Abstract
Objective
Individual muscle activation patterns may be controlled by motor modules constructed by the central nervous system to simplify motor control. This study compared modular control of gait between persons with Parkinson’s disease (PD) and neurologically-healthy older adults (HOA) and investigated relationships between modular organization and gait parameters in persons with PD.
Methods
Fifteen persons with idiopathic PD and fourteen HOA participated. Electromyographic recordings were made from eight leg muscles bilaterally while participants walked at their preferred walking speed for ten minutes on an instrumented treadmill. Non-negative matrix factorization techniques decomposed the electromyographic signals, identifying the number and nature of modules accounting for 95% of variability in muscle activations during treadmill walking.
Results
Generally, fewer modules were required to reconstruct muscle activation patterns during treadmill walking in PD compared to HOA (p<.05). Control of knee flexor and ankle plantarflexor musculature was simplified in PD. Activation timing was altered in PD while muscle weightings were unaffected. Simplified neuromuscular control was related to decreased walking speed in PD.
Conclusions
Neuromuscular control of gait is simplified in PD and may contribute to gait deficits in this population.
Significance
Future studies of locomotor rehabilitation in PD should consider neuromuscular complexity to maximize intervention effectiveness.
Keywords: Motor modules, motor control, gait, Parkinson’s disease, non-negative matrix factorization, electromyography
Introduction
Walking is a complex neuromechanical process involving activity across a range of muscles. The central nervous system forms motor modules to coordinate activation of different muscles and muscle groups simultaneously (Ivanenko et al., 2005; Neptune et al., 2009). Motor modules are individual groups of synchronously-activated skeletal muscles that demonstrate coincident firing patterns to reduce complexity of motor control. These motor modules are composed such that activation of a single motor module co-activates a series of individual muscles to efficiently execute the desired task. During walking in neurologically-healthy older adults (HOA), five motor modules generally account for 95% of the cycle-by-cycle variability in the activation of all unilateral lower extremity muscles across a range of walking speeds (Ivanenko et al., 2004). Although researchers have used several computational methods to identify these modules (i.e., principal component analysis, independent component analysis, and non-negative matrix factorization (NNMF)), these different algorithms have identified similar modules. This finding suggests that these analyses do not produce arbitrary fits to the data but rather describe underlying physiological aspects of muscle activation patterns (Ivanenko et al., 2005; Tresch et al., 2006). Modular organization findings suggest that relatively few modules are scaled continuously to consolidate a larger number of constantly-changing individual muscle activation and deactivation patterns, ultimately producing the desired human gait kinematics and kinetics (Ivanenko et al., 2005).
Irregularities in the modular control mechanisms can cause significant deficits in gait, as recent research has revealed a reduction in the number of modules accessed on the paretic side by persons with post-stroke hemiparesis compared to HOA (Clark et al., 2010) and that the number of modules significantly correlates with clinical and laboratory based assessments of walking performance (Bowden et al., 2010). Persons post-stroke typically demonstrate a reduction in corticospinal drive (Nielsen et al., 2008), which influences muscle activity patterns during walking (Petersen et al., 2001; Pijnappels et al., 1998). Researchers have suggested that a reduction in the corticospinal drive post-stroke could reduce the ability to activate gait-related modules (Clark et al., 2010). Thus, consolidation of motor modules during gait appears to reduce movement complexity and locomotor performance in this population (Bowden et al., 2010). These results further suggest that the modular organization of muscle activation serves as a quantitative indicator of complexity changes in muscle firing patterns and neurological coordination of gait.
To date, research on motor modules in populations characterized by neuropathology has been largely limited to persons post-stroke. However, Parkinson’s disease (PD) is one of the most common neurodegenerative movement disorders worldwide and also often causes debilitating effects on gait. PD is characterized by motor deficits resulting from the degradation of dopaminergic neurons in the basal ganglia (Obeso et al., 2008). The basal ganglia receive input from multiple cortical areas and extend projections to premotor and supplementary motor areas that play significant roles in motor planning and early motor execution (Alexander et al., 1986). The basal ganglia also share reciprocal connections with the pedunculopontine nucleus (PPN), a brainstem nucleus with significant influence on gait (Mena-Segovia et al., 2004). Indeed, neuromuscular control and execution of gait is altered in PD, as persons with PD exhibit increased co-activation of antagonistic muscles (Dietz et al., 1995) and reductions in amplitude of the distal lower-extremity musculature (Cioni et al., 1997; Mitoma et al., 2000). These neural control deficits are particularly debilitating, as slower walking speeds (Knutsson, 1972), increased gait variability (Hausdorff, 2009), and increased frequency of falling (Pickering et al., 2007) are hallmarks of parkinsonian gait. While abnormalities in EMG signals have been observed in individual muscles in PD, no studies have evaluated the complexity of neuromuscular organization during gait based on analysis of motor modules that control individual EMG signals in this population.
The purposes of this study were 1) to investigate whether parkinsonian gait is accomplished using a similar set of motor modules available to HOA, 2) to compare the activation profiles and muscle weighting vectors that comprise the motor modules between persons with PD and HOA, and 3) to investigate relationships between motor modules and biomechanical gait characteristics in HOA and persons with PD. As gait deficits accompany impaired ability to plan and execute motor actions efficiently in persons with PD, we hypothesized that the number of motor modules controlling gait would be reduced in PD and that this reduction may be associated with biomechanical gait deficits frequently observed in PD.
