Abstract
Upon exposure to Ag on the day of birth, neonatal mice mount balanced primary Th1 and Th2 responses with the former displaying up-regulated IL-13 receptor alpha 1 (IL-13Rα1) expression. This chain associates with IL-4Rα to form a heteroreceptor (IL-4Rα/IL-13Rα1) that marks the Th1 cells for death by IL-4 produced by Th2 cells during re-challenge with Ag, hence, the Th2 bias of murine neonatal immunity. The up-regulation of IL-13Rα1 on neonatal Th1 cells was due to the paucity of IL-12 in the neonatal environment. Herein, we show that by day 8 after birth, naïve splenic T cells are no longer susceptible to IL-13Rα1 up-regulation even when exposed to Ag within the neonatal environment. Furthermore, during the 8-day lapse, the naïve splenic T cells spontaneously and progressively up-regulate the IL-12Rβ2 chain, perhaps due to colonization by commensals which induce production of IL-12 by cells of the innate immune system such as dendritic cells. In fact, mature T cells from the thymus, a sterile environment not accessible to microbes, did not up-regulate IL-12Rβ2 and were unable to counter IL-13Rα1 up-regulation. Finally, the 8 day naïve T cells were able to differentiate into Th1 cells even independently of IL-12 but required the cytokine to counter up-regulation of IL-13Rα1. Thus, in neonatal mice, IL-12, which accumulates in the environment progressively, utilizes IL-12Rβ2 to counter IL-13Rα1 expression in addition to promoting Th1 differentiation.
Introduction
For a long time, the neonatal period was considered a window during which exposure to Ag induces T cell tolerance and re-challenge later with the same Ag leads to unresponsiveness (1). Clonal deletion of T cells was initially considered the main mechanism for neonatal tolerance (2). However, careful examination of secondary neonatal responses revealed the development of immunity, but this was mostly in the form of Th2 rather than Th1 cells (3-7). The excess of Th2 cells in neonates likely confers susceptibility to allergic reactions while the diminished Th1 responses sustain vulnerability to microbial infections (8). Lately, it has been shown that both Th1 and Th2 cells develop in the primary neonatal T cell response (7, 9). However, the Th1 cells displayed up-regulation of IL-13Rα1 (9), which was due to diminished IL-12 production by neonatal dendritic cells (10). Also, IL-13Rα1 expression on Th1 cells represents a developmental trait as T cells from adult mice do not up-regulate IL-13Rα1 when challenged with Ag within the neonatal environment where IL-12 is limited (10). Thus, there is a T cell intrinsic factor that contributes to the regulation of IL-13Rα1 expression on Th1 cells. Here, we show that by 8 days (8d) of age, the primary Th1 cells lose susceptibility to IL-13Rα1 up-regulation and develop secondary responses when re-challenged with Ag. The mechanism underlying the inability of neonatal Th1 cells to up-regulate IL-13Rα1 at day 8 lies on a spontaneous up-regulation of the IL-12Rβ2 chain on naïve T cells prior to differentiation. This, however, occurred only when the naïve T cells were derived from the spleen (SP)2, an organ accessible to microbes, but not from the thymus (THY), a sterile site guarded from environmental microbes by the blood-thymus barrier(11). IL-12Rβ2 up-regulation on naïve SP T cells was gradual and dependent on IL-12, a cytokine produced by innate immune system cells upon activation through toll-like receptors by microbial compounds such as CpG and LPS (12-14). In fact, these TLR-ligands triggered IL-12Rβ2 expression by naïve neonatal T cells when injected into 1d old mice and, upon exposure to Ag, these cells did not up-regulate IL-13Rα1 but developed secondary responses. Moreover, when naïve T cells from 8d old mice were exposed to Ag in host mice that were deficient in IL-12, differentiation into Th1 cells occurred but IL-13Rα1 expression persisted, indicating that signaling through IL-12Rβ2, presumably by IL-12, is needed to counter IL-13Rα1 up-regulation. Together, these observations suggest that IL-12 produced by innate cells is required not only to drive differentiation of naïve T cells towards Th1, but also to counter the up-regulation of IL-13Rα1 on these cells, resulting in resistance to IL-4-driven apoptosis during re-challenge with Ag.
Material and Methods
Mice
IL-13Rα1+/+-GFP and IL-13Rα1-/- mice
129Sv/Pas expressing the green fluorescence protein (GFP) under the control of the IL-13Rα1 promoter were generated in our laboratory and were previously described (15). These knock-in mice were then used to generate IL-13Rα1+/+-GFP Balb/c mice by speed congenic technology. Balb/c mice in which exons 7,8, and 9 of the IL-13Rα1 locus were deleted and replaced by a neomycin gene were also generated in the laboratory by homologous recombination as described (15). DO11.10/Rag2-/- transgenic Balb/c mice expressing ovalbumin (OVA)-specific T cell receptor (TCR) were previously described (9). IL-12-/- Balb/c mice deficient for IL-12p35 gene (16) were from the Jackson laboratory. All animals were used according to protocols approved by the University of Missouri animal care and use committee.
