ CD8 and gB-Tet− CD8+ T cells (Fig. 5A). However, latently infected TG of mice that are genetically deficient in B7H1 (B7H1−/− mice) exhibit a significantly increased number of gB-Tet− CD8 and a slight, but significant reduction in gB-Tet+ CD8 (Fig. 5A). This results in a significant increase in the frequency of gB-Tet− cells (Fig. 5B). The immunodominant gB-Tet+ CD8 population consistently comprised approximately 50% of the TG resident CD8+ T cell population in infected TG of wild type mice from acute infection through latency (Fig. 5C). In contrast, the immunodominant gB-Tet+ CD8 population represented a slightly reduced proportion of the TG-resident CD8+ T cells in acutely infected TG (8 dpi), and progressively declined during latency (Fig. 5C). Interestingly, during latency 80% of the TG-resident gB-Tet− CD8 expressed PD-1 in B7H1−/− mice compared to less than 20% in wild type mice (Fig. 5D), and the level of PD-1 expression (MFI) was also significantly higher (Fig. 5E). In contrast the frequency and level of expression of PD-1+ on immunodominant gB-Tet+ CD8 was only slightly increased in B7H1−/− mice.
Figure 5. Significantly increased number of PD-1hi gB-Tet− CD8+ T cells in B7-H1−/− TG.
TG from wild type or B7- H1−/− C57BL/6 mice were obtained at 30 dpi (A, B, D, E) or at indicated times after infection (C), and dispersed cells were stained for CD45, CD8, gB(498–505)-tetramer (gB-Tet), and PD-1, and analyzed by flow cytometry. A, bars represent the mean ± SEM number of gB-Tet+ and gB-Tet− CD8+ T cells in TG of WT and B7-H1−/− mice (n = 10 mice). B, Bars represent the mean ± SEM percentage of gB-Tet− CD8+ T cells (n = 10 mice). C, Line graph showing the mean ± SEM percentage of gB-Tet+ CD8+ T cells at indicated time points in TG of WT and B7-H1−/− mice (n = 5 mice). D, Representative dot plots showing PD-1 staining on gB-Tet+ and gB-Tet− CD8+ T cells in TG of Wild type and B7H1−/− mice. Bars indicate the mean ± SEM percentage of PD-1+ gB-Tet+ and gB-Tet− CD8 T cells from WT and B7-H1−/− mice (n= 10 mice). E, Bar graph showing PD-1 MFI for the gB-Tet+ and gB-Tet− CD8+ T cells in wild type and B7-H1−/− TG (n = 10 mice). All data are pooled from three independent experiments. *p ≤0.05, **p ≤ 0.01, and ***p ≤ 0.001