Methods
Participants
Fifteen persons with idiopathic PD (66.6±7.8 yrs, 172±9.5 cm, 80.2±13.6 kg,) and fourteen age-matched HOA (66.2±7.1 yrs, 166±13.3 cm, 69.7±17.8 kg) participated. Inclusion criteria for the PD participants included diagnosis of idiopathic PD by a fellowship-trained movement disorders neurologist based on UK Brain Bank criteria (Gibb and Lees, 1988). HOA inclusion criteria included lack of a history of neurological impairment and within a two-year age-match of one of the PD participants. None of the participants had experienced any lower-extremity orthopedic injury for at least one year prior to inclusion. Each participant signed an informed consent form approved by the university’s institutional review board.
Procedures
Thirty-five passive reflective markers were attached to lower and upper body bony landmarks in accordance with the Vicon Plug-in-Gait marker set. Bipolar surface electrodes were placed bilaterally over the soleus (SOL), medial gastrocnemius (GAS), tibialis anterior (TA), vastus medialis (VM), rectus femoris (RF), semimembranosus (SM), biceps femoris (BF), and the gluteus medius (GM). Surface EMG signals were recorded with a telemetric EMG system (Konigsburg Instruments, Pasadena, CA) at a sampling rate of 1200 Hz. Kinematic data were collected using a 7-camera motion capture system (120 Hz; Vicon Nexus, Oxford, UK) while participants walked on a split-belt instrumented treadmill (Bertec Corporation, Columbus, OH). The EMG, kinematic, and kinetic data were time-synchronized and collected simultaneously. Preferred walking speed (PWS) was determined using a technique outlined by Dingwell and colleagues (Dingwell et al., 2007). Participants walked for ten minutes at the PWS with EMG, kinematic, and kinetic data collected over the last four minutes.
Data Analysis
The raw EMG signals were high-pass filtered (35Hz) with a zero lag fourth-order Butterworth filter, demeaned, rectified, and finally low-pass filtered (7Hz) with a zero lag fourth-order Butterworth filter. Our filter selection is similar to previous research on motor modules during gait (Clark et al., 2010). To account for differences in signal amplitude, we normalized the amplitude of the processed EMG signal from each muscle to its peak value during treadmill walking. We also normalized each EMG signal temporally to 0 to 100% of the gait cycle. For each subject and leg, the processed physiological EMG signals (EMG0) for all muscles were combined into a matrix consisting of eight rows (one for each muscle being recorded) and a separate column for each processed EMG0 data point. The total number of columns for a given subject and leg was 101 (the number of data points after temporal normalization to 100% of the gait cycle) multiplied by the total number of gait cycles collected across the four minutes of treadmill data.
Our NNMF analysis was conducted using previously-published procedures (Clark et al., 2010; Dingwell et al., 2007; Ting and Chvatal, 2010). Specifically, the NNMF algorithm in Matlab (The Mathworks, Natick, MA) was altered to implement the methodology described in Ting and Chvatal (Ting and Chvatal, 2010). For each subject, we applied our NNMF algorithm to the previously-described matrix containing the EMG0 data across all gait cycles from the four minutes of PWS trials. The number of modules, n, was initially specified. The NNMF algorithm subsequently decomposed the EMG0 signals into a smaller number of motor modules, where each module was defined by a muscle weighting vector and a single corresponding time-varying activation profile. The muscle weighting vector was an 8 × n matrix that identified the relative contributions of individual muscles to each module. The activation profiles were collected in an n × 101 matrix that represented the firing patterns of the modules across the 101 points of the temporally-normalized gait cycle. Reconstructed EMG signals (EMGr) were then generated by multiplying the 8 × n matrix of muscle weightings by the n × 101 matrix of activation timing profiles on a cycle-by-cycle basis. Each gait cycle was analyzed separately with the assumption that muscle weightings were fixed for that cycle while activation profiles were allowed to vary across gait cycles (Ting and Chvatal, 2010). The NNMF algorithm minimized the sum of squares of the errors (∑ (EMG0-EMGr)2) by adjusting each module’s muscle weighting vector and activation profile given the specified number of modules.
Modules
The NNMF analyses were performed assuming one through six modules. The minimum number of modules needed to reconstruct the EMG0 in each leg of each subject was determined by first calculating the percent variability accounted for (%VAF = 1-(EMG0-EMGr)2/EMG02) for all muscles analyzed together (Ting and Macpherson, 2005). The number of modules assumed was increased until modular configuration eclipsed 95% VAF of all muscles combined (Ivanenko et al., 2004; Ivanenko et al., 2003). For instance, if for a given leg a four-module configuration achieved a maximum total %VAF of 93% and upon expansion to a five-module configuration achieved 96% VAF, this leg would be classified as reaching 95% VAF at five modules. In addition to calculating the %VAF for all muscles analyzed together, we also calculated %VAF for individual muscles (Ting and Chvatal, 2010). This type of analysis provides insight into which individual muscle activation patterns are poorly reconstructed by the NNMF, thus affecting the complexity of the entire eight-muscle NNMF EMG reconstructions.
We organized the motor modules based on the dominant contributors of their respective muscle weighting vectors to maintain consistency for comparison between groups. The dominant contributor to each module was defined as the muscle with the highest individual weight within the module’s muscle weighting vector. For example, module one was defined by SOL as the dominant contributor since SOL had the highest weight within this module; module two was defined by TA as the dominant contributor, etc. Each leg of each participant was aligned to these definitions. After the motor modules had been organized for each participant, the amplitude and timing of the peaks of the activation profiles in each motor module were calculated. All parameters were calculated for each leg individually and thus every leg remained independent in the statistical analyses (i.e., each participant contributed two legs to the group).