Antigen
Chicken OVA 323-339 peptide (SQAVHAAHAEINEAGR) was purchased from Sigma (St. Louis, MO). Ig-OVA, expressing OVA peptide within the variable region of the heavy chain, and Ig-W, the wild type IgG2b backbone used to generate Ig-OVA, were previously described (9, 10, 17). CpG (ODN 1826) and LPS were purchased from InvivoGen (San Diego, CA).
T cell purification and adoptive transfer
Splenic (SP) naïve CD4+ T cells were purified from DO11.10/Rag2-/- mice at the indicated age by positive selection on anti-CD4 antibody coupled magnetic beads (Miltenyi Biotech; Auburn, CA). For purification of thymic (THY) naïve CD4 T cells, the culture was depleted of CD8 T cells (whether single or double positive) on anti-CD8 antibody coupled microbeads prior to positive selection of CD4 T cells on anti-CD4 microbeads. For transfer into host mice, 1 × 105 purified CD4 T cells were adoptively transferred into 1d old hosts by i.v. injection through the facial vein using a 30-gauge needle as described (17). Usually 32 to 40 mice are used to generate 8 neonatal hosts.
Analysis of T cell responses
Primary or ex vivo responses
Mice recipient of T cell transfer on the day of birth are injected i.p. with a saline solution containing 100 μg Ig-OVA. Two weeks later the splenic cells containing both T cell and APCs were stimulated for a short 4 h period of time (ex vivo conditions) with 10 μM OVAp to enhance cytokine accumulation and facilitate intracellular detection. CD4 T cells were then isolated on anti-CD4-microbeads, labeled with KJ1-26 antibody, and tested for intracellular production of IFNγ.
Recall responses
Mice recipient of T cell transfer on the day of birth are injected i.p. with a saline solution containing 100 μg Ig-OVA. Two weeks later the splenic cells (1 × 106 cells/well) containing both T cells and APCs were stimulated with 10 μM OVAp for 24 h instead of 4h to facilitate cytokine secretion. Production of IFNγ was determined by ELISPOT and ELISA.
Secondary responses
Mice recipient of T cell transfer on the day of birth are injected i.p. with a saline solution containing 100 μg Ig-OVA. Two months later, the mice were challenged with 125 μg OVAp in CFA. Ten days later, the splenic (1 × 106 cells/well) and lymph node (5 × 105 cells/well) containing both T cells and APCs were stimulated with 10 μM OVAp for 24 h and production of IFNγ was determined by ELISPOT and ELISA.
Flow cytometry analyses
Cell surface staining for IL-12Rβ2
Purified splenic CD4+ T cells were incubated for 20 min at 4°C with 5μg/mL 2.4G2 mAb to block FcγRs. The cells were stained with FITC-labeled KJ1-26, PeCy7-labeled anti-CD4, APC-labeled anti-CD62L (BD Biosciences) and PE-labeled anti- IL-12Rβ2 (R & D Systems) for 30 min at 4°C. The culture was then washed with staining buffer and detection of IL-12Rβ2 expression was carried out by flow cytometry using a Beckman Coulter Cy An (Brea, CA). Quadrants in density plots are drawn relative to isotype controls. The data was analyzed using FlowJo version 8.8.6 (Tree Star).
Detection of apoptosis by staining with Annexin V
Apoptosis of primary Th1 cells was measured by Annexin V binding to KJ1-26+IFNγ+ T cells as was previously described (9, 10). The cells were first incubated with the clonotypic mAb KJ1-26 and Annexin V (BD Biosciences) as described for cell surface staining and cytokine secretion was blocked by 10μg/mL BFA. The cells were then fixed with 2% formaldehyde, permeabilized with 2% saponin, and incubated with anti-IFNγ antibody. Quadrants in dot plots are drawn relative to isotype controls. The data was collected and analyzed as described for cell surface staining.
Detection of IL-13Rα1
IL-13Rα1-GFP mice were used as a source of naïve CD4 T cells for transfer into host mice. For detection of IL-13Rα1 expression on primary Th1 cells in host mice, the cells were stained with KJ1-26, anti-CD4 and anti-IFNγ antibodies and the KJ1-26+CD4+IFNγ+ T cells were analyzed for GFP expression by flow cytometry.