Increased survival accounts for the selective accumulation of gB-Tet− CD8+ T cells in TG of B7H1−/− mice
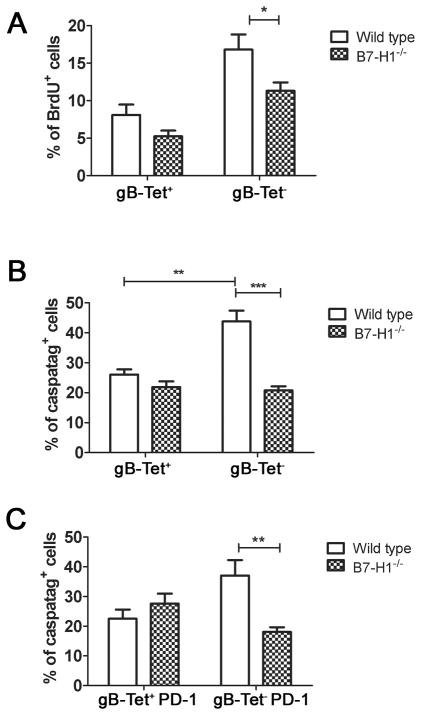
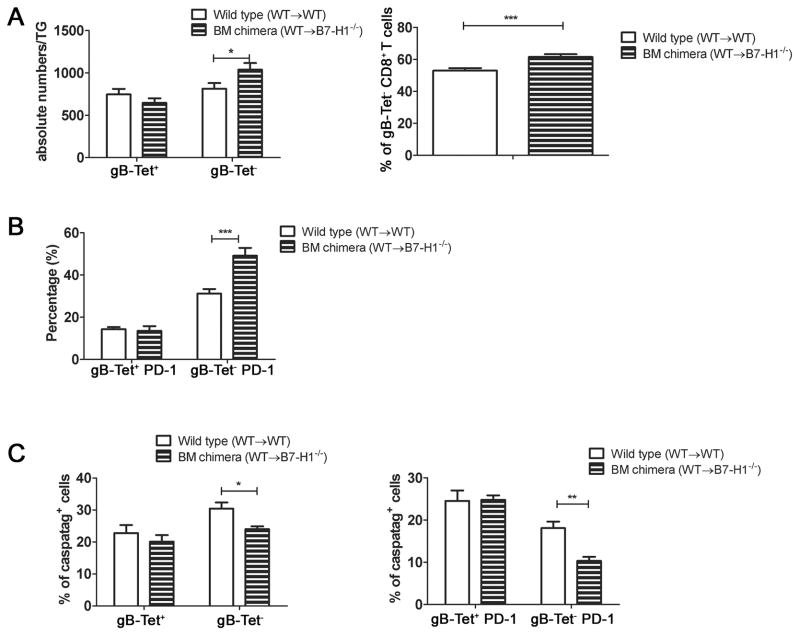
The increased number and frequency of gB-Tet− CD8+ T cells in the latently infected TG of B7H1−/− mice could be due to a selective increase in proliferation and/or survival of the gB-Tet− CD8 relative to the gB-Tet+ CD8+ T cells. We found that the rate of cell division (BrdU incorporation) among gB-Tet− CD8 was slightly reduced in B7-H1−/− mice (Fig. 6A), but their reduced rate of proliferation was over compensated by a 51% reduction in their rate of apoptosis based on caspatag staining (Fig. 6B&C). In contrast, neither the rate of proliferation nor the rate of apoptosis of gB-Tet+ CD8 was significantly affected in B7-H1−/− mice. In latently infected TG, PD-L1/B7H1 is expressed on CD45+ bone marrow-derived cells as well as on neurons (data not shown), and the relative contribution of expression on these two cell types to the survival of the gB-Tet− CD8+ T cells is unclear. Therefore, mice capable of expressing B7-H1 on bone marrow-derived cells, but not on neurons were created by transferring WT bone marrow into irradiated B7-H1−/− recipients. These mice showed a selective increase in TG-resident gB-Tet− CD8+ T cells relative to controls (Fig. 7A), although the increase was much less dramatic than that seen in B7-H1−/− mice (Fig. 5A&B). The selective lack of B7-H1 on neurons also resulted in an increased frequency of PD-1+ gB-Tet− CD8+ T cells in latently infected TG (Fig. 7B), though again the difference was not as dramatic as that seen in the B7-H1−/− mice (Fig. 5D). The increased size of the gB-Tet− CD8+ T cell population in ganglia of chimeras lacking B7-H1 only on neurons was associated with a decreased rate of apoptosis (Fig. 7C). Thus, B7-H1 expression on non-hematopoetic cells is at least partially responsible for the selective accumulation of gB-Tet− CD8 in latently infected TG of B7-H1−/− mice.
Figure 6. PD-1/B7-H1 regulates survival of gB-Tet− CD8+ T cells.