Gait Kinetics
Inverse dynamics techniques within Vicon Nexus were used to calculate sagittal plane joint moments at the hip, knee, and ankle. The ground reaction forces (GRFs) were collected using force plates embedded within the split-belt treadmill (Bertec Corporation, Columbus, OH) sampling at 1200 Hz. The moments and GRFs were normalized to participant body mass and temporally to the gait cycle. The propulsive phase was defined as the second half of stance phase (from 50% of stance until toe-off) (Turns et al., 2007). The propulsive GRF impulse was calculated as the time integral of the anterior-posterior GRF component over the propulsive phase. For each participant, all variables were averaged across all strides of the four-minute treadmill trials.
Statistical Analyses
Pearson’s Chi-Squared test analyzed differences in the proportions of HOA and PD accessing varying numbers of modules at 95%VAF. Independent samples t-tests were performed to compare the following variables between groups (PD and HOA):
Amplitude of the individual contribution of each muscle to the muscle weighting vectors for four- and five-module configurations.
The amplitude and timing of activation profile peaks for four- and five-module configurations.
%VAF for individual muscles for four- and five-module configurations.
Propulsive GRF and sagittal hip, knee, and ankle moment impulses.
Preferred treadmill walking speed.
We also calculated Cohen’s d effect sizes for these between-group comparisons as an estimate of the meaningfulness of differences. An effect size of .2 is typically considered small, .5 medium, and .8 large (Cohen, 1988).
One-tailed Pearson’s correlation coefficients were calculated to investigate the following relationships:
The number of modules required to reach 95% VAF vs. sagittal hip, knee, and ankle moment impulses during the propulsive phase of gait.
The number of modules required to reach 95% VAF vs. walking speed.
The total %VAF for four- and five-module configurations vs. sagittal hip, knee, and ankle moment impulses during the propulsive phase of gait.
The total %VAF for four- and five-module configurations vs. walking speed.
Results
Neuromuscular complexity
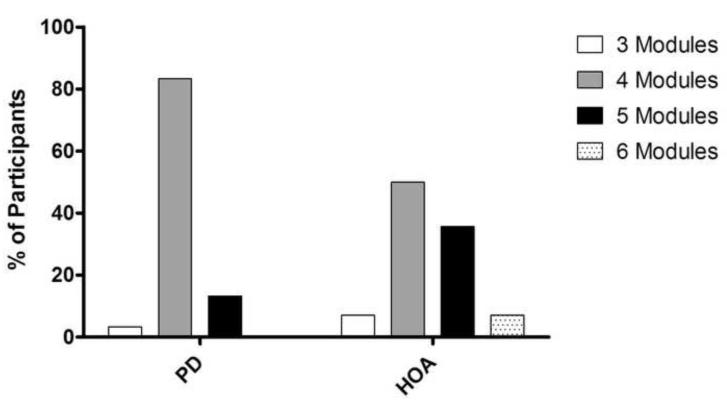
Persons with PD generally required fewer modules than did HOA to achieve 95%VAF at PWS. The majority of PD participants utilized four modules to reconstruct 95% of the unilateral variability in eight lower extremity muscle activations. Of the 30 PD legs, 3.3% required three modules, 83.3% required four modules, and 13.3% required five modules. In contrast, HOA participants generally required more modules for the reconstruction (Figure 1; p<.05). Of the 28 HOA legs, 7.1% required three modules, 50% required four modules, 35.7% required five modules, and 7.1% required six modules.
Figure 1.
Number of modules needed to account for 95% the individual gait cycle variability at PWS by percentage of participants in HOA and PD. The majority of PD participants needed four modules, while HOA required significantly more (p<.05).
Muscle weighting vectors
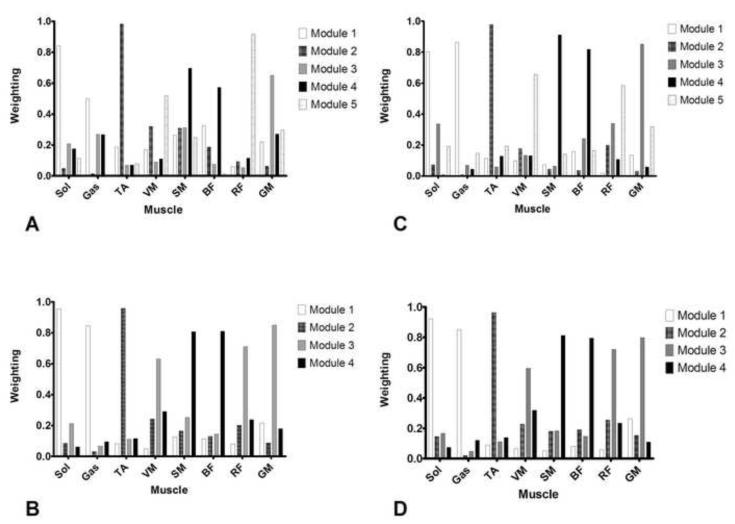
The individual muscle weights within any of the modules’ muscle weighting vectors were similar between groups. Regardless of whether the participants required four or five modules to reconstruct 95%VAF in EMG0, the muscle weightings in each module were comparable between groups; contributions of each muscle to PD modules were very similar to contributions of each muscle to HOA modules when the number of modules was constant (Figure 2).
Figure 2.
Module muscle weightings when 95% of variability was accounted for. A (5 modules assumed) and B (4 modules assumed) represent the averaged reconstructed modules for PD participants. C (5 modules assumed) and D (4 modules assumed) represent the average reconstructed modules for HOA participants. There was no significant difference between each reconstructed muscle at four or five modular output between the groups (all p>.05).
Amplitude and timing of activation profile peaks
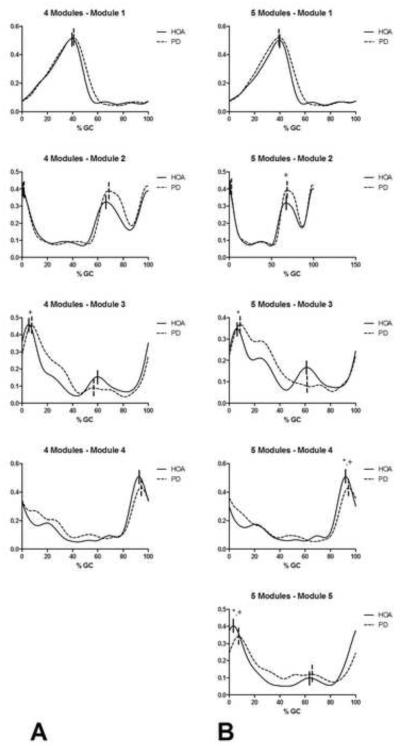
When four modules were assumed, we typically observed one distinguishable peak in the activation profiles in the first and fourth modules and two peaks in the activation profiles in the second and third modules (Figure 3). If one of the peaks of the activation profiles of the second or third modules was absent in the activation profile of any individual leg, this peak was omitted from the analysis for that leg. This issue did not occur in more than three participants in either group in any of the analyses and typically did not occur at all as the general shapes of the activation profiles were quite consistent across participants.
Figure 3.
Module activation profiles when 95% of variability was accounted for. Profiles in columns A and B correspond to those observed when four and five modules were assumed, respectively. The solid vertical lines indicate the peaks of the HOA profiles while the dashed vertical lines indicate the peaks of the PD profiles. *indicates a significant difference (p<.05) in the magnitude of the peaks while + indicates a significant difference (p<.05) in the timing of the peaks.
We observed that the first peak in the activation profile of the third module occurred significantly later in the gait cycle of PD compared to HOA (PD: 9.14±4.74% of gait cycle (%GC), HOA: 5.86±2.24%GC; p<.05, d=0.90). We also observed trends suggesting that the magnitude of the second peak of the third module was lower (PD: 0.138±0.090, HOA: 0.187±0.111; p=.082, d=0.49) and temporally occurred later in the gait cycle (PD: 64.54±7.00%GC, HOA: 60.93±7.43%GC; p=.072, d=0.51). Further, a trend suggested that the magnitude of the peak of the fourth module (PD: 0.489±0.119, HOA: 0.547±0.089; p=.051, d=0.56) was lower in PD compared to HOA.
When five modules were assumed, we typically observed one distinguishable peak in the activation profiles in the first and fourth modules and two peaks in the activation profiles of the second, third, and fifth modules (Figure 3). When comparing the activation profiles of PD to HOA, we observed delayed onset of the peaks across several modules. The second peak within the second module (PD: 71.50±4.99%GC, HOA: 66.18±7.65%GC; p<.05, d=0.84), the peak of the fourth module (PD: 95.33±3.43%GC, HOA: 93.30±2.40%GC; p<.05, d=0.70), and the first peak of the fifth module (PD: 11.17±6.96%GC, HOA: 6.67±7.40%GC, p<.05, d=0.64) occurred significantly later in the gait cycle in PD. Further, the magnitude of the first peak of the third module was significantly higher in PD (PD: 0.457±0.122, HOA: 0.388±0.120; p<.05, d=0.58) while the peak of the fourth module (PD: 0.483±0.133, HOA: 0.554±0.088; p<.05, d=0.64) and the first peak of the fifth module were significantly lower (PD: 0.353±0.208, HOA: 0.456±0.098; p<.05, d=0.64). We also observed trends suggesting that the first peak of the third module occurred later (PD: 15.62±10.28%GC, HOA: 10.82±9.20%GC; p=.073, d=0.50) and that the magnitude of the second peak of the third module was lower (PD: 0.157±0.126, HOA: 0.227±0.120; p=.055, d=0.58) in PD compared to HOA.
EMG0%VAF by individual EMGr muscle reconstructions
When four modules were assumed (Table 1), the %VAF of GAS (PD: 0.951±0.023, HOA: 0.927±0.037; p<.05, d=0.80) and SM (PD: 0.961±.020, HOA: 0.936±0.027; p<.05, d=1.09) were higher in PD compared to HOA. When five modules were assumed, the %VAF of GAS (PD: 0.968±0.017, HOA: 0.951±0.026; p<.05, d=0.77), SM (PD: 0.976±0.016, HOA: 0.958±0.018; p<.05, d=1.09), and BF (PD: 0.971±0.019, HOA: 0.960±0.018; p<.05, d=0.57) were higher in PD compared to HOA. Thus, the NNMF analysis was able to reconstruct GAS, SM, and BF EMG0 signals more accurately in PD compared to HOA when the number of modules was constant (Ting and Chvatal, 2010).
Table 1. Percent variability accounted for (%VAF) by each individual muscle and module.