At 30 dpi wild type and B7-H1−/− mice received 2 daily intraperitoneal injections of BrdU (1mg/mouse). At 32 dpi TG were excised and dispersed cells stained for CD45, CD8, gB(498–505)-specific tetramer (gB-Tet), and PD-1 and for BrdU (A) or caspatag (B, C). A, Bars represent the mean percentage ± SEM of BrdU+ cells in gB-Tet+ and gB-Tet− CD8 T cells from wild type and B7-H1−/− mice (n= 5 mice). B & C, Bars represent the mean percentage ± SEM of caspatag+ cells within the indicated cell populations (n= 5 mice). Data are representative of two independent experiments. *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001
Figure 7. B7-H1 on neurons is partially responsible for controlling the survival of PD-1+ gB-Tet− CD8+ T cells.
TG were obtained at 30 dpi from bone marrow chimeric mice that were capable of expressing B7-H1 on all cells (WT to WT) or only on bone marrow derived cells (WT to B7-H1−/−). Dispersed cells were stained for PD-1 and with gB498–505-tetramers (gB-Tet) and analyzed by flow cytometry. A, Bars represent mean ± SEM absolute numbers (left) or percentage (right) gB-Tet+ and gB-Tet− CD8+ T cells. B, Bars represent the mean ± SEM percentage of PD-1+ gB-Tet+ and gB-Tet− CD8+ T cells. C, Bars represent the mean ± SEM of Caspatag+ gB-Tet+ and gB-Tet− CD8+ T cells (left) and the percentage of caspatag+ PD-1+ gB-Tet+ and gB-Tet− CD8+ T cells (right). All data are representative of two independent experiments. Bars represent mean ± SEM (n = 5 mice). *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001.
In vivo disruption of PD-1/B7H1 signaling does not rescue the function of gB-Tet− CD8+ T cells in latently infected TG
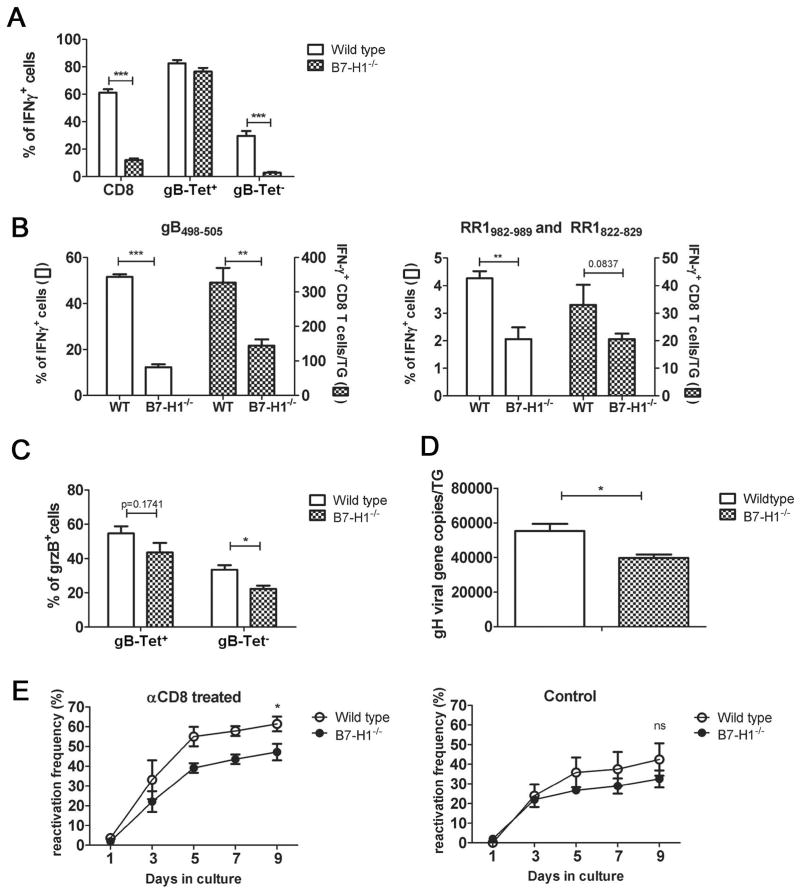
Dispersed TG cells from latently infected wild type or B7-H1−/− mice were stained directly ex vivo with tetramers containing the immunodominant gB498–505 epitope, stimulated with HSV-1 infected B6WT3 cells for 6 hrs, and then stained for intracellular IFN-γ. Flow cytometry analysis of CD8+ T cells from TG of wild type mice showed that IFN-γ was produced by 80% of the immunodominant gB-Tet+ CD8 but only approximately 30% of the gB-Tet− CD8 following stimulation (Fig. 8A). This is consistent with our previous observation that in wild type mice gB-Tet+ CD8 retain functionality, whereas the gB-Tet− cells become partially exhausted (St. Leger et al, submitted). In latently infected TG from B7-H1−/− mice, the frequency of gB-Tet+ CD8 is reduced to approximately 10% of the total CD8+ T cells due primarily to a dramatic increase in the gB-Tet− cells (Fig. 5A&B). The overall frequency of IFN-γ producing CD8+ T cells in TG of B7-H1−/− mice was reduced to approximately 12%, with approximately 80% of gB-Tet+ CD8 responding, but less than 3% of gB-Tet− CD8 responding. The absolute number of IFN-γ producing subdominant RR1(982–989) and RR1(822–829)-specific CD8+ T cells was equivalent in the TG of wild type and B7-H1−/− mice (Fig. 8B right), suggesting that the cells that survive as a result of disrupting the PD-1/B7H1 interaction are largely non-functional. This is further supported by the observation that the percentage of grzB+ gB-Tet− CD8 is significantly decreased in B7-H1−/− mice (Fig 8C) as well as that of IFN-γ+ gB-Tet− CD8 (Fig 8A).