When four modules were assumed, the %VAF of the GAS and SM were higher in PD compared to HOA (p<.05). When five modules were assumed, the %VAF of the GAS, SM, and BF were higher in PD compared to HOA (p<.05). Bold text and asterisks indicate p<.05
| Muscle | Modules | %VAF PD | %VAF HOA | Significance |
|---|---|---|---|---|
| SOL | 4 | 95.6±2.1 | 95.0±2.7 | 0.38 |
| 5 | 97.1±1.7 | 97.1±1.6 | 0.82 | |
| GAS | 4 | 95.1±2.2 | 92.7±3.7 | 0.005* |
| 5 | 96.8±1.7 | 95.1±2.6 | 0.006* | |
| TA | 4 | 97.0±3.3 | 95.1±5.1 | 0.10 |
| 5 | 99.2±1.3 | 98.5±2.6 | 0.25 | |
| VM | 4 | 94.2±2.1 | 92.7±3.9 | 0.07 |
| 5 | 96.4±1.8 | 95.3±3.4 | 0.15 | |
| SM | 4 | 96.1±2.0 | 93.6±2.6 | <0.001* |
| 5 | 97.6±1.5 | 95.8±1.8 | <0.001* | |
| BF | 4 | 95.0±3.2 | 93.7±2.6 | 0.09 |
| 5 | 97.1±1.9 | 96.0±1.8 | 0.04* | |
| RF | 4 | 95.4±2.3 | 94.6±2.3 | 0.19 |
| 5 | 96.8±1.7 | 96.3±2.2 | 0.37 | |
| GM | 4 | 93.2±3.3 | 94.5±3.9 | 0.17 |
| 5 | 96.9±1.9 | 97.7±1.9 | 0.09 |
Gait biomechanics
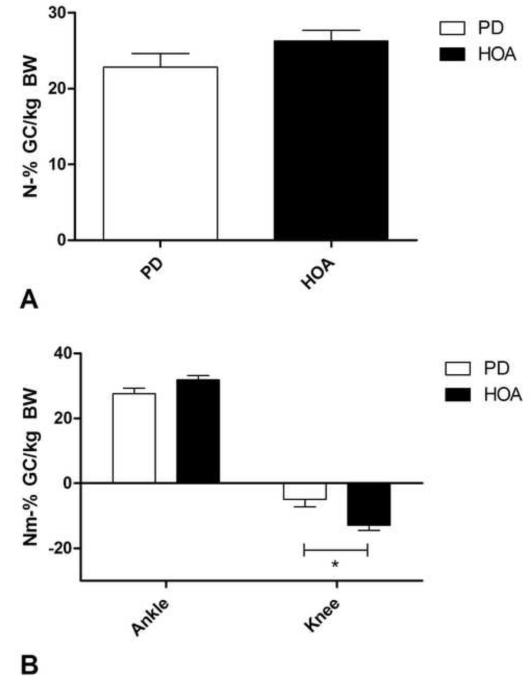
Walking speed was lower in PD but not significantly different between the two groups (PD: 0.90±0.34 m/s, HOA: 1.04±0.26 m/s, p=.101, d=0.47). We observed a significant decrease in sagittal hip (PD: −8.85±16.34 Nm•%GC/kg, HOA: 0.96±9.09 Nm•%GC/kg; p<.05, d=0.73) and knee (PD: −4.92±11.62 Nm•%GC/kg, HOA: −12.86±6.89 Nm•%GC/kg; p<.05, d= 0.82) moment impulses in PD compared to HOA during the propulsive phase. We also observed a statistical trend suggesting lower sagittal ankle moment impulses in PD during propulsion (PD: 27.52±8.61 Nm•%GC/kg, HOA: 31.82±5.53 Nm•%GC/kg, p=.07, d=0.59). Propulsive GRF impulse was lower in PD but not significantly different from HOA (PD: 22.85±8.59 N•%GC/kg, HOA: 26.28±6.27 N•%GC/kg, p=.14, d=0.46) (Figure 4).
Figure 4.
A) displays the propulsive GRF impulse in PD and HOA. Propulsive GRF impulse was lower in PD but not significantly so (p=.14). B) displays the sagittal knee and ankle moment impulses in PD and HOA during propulsion. We observed a significantly lower sagittal knee moment impulse (p<.05) and a trend towards a lower sagittal ankle moment impulse during the propulsive phase of gait in PD (p=.07).
Relationships between neuromuscular complexity and gait mechanics
We did not observe any significant relationships between the number of modules required to reach 95%VAF in EMG0 and either walking speed, propulsive sagittal hip moment impulse, propulsive sagittal knee moment impulse, propulsive sagittal ankle moment impulse, or propulsive GRF impulse. However, we did observe significant associations between walking speed and the total %VAF in both four- and five-module configurations within the PD group (r=−0.363, p=0.024; r=−0.435, p=0.008, respectively). These associations were not observed in HOA.
Discussion
This study demonstrated that some persons with PD exhibit a reduced number of motor modules during gait when compared to HOA. Our results provide novel evidence that neuromechanical control of gait tends to be less complex in PD and the motor module activation profiles differ between PD and HOA during gait. Ninety-five %VAF was achieved with four or fewer modules in 86% of PD limbs but only 57% of HOA limbs. Clark and colleagues demonstrated that simplification of modular control resulted in slower walking speeds, reduced propulsion, and increased gait asymmetry in persons post-stroke (Clark et al., 2010). In the present study, we observed a relationship between simplified neuromuscular complexity at four- and five-module configurations and slower walking speeds in persons with PD. Indeed, our findings are somewhat similar to those observed by Clark and colleagues in persons post-stroke (Clark et al., 2010). As previously observed in persons post-stroke, the reduction of the number of modules may represent potential merging of multiple modules available to healthy participants. For example, the GM shifts from characterizing predominantly its own module in the five-module configuration (module 3, Figure 2) to a module with strong contributions from the quadriceps and, to a lesser extent, the hamstrings in the four-module configuration (module 3, Figure 2). While organization of weighting vectors for the four- and five-module configurations was not different between PD and HOA, our finding that a significantly larger proportion of persons with PD achieve 95%VAF with four modules indicates that persons with PD may merge control of the GM with control of the knee extensors to a greater degree than in HOA. However, the deficits do not appear to be as severe as those previously observed in persons post-stroke as only one of the ambulatory persons with mild-to-moderate PD included in this study reached 95%VAF with fewer than four modules.
This potential merging of control between the GM and quadriceps musculature may have important functional consequences on the control of locomotion in PD. Though these muscles have somewhat similar activation patterns, they act primarily on different joints and thus serve different roles at similar points in the gait cycle. GM stabilizes the hip throughout a large portion of stance while the knee extensors control knee flexion and extend the knee primarily during weight acceptance (Winter, 1991). Therefore, it seems conceivable that it would be beneficial to maintain independent control over these two modules, though it would also be possible to exert functional control over gait by merging the two (as may be the case in PD and stroke).
Various studies on motor modules during gait have presented conflicting results in terms of the number of modules necessary to account for sufficient variability within EMG signals - some have identified five modules (Ivanenko et al., 2005; Ivanenko et al., 2004; Ivanenko et al., 2003) and others have identified four (Clark et al., 2010). We identified five modules and suggest that this finding may be explained by our selection of a 95%VAF threshold whereas Clark and colleagues identified four modules utilizing a less-stringent VAF threshold of 90%. The muscle weighting vectors identified in this study were not significantly different between HOA and PD, suggesting that persons with PD retain access to the same muscle grouping patterns as HOA during modular activation. Further, the muscle weighting vectors presented here are very similar to those demonstrated in previous studies of human walking in healthy persons (Ivanenko et al., 2004; Clark et al., 2010; Cappellini et al., 2006). On the contrary, several of the activation profile peaks differed in both timing and amplitude in PD when compared to HOA. Indeed, when five modules were assumed, the peaks of the activation profiles were altered in all modules except module one when comparing PD to HOA. Specifically, the peak of the activation profile in module four (the module whose muscle weighting vector was defined by knee flexor musculature as the dominant contributors) was diminished in magnitude and temporally shifted in PD. This finding is of potential clinical significance as both knee flexor muscles recorded in this study (SM and BF) demonstrated simplified control in PD when compared to HOA, as evidenced by higher %VAF by individual musculature.
It therefore appears that persons with PD have access to similar muscle weighting vectors as HOA but demonstrate altered control of temporal modular activation patterns. Considering our present findings regarding coincident reduction in control complexity of SM and BF and modification of activation profiles in modules characterized by these muscles, we suggest that altered timing of modular activation may be a significant contributor to the simplified neuromuscular control of gait observed in PD. However, altered activation profiles were observed in the absence of simplified control in other modules and thus our hypothesis requires further investigation.
Implications for Supraspinal Control of Locomotion
Prior investigations of animal locomotion have suggested that organization of muscle coordination is encoded within central pattern generators (CPGs) of the spinal cord (Grillner, 2006; McCrea and Rybak, 2008; Kiehn, 2011). Our results add to the limited literature of supraspinal importance in human modular organization of gait. Moreover, the simplified modular organization that we observed in PD participants is likely related to motor control deficits resulting from dysfunction of the basal ganglia and PPN. Importantly, the degradation of dopaminergic neurons in the basal ganglia (Obeso et al., 2008) leads to consequent impairments in descending pathways in persons with PD (Spann and Grofova, 1989). For instance, the PPN of the midbrain locomotor region receives input from the basal ganglia and has been demonstrated to play a significant role in brainstem control over CPGs during locomotion (Grillner et al., 2008). Indeed, degeneration of cholinergic neurons in the PPN has been reported in persons with PD (Hirsch et al., 1987) and linked to decreased gait performance in this population (Karachi et al., 2010). Thus, it has been suggested that locomotor control may be diminished in PD as a result of PPN cholinergic degeneration coupled with disruptions in the bidirectional signals between the PPN and basal ganglia due to dopaminergic degeneration of basal ganglia (Mena-Segovia et al., 2004). It is important to consider that the methodology used in this study is limited to measures of peripheral muscle activation only and does not directly examine central processes; however, we postulate that basal ganglia and downstream brainstem dysfunction in PD may simplify the complexity of neuromuscular modular organization during gait even in an optimally-medicated state.
Specific Regions of Deficits
To further our understanding of the functional effects of the neuromuscular simplification observed in PD, we analyzed the %VAF within each individual muscle and module. Of the eight muscles collected, only ankle plantarflexor and knee flexor musculature (GAS, SM, and BF) reached a higher %VAF (and hence lower complexity) in PD than in HOA for the same number of modules. Indeed, we demonstrated that propulsive knee moment impulses (which are typically knee flexor impulses) were diminished in PD when compared to HOA. Thus, we suggest that therapeutic mechanisms targeting the knee flexor musculature in PD may enhance (or at least compensate for) overall neuromuscular complexity of locomotion.