Figure 8. Expanded gB-Tet− CD8+ T cells in B7-H1−/− mice are not functional.
Dispersed TG cells from wild type and B7-H1−/− mice (30 dpi) were (A) pre-stained with gB(498–505) tetramers (gB-Tet) before stimulation and then stained for CD45, CD8, and intracellular IFN-γ following 6 hrs of stimulation. Bars represent the mean ± SEM percentage of IFN-γ+ CD8+ T cells/TG (n = 10 mice); or (B) stimulated for 6 hours with B6WT3 cells pulsed with gB(498–505) peptide (B left) or pooled RR1(982–989) and RR1(822–829) peptides (B right) followed by staining for CD45, CD8, and intracellular IFN-γ; or (C) stained directly for CD45, CD8, gB-Tet and intracellular GrzB. Bars represent the mean ± SEM percentage of GrzB+ cells in gB-Tet+ or gB-Tet− CD8populations (n = 5 mice). (D) DNA was extracted from TG of wild type and B7-H1−/− mice (30 dpi), and HSV-1 genome copy number was determined by quantitative PCR. Bars represent the mean ± SEM genome copy number/TG (n = 5 mice). (E) TG of wild type and B7-H1−/− mice (30 dpi) were dispersed and cultured with (left) or without (right) anti-CD8 mAb. Culture supernatants were serially sampled and assayed for infectious virus on monolayers of Vero cells to detect HSV-1 reactivation from latency. Symbols represent the mean ± SEM frequency of reactivation at each time point (n = 15 mice). Data for A, B & D are pooled from at least two independent experiments and data for C is representative of two independent experiments. *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001.
The increased size of the TG-resident CD8+ T cell population in B7H1−/− mice does not result in improved protection from HSV-1 reactivation from latency
In WT C57BL/6 mice, HSV-1 reactivation from latency in ex vivo TG cultures correlates directly with viral genome copy number, and inversely with the number of immunodominant CD8+ T cells in the TG at the time of excision cultures (24, 28). The B7-H1−/− TG showed a slight, but significant reduction in HSV-1 genome copy number during acute (8 dpi, not shown) and latent infection (30 dpi, Fig. 8D). The B7-H1−/− TG also showed a slight, but significant reduction in reactivation frequency compared to WT TG in cultures in which CD8+ T cell function was compromised (Fig. 8E left). The B7-H1−/− TG contained significantly more CD8+ T cells at the time of excision (Fig. 5A), but the increase represented non-functional gB-Tet− CD8+ T cells. Therefore, we predicted that the increased number would not result in increased protection from reactivation in TG cultures in which CD8+ T cell function was intact. Indeed, CD8+ T cells reduced the reactivation by an identical amount (31% reduction compared to cultures with anti-CD8 mAb) in WT and B7-H1−/− TG (Fig. 8E right).