Future Directions
We have shown that there is a decrease in locomotor output complexity during gait in persons with PD compared to HOA. The question then prevails - is it possible for PD participants to regain a higher level of locomotor output complexity? Recent studies have demonstrated the capacity for neurons to adapt structurally and functionally not only during development but even after a traumatic brain injury or disease (Kleim, 2011). For instance, previous research on animals has suggested that synaptogenesis in the motor cortex increased with exercise complexity (Kleim et al., 1996). Further work by Nudo and colleagues demonstrated the potential for motor training to elicit neuroplastic changes and reorganization in the pathological brain (Nudo et al., 1996). Accordingly, we postulate that there may exist some potential for complex locomotor exercise protocols to facilitate restoration of neuromuscular complexity in persons with PD. Future evaluation of intervention techniques using motor modules as a dependent variable may be useful in identifying the most effective rehabilitation mechanisms for persons with PD. Furthermore, the field of rehabilitation has recognized the importance of individualizing therapy for individual patients. Characterizing the organization and activation patterns of each patient’s available motor modules may guide the development of specific therapies that maximize the opportunity for neural plasticity and ultimately enhance functional outcome (Kleim, 2011).
Limitations
Our modular analysis of motor control complexity during gait in persons with PD possesses several important limitations. Our analysis was limited to treadmill as opposed to overground walking. Though treadmill gait differs slightly from overground gait (Alton et al., 1998), it is unlikely to have significantly affected our results as the same approach was applied to both PD and HOA participants. In addition, PWS of persons with PD were slower than those of HOA, though modular composition in previous studies remained relatively unchanged over a wide range of walking speeds within the range of speeds selected by our participants (Ivanenko et al., 2004; Clark et al., 2010). We also tested all participants with PD in their self-reported best-medicated state. Thus, dopaminergic treatment may have had an effect on locomotor complexity in the PD group. However, we would expect dopaminergic treatment to have a positive effect on locomotor complexity if indeed any effect exists. It should also be acknowledged that no correction for multiple comparisons was applied to the correlational analyses. However, even if a strict Bonferroni correction was applied to our analysis, the result that total %VAF at 5 modules is related to walking speed in PD remains significant and our interpretation of these findings remains the same. Further, all of the comparisons elicit large Cohen effect sizes, suggesting that the observed differences are in fact meaningful. It is also possible that we would have identified additional modules had we collected from a more diverse set of motor tasks or a larger set of muscles.
Conclusions/Implications
The purpose of this study was to investigate whether PD subjects control gait using a similar organization of motor modules available to neurologically-healthy peers. We were able to analyze motor module organization in persons with PD and age-matched controls using an NNMF analysis of EMG data collected during treadmill walking at preferred walking speeds. We demonstrated that persons with PD generally demonstrate a reduction in the number of motor modules during gait when compared to aged-matched neurologically-healthy peers. Furthermore, we identified the specific reduced modular complexity of ankle plantarflexor and knee flexor regions in PD and consequential functional disability. Our results suggest that modular organization of muscle activation serves as a quantitative indicator of complexity changes in muscle firing patterns and neurological coordination of walking ability in PD. Future interventions should consider addressing neuromuscular complexity as an important deficit in persons with PD.
Highlights.
Persons with Parkinson’s disease required fewer motor modules than did healthy older adults to reconstruct 95% of the variability in the physiological processed EMG signals during treadmill walking.
Compared to healthy older adults, activation profiles were altered in persons with Parkinson’s disease while muscle weighting vectors were unchanged.
These patterns of simplified neuromuscular control complexity were associated with decreased gait performance in persons with Parkinson’s disease.
Acknowledgements
This work was supported in part by NIH grant R21033284-01A2 and the National Parkinson Foundation Center of Excellence at the University of Florida.
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alton F, Baldey L, Caplan S, Morrissey MC. A kinematic comparison of overground and treadmill walking. Clin Biomech. 1998;13:434–440. doi: 10.1016/s0268-0033(98)00012-6. [DOI] [PubMed] [Google Scholar]
- Bowden MG, Clark DJ, Kautz SA. Evaluation of abnormal synergy patterns poststroke: relationship of the Fugl-Meyer Assessment to hemiparetic locomotion. Neurorehabil Neural Repair. 2010;24:328–37. doi: 10.1177/1545968309343215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. J Neurophysiol. 2006;95:3426–3437. doi: 10.1152/jn.00081.2006. [DOI] [PubMed] [Google Scholar]
- Cioni M, Richards CL, Malouin F, Bedard PJ, Lemieux R. Characteristics of the electromyographic patterns of lower limb muscles during gait in patients with Parkinson’s disease when OFF and ON L-Dopa treatment. Ital J Neurol Sci. 1997;18:195–208. doi: 10.1007/BF02080464. [DOI] [PubMed] [Google Scholar]
- Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol. 2010;103:844–857. doi: 10.1152/jn.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates, Inc; 1988. The t Test for Means; pp. 20–27. Chapter 2. [Google Scholar]