DISCUSSION
The capacity of immunodominant gB498–505-specific CD8+ T cells to prevent HSV-1 reactivation from latency is well established (15, 25). These cells have been the primary focus of such studies because of their high frequency and known HSV-specificity. However, it now appears that essentially all of the CD8+ T cells in the infected TG are HSV-specific, while only half recognize the gB498–505 epitope (23, 26). Our recent study demonstrated that the subdominant CD8+ T cells in latently infected TG exhibit partial functional exhaustion associated with IL-10-mediated inhibition of their rate of cell division, functionality, and ability to inhibit HSV-1 reactivation from latency (St. Leger et al, submitted).
Protracted expression of the inhibitory PD-1 receptor has been associated with CD8+ T cell functional exhaustion in several tumor models and models of chronic viral infection (4, 5, 7, 8, 29–32). Approximately 10–20% of both immunodominant and subdominant HSV-specific effector CD8+ T cells that infiltrate the TG during acute infection (8 dpi) express high levels of PD-1 (data not shown). During latency, the frequency and level of expression of PD-1 is maintained on gB-Tet− TG-resident CD8+ T cells, but declines among the immunodominant CD8+ T cells. The reason for the differential loss of PD-1 on immunodomonant memory CD8+ T cells is not clear, but two possible explanations arise from our previous studies. We demonstrated that depletion of CD4+ T cells during the priming of the HSV-specific CD8+ T cell response resulted in a higher frequency of PD1+ immunodiminant gB-Tet+ CD8 in acutely infected TG (8 dpi) that persisted in the memory population up to 56 dpi (22). The increased PD-1 expression on the gB-Tet+ CD8 was associated with reduced functionality. Thus, differential PD-1 expression and functional exhaustion by gB-Tet− HSV-specific memory CD8+ T cells might reflect reduced CD4+ T cell help during priming, although to our knowledge a mechanism that would permit selective CD4+ T cell help for immunodominant CD8+ T cells has not been described.
An alternative explanation for the elevated PD-1 expression on TG resident gB-Tet− memory CD8+ T cells might be that the epitopes recognized by these cells are expressed at a higher level in latently infected neurons. This would be consistent with the observation that these cells exhibit a higher level of proliferation than the immunodominant cells. Overexposure to subdominant epitopes could lead to exhaustion characterized by up-regulation of PD-1 and ultimate physical deletion.
CD8+ T cells in latently infected TG interact closely with neurons; the only cells that retain latent virus (15, 16). Since PD-1 inhibition of TCR signaling reportedly requires expression of the TCR ligand and PD-1 ligand on the same cell (33), we examined PD-L1/B7-H1 expression on neurons in infected TG. The frequency of PD-L1/B7-H1 positive neurons increased dramatically during acute infection; and remained higher than non-infected TG during latency. Since the number of PD-L1/B7-H1+ neurons exceeded the likely number of infected neurons in acutely infected TG, and their number declined in parallel with the declining numbers of TG-resident CD8+ T cells, we hypothesized that neuronal expression of PD-L1/B7-H1 might be regulated by a soluble mediator produced by CD8+ T cells. IFN-γ is persistently present in HSV-1 acutely and latently infected TG (34–38), and was shown to regulate PD-L1 expression on neurons in the brains of coronavirus infected mice (27). Indeed, we found IFN-γ has a requisite role in regulating PD-L1 expression on neurons in infected TG.
The ratio of viral genomes to neurons was 3-fold higher among PD-L1+ neurons compared to PD-L1− neurons, consistent with the notion that neurons with a higher load of latent virus are more likely to stimulate an IFN-γ response (presumably by CD8+ T cells). However, even the PD-L1+ neurons contained only 30 genome copies per 100 cells, suggesting that a maximum of 30% of PD-L1+ neurons harbor latent virus. Presumably, the ≥ 70% of PD-L1+ neurons that did not harbor latent virus were exposed to IFN-γ that diffused away from an encounter between a latently infected neuron and a CD8+ T cell or other IFN-γ producing cell as previously described (39).
Although PD-L1 negative neurons contain 3-fold fewer viral genomes than PD-L1 positive neurons, there are approximately 3-fold more PD-L1 negative neurons. Therefore, approximately half of latent HSV-1 genomes are harbored in neurons that have not been recently exposed to IFN-γ. This finding is consistent with the notion that a significant proportion of latent viral genomes are maintained in a latent state without immune intervention.
The process of exhaustion appears to advance through stages marked by progressively reduced capacity for lytic granule-mediated target cell lysis, reduced production of cytokines, and ultimately physical deletion of the exhausted cells (7). Cells at advanced stages of exhaustion are marked by increased levels of PD-1 expression (30). Our recent study demonstrated that immunodominant gB-Tet+ CD8 remain fully functional as assessed by cytokine production and lytic granule release when stimulated with gB498–505 peptide pulsed targets directly ex vivo, whereas a significant proportion of subdominant CD8+ T cells lose functionality (St Leger et al, submitted). We further demonstrated that in vivo blockade of the IL-10 receptor had little effect on the immunodominant gB-Tet+ CD8, but significantly increased the proliferation and size of the gB-Tet− CD8+ T cell pool as well as the number of gB-Tet− CD8+ T cells capable of producing cytokines and expressing GrzB in latently infected TG. Here we show a higher frequency and higher level of expression of PD-1 in gB-Tet− relative to immunodominant CD8+ T cells in latently infected TG. Interfering with the PD-1/PD-L1 interaction resembles blockade of the IL-10 receptor in selectively increasing the size of the gB-Tet− CD8+ T cell pool in latently infected TG. However, PD-1/PD-L1 blockade differs from IL-10 receptor blockade in that it increases the size of the gB-Tet− CD8+ T cell pool by increasing survival rather than increasing proliferation. Moreover, the cells that survive as a result of PD-1/PD-L1 blockade express high levels of PD-1 and are not functional, resulting in a dramatic decrease in the frequency of functional gB-Tet− CD8+ T cells in latently infected TG.
Several observations suggest that gB-Tet− CD8+ T cells in HSV-1 latently infected TG are selectively undergoing functional exhaustion and physical deletion. First, the gB-Tet− CD8+ T cells express less GrzB than the immunodominant cells, and GrzB expression is inversely related to PD-1 expression. Second, the ability to produce IFN-γ is maintained by virtually all immunodominant, but only a small portion of gB-Tet− CD8+ T cells in latently infected TG. Third, although the TG-resident gB-Tet− CD8+ T cells proliferate at a higher rate than the immunodominant cells, the two populations maintain a strict 1:1 ratio through an increased rate of apoptosis in the gB-Tet− population. Finally, disrupting the PD-1/PD-L1 interaction results in a dramatic accumulation of TG-resident gB-Tet− CD8+ T cells accompanied by a decrease in their rate of apoptosis. The surviving cells show a high frequency and level of expression of PD-1, a phenotype that is suggestive of cells in the late stages of exhaustion that would normally be deleted. Moreover, disrupting the PD-1/PD-L1 interaction does not result in an increased frequency or absolute number of functional CD8+ T cells as assessed by IFN-γ production or GrzB expression directly ex vivo. Similar results have been shown in HCV-infected patients who received PD-1/PD-L1 blockade (40). Thus, the PD-1/PD-L1 interaction alone is sufficient to reduce the survival of gB-Tet− HSV-specific CD8+ T cells, while their function may be regulated by the redundant activity of multiple inhibitory receptors. Based on these findings, we propose that the gB-Tet− TG-resident CD8+ T cells are constantly progressing through stages of exhaustion ranging from functional compromise to physical deletion, with IL-10R regulating the former and PD-1 regulating the latter.