- Dietz V, Zijlstra W, Prokop T, Berger W. Leg muscle activation during gait in Parkinson’s disease: adaptation and interlimb coordination. Electroencephalogr Clin Neurophysiol. 1995;97:408–415. doi: 10.1016/0924-980x(95)00109-x. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Napolitano DF, Chelidze D. A nonlinear approach to tracking slow-time-scale changes in movement kinematics. J Biomech. 2007;40:1629–1634. doi: 10.1016/j.jbiomech.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. A comparison of clinical and pathological features of young- and old-onset Parkinson’s disease. Neurology. 1988;38:1402–1406. doi: 10.1212/wnl.38.9.1402. [DOI] [PubMed] [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallén P, Saitoh K, Kozlov A, Robertson B. Neural bases of goal-directed locomotion in vertebrates--an overview. Brain Res Rev. 2008;57:2–12. doi: 10.1016/j.brainresrev.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM. Gait dynamics in Parkinson’s disease: common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos. 2009;19:026113. doi: 10.1063/1.3147408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Graybiel AM, Duyckaerts C, Javoy-Agid F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc Natl Acad Sci U S A. 1987;84:5976–5980. doi: 10.1073/pnas.84.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Grasso R, Zago M, Molinari M, Scivoletto G, Castellano V, et al. Temporal components of the motor patterns expressed by the human spinal cord reflect foot kinematics. J Neurophysiol. 2003;90:3555–3565. doi: 10.1152/jn.00223.2003. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Poppele RE, Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol. 2004;556:267–282. doi: 10.1113/jphysiol.2003.057174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Cappellini G, Dominici N, Poppele RE, Lacquaniti F. Coordination of locomotion with voluntary movements in humans. J Neurosci. 2005;25:7238–7253. doi: 10.1523/JNEUROSCI.1327-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachi C, Grabli D, Bernard FA, Tandé D, Wattiez N, Belaid H, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest. 2010;120:2745–2754. doi: 10.1172/JCI42642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O. Development and functional organization of spinal locomotor circuits. Curr Opin Neurobiol. 2011;21:100–109. doi: 10.1016/j.conb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA. Neural plasticity and neurorehabilitation: teaching the new brain old tricks. J Commun Disord. 2011;44:521–528. doi: 10.1016/j.jcomdis.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Knutsson E. An analysis of Parkinsonian gait. Brain. 1972;95:475–486. doi: 10.1093/brain/95.3.475. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev. 2008;57:134–146. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27:585–588. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Mitoma H, Hayashi R, Yanagisawa N, Tsukagoshi H. Characteristics of parkinsonian and ataxic gaits: a study using surface electromyograms, angular displacements and floor reaction forces. J Neurol Sci. 2000;174:22–39. doi: 10.1016/s0022-510x(99)00329-9. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Clark DJ, Kautz SA. Modular control of human walking: a simulation study. J Biomech. 2009;42:1282–1287. doi: 10.1016/j.jbiomech.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JB, Brittain JS, Halliday DM, Marchand-Pauvert V, Mazevet D, Conway BA. Reduction of common motoneuronal drive on the affected side during walking in hemiplegic stroke patients. Clin Neurophysiol. 2008;119:2813–2818. doi: 10.1016/j.clinph.2008.07.283. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Marin C, Rodriguez-Oroz C, Blesa J, Benitez-Temiño B, Mena-Segovia J, et al. The basal ganglia in Parkinson’s disease: current concepts and unexplained observations. Ann Neurol. 2008;64:S30–46. doi: 10.1002/ana.21481. [DOI] [PubMed] [Google Scholar]
- Petersen NT, Butler JE, Marchand-Pauvert V, Fisher R, Ledebt A, Pyndt HS, et al. Suppression of EMG activity by transcranial magnetic stimulation in human subjects during walking. J Physiol. 2001;537:651–656. doi: 10.1111/j.1469-7793.2001.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering RM, Grimbergen YA, Rigney U, Ashburn A, Mazibrada G, Wood B, et al. A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov Disord. 2007;22:1892–1900. doi: 10.1002/mds.21598. [DOI] [PubMed] [Google Scholar]
- Pijnappels M, Van Wezel BM, Colombo G, Dietz V, Duysens J. Cortical facilitation of cutaneous reflexes in leg muscles during human gait. Brain Res. 1998;787:149–153. doi: 10.1016/s0006-8993(97)01557-6. [DOI] [PubMed] [Google Scholar]
- Spann BM, Grofova I. Origin of ascending and spinal pathways from the nucleus tegmenti pedunculopontinus in the rat. J Comp Neurol. 1989;283:13–27. doi: 10.1002/cne.902830103. [DOI] [PubMed] [Google Scholar]
- Ting LH, Macpherson JM. A limited set of muscle synergies for force control during a postural task. J Neurophysiol. 2005;93:609–613. doi: 10.1152/jn.00681.2004. [DOI] [PubMed] [Google Scholar]
- Ting LH, Chvatal SA. Decomposing muscle activity in motor tasks: methods and interpretation. In: Danion F, Latash ML, editors. Motor Control: Theories, Experiments, and Applications. Oxford University Press; New York, NY: 2010. pp. 102–138. [Google Scholar]
- Tresch MC, Cheung VC, d’Avella A. Matrix factorization algorithms for the identification of muscle synergies: evaluation on simulated and experimental data sets. J Neurophysiol. 2006;95:2199–2212. doi: 10.1152/jn.00222.2005. [DOI] [PubMed] [Google Scholar]
- Turns LJ, Neptune RR, Kautz SA. Relationships between muscle activity and anteroposterior ground reaction forces in hemiparetic walking. Arch Phys Med Rehabil. 2007;88:1127–1135. doi: 10.1016/j.apmr.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter DA. Electromyography in human gait. In: Winter DA, editor. The Biomechanics and Motor Control of Human Gait: Normal, Elderly, and Pathological. Second Edition University of Waterloo Press; 1991. pp. 53–74. [Google Scholar]