TG-resident CD8+ T cells can inhibit HSV-1 reactivation from latency in ex vivo TG cultures through release of IFN-γ and lytic granules containing GrzB (24, 25). Since immunodominant gB-Tet+ CD8 selectively retain these functions during latency, it is likely that gB-Tet+ CD8 play a primary role in preventing HSV-1 reactivation. Disruption of the PD-1/PD-L1 interaction in latently infected TG of B7-H1−/− mice had little effect on the immunodominant gB-Tet+ CD8 T cell, and only resulted in an increase in non-functional gB-Tet− CD8 T cells. Thus, we observed an identical level of CD8+ T cell protection from reactivation in ex vivo cultures of TG from wild type and B7-H1−/− mice. While a portion of CD8+ T cells in human TG express PD-1 (41), our findings suggest that blocking PD-1 function alone would probably not have therapeutic efficacy. However, combined blockade of PD-1, IL-10R, and perhaps other inhibitory receptors seems to have exciting potential for preventing HSV-1 reactivation from latency and recurrent herpetic disease.
Acknowledgments
We would like to thank Nancy Zurowski for flow cytometry acquisition and the National Institue of Allergy and Infectious Diseases Tetramer Core Facility (Emory University Vaccine Center, Atlanta, GA) for supplying tetramers.
Footnotes
P30-EY08098 (R.L.H.), R01-EY005945 (R.L.H.), T32-EY017271 (A.J.S.), R56AI85110 (T.L.C.) an unrestricted grant from Research to Prevent Blindness (New York, NY), the Eye and Ear Foundation of Pittsburgh
References
- 1.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. Journal of virology. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. The Journal of experimental medicine. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. Journal of virology. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nature medicine. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature immunology. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 9.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 Tcell exhaustion during chronic viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura H, Agata Y, Kawasaki A, Sato M, Imamura S, Minato N, Yagita H, Nakano T, Honjo T. Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4-CD8-) thymocytes. International immunology. 1996;8:773–780. doi: 10.1093/intimm/8.5.773. [DOI] [PubMed] [Google Scholar]
- 11.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. International immunology. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 12.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annual review of immunology. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloom DC, Giordani NV, Kwiatkowski DL. Epigenetic regulation of latent HSV-1 gene expression. Biochimica et biophysica acta. 2010;1799:246–256. doi: 10.1016/j.bbagrm.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knipe DM, Cliffe A. Chromatin control of herpes simplex virus lytic and latent infection. Nature reviews Microbiology. 2008;6:211–221. doi: 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- 15.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, Langerak AW, Kinchington PR, Osterhaus AD. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:978–983. doi: 10.1073/pnas.022301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer MF, Coen DM. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. Journal of virology. 1995;69:1389–1399. doi: 10.1128/jvi.69.3.1389-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. The Journal of experimental medicine. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen SJ, Hamrah P, Gate D, Mott KR, Mantopoulos D, Zheng L, Town T, Jones C, von Andrian UH, Freeman GJ, Sharpe AH, BenMohamed L, Ahmed R, Wechsler SL, Ghiasi H. The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus 1. Journal of virology. 2011;85:4184–4197. doi: 10.1128/JVI.02290-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackay LK, Wakim L, van Vliet CJ, Jones CM, Mueller SN, Bannard O, Fearon DT, Heath WR, Carbone FR. Maintenance of T cell function in the face of chronic antigen stimulation and repeated reactivation for a latent virus infection. Journal of immunology. 2012;188:2173–2178. doi: 10.4049/jimmunol.1102719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank GM, Lepisto AJ, Freeman ML, Sheridan BS, Cherpes TL, Hendricks RL. Early CD4(+) T cell help prevents partial CD8(+) T cell exhaustion and promotes maintenance of Herpes Simplex Virus 1 latency. Journal of immunology. 2010;184:277–286. doi: 10.4049/jimmunol.0902373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St Leger AJ, Peters B, Sidney J, Sette A, Hendricks RL. Defining the herpes simplex virus-specific CD8+ T cell repertoire in C57BL/6 mice. Journal of immunology. 2011;186:3927–3933. doi: 10.4049/jimmunol.1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu T, Khanna KM, Carriere BN, Hendricks RL. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. Journal of virology. 2001;75:11178–11184. doi: 10.1128/JVI.75.22.11178-11184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheridan BS, Cherpes TL, Urban J, Kalinski P, Hendricks RL. Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes. Journal of virology. 2009;83:2237–2245. doi: 10.1128/JVI.01699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phares TW, Ramakrishna C, Parra GI, Epstein A, Chen L, Atkinson R, Stohlman SA, Bergmann CC. Target-dependent B7-H1 regulation contributes to clearance of central nervous system infection and dampens morbidity. Journal of immunology. 2009;182:5430–5438. doi: 10.4049/jimmunol.0803557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoshino Y, Pesnicak L, Cohen JI, Straus SE. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. Journal of virology. 2007;81:8157–8164. doi: 10.1128/JVI.00474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blattman JN, Wherry EJ, Ha SJ, van der Most RG, Ahmed R. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. Journal of virology. 2009;83:4386–4394. doi: 10.1128/JVI.02524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maier H, Isogawa M, Freeman GJ, Chisari FV. PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. Journal of immunology. 2007;178:2714–2720. doi: 10.4049/jimmunol.178.5.2714. [DOI] [PubMed] [Google Scholar]
- 32.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 33.Bennett F, Luxenberg D, Ling V, Wang IM, Marquette K, Lowe D, Khan N, Veldman G, Jacobs KA, Valge-Archer VE, Collins M, Carreno BM. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. Journal of immunology. 2003;170:711–718. doi: 10.4049/jimmunol.170.2.711. [DOI] [PubMed] [Google Scholar]
- 34.Cantin E, Tanamachi B, Openshaw H. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. Journal of virology. 1999;73:3418–3423. doi: 10.1128/jvi.73.4.3418-3423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cantin EM, Hinton DR, Chen J, Openshaw H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. Journal of virology. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen SH, Garber DA, Schaffer PA, Knipe DM, Coen DM. Persistent elevated expression of cytokine transcripts in ganglia latently infected with herpes simplex virus in the absence of ganglionic replication or reactivation. Virology. 2000;278:207–216. doi: 10.1006/viro.2000.0643. [DOI] [PubMed] [Google Scholar]
- 37.Halford WP, Gebhardt BM, Carr DJ. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. Journal of immunology. 1996;157:3542–3549. [PubMed] [Google Scholar]
- 38.Shimeld C, Whiteland JL, Williams NA, Easty DL, Hill TJ. Cytokine production in the nervous system of mice during acute and latent infection with herpes simplex virus type 1. The Journal of general virology. 1997;78(Pt 12):3317–3325. doi: 10.1099/0022-1317-78-12-3317. [DOI] [PubMed] [Google Scholar]
- 39.Sanderson NS, Puntel M, Kroeger KM, Bondale NS, Swerdlow M, Iranmanesh N, Yagita H, Ibrahim A, Castro MG, Lowenstein PR. Cytotoxic immunological synapses do not restrict the action of interferongamma to antigenic target cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7835–7840. doi: 10.1073/pnas.1116058109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, Olthoff K, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–1937. 1937 e1921–1922. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Velzen M, Laman JD, Kleinjan A, Poot A, Osterhaus AD, Verjans GM. Neuron-interacting satellite glial cells in human trigeminal ganglia have an APC phenotype. Journal of immunology. 2009;183:2456–2461. doi: 10.4049/jimmunol.0900890. [DOI] [PubMed] [Google Scholar]