Measurement of cytokines
ELISA
Splenic cells containing both T cells and APCs were incubated with antigen in 96-well round-bottom plates for 24 hours. IFNγ production was measured by ELISA using anti-cytokine antibodies according to manufacturer's instructions.
ELISPOT
Detection of IFNγ by ELISPOT was carried out as described (5). Briefly, HA-multiscreen plates (Millipore, Bedford, MA) were coated with capture antibody and free sites were saturated with DMEM culture media containing 10% fetal calf serum. Subsequently, 1 × 106 splenic cells were added and the culture was stimulated with OVA peptide with or without blocking antibody. Biotinylated anti-IFNγ antibody (1μg/ml) was added and bound antibody was revealed with avidin-peroxidase. Spots were counted using Immunospot software (Cellular Technology Ltd, Cleveland, OH).
Treatment with rIL-12
Injection of rIL-12 (PeproTech Rocky Hill, NJ) into IL-12-/- mice consisted of 100 ng of cytokine in PBS given i.p. on the day of birth.
Neutralization of IL-12 in vivo
For neutralization of IL-12 in vivo the mice were given i.p. 50 μg anti-IL-12 antibody (clone C17.8, BD Biosciences) or control Rat IgG on day 6, 7, and 8 after birth.
Treatment with LPS and CpG
1d old DO11.10/Rag2-/- mice were injected i.p. with 10 μg LPS or 20 μg CpG in PBS.
Real-time PCR
RT and DNA amplification were performed on a StepOne Instrument cycler (Applied Biosystems) using Power SYBR Green RNA-to-CT 1-Step Kit (Applied Biosystems) according to the manufacturer's instructions on naïve CD4 T cells. The forward and reverse primers were: 5’-AGTCCGTGTTACTGCCATCAACGA-3’ and 5’-TGTACCTCTGCTCCCAGAAGCATT-3’, respectively. The results were analyzed by the comparative CT method using the StepOne software. β-actin was included as a normalizer.
Results
At day 8 after birth neonatal T cells are no longer susceptible to IL-13Rα1 up-regulation
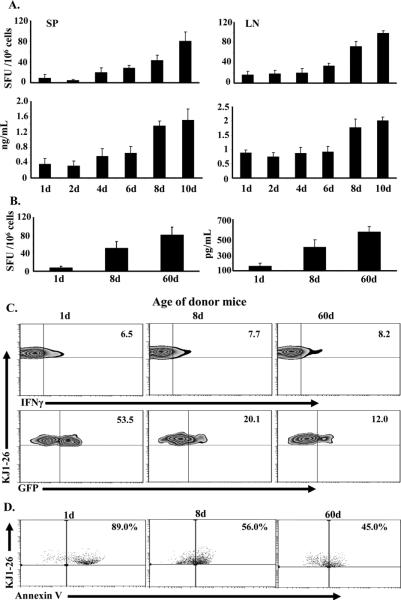
Upon exposure to Ag, naïve neonatal T cells differentiate into Th1 and Th2 cells (7) but the former up-regulate IL-13Rα1 expression (9, 10). In contrast, when naïve T cells from adult mice are transferred into newborns and exposed to Ag, they differentiate into Th1 cells that do not up-regulate IL-13Rα1 (9). This suggests that the susceptibility to up-regulation of IL-13Rα1 represents a developmental trait that subsides over time. To determine the point in time at which the naïve T cells are no longer susceptible to IL-13Rα1 up-regulation, newborn mice were adoptively transferred with naïve DO11.10 TCR transgenic T cells from 1 to 10d old mice, and IL-13Rα1 expression was analyzed after exposure to OVA peptide. Since IL-13Rα1, along with IL-4Rα, serves as a death heteroreceptor for Th1 cells to bias secondary neonatal responses towards Th2 cells (9, 10), we began by defining the time point at which exposure to Ag overcomes the Th2 bias and restores Th1 secondary immunity. The results show that hosts recipient of neonatal DO11.10 T cells from 1, 2, 4, or 6d old mice had minimal secondary IFNγ (Th1) responses in the SP and lymph node (LN) as measured by ELISA and ELISPOT (Fig. 1A). In contrast, those transferred with T cells from 8 or 10d old mice had increased IFNγ responses (Fig. 1A). Consistent with these results, the recall IFNγ responses were also significant when the transfer was made with T cells from 8d, but not 1d, old mice (Fig. 1B). Moreover, while all hosts had similar percentages of primary Th1 cells, IL-13Rα1 expression on these Th1 cells was significantly lower in mice recipient of naïve T cells from 8d (20.1%) or 60d (12.0%) old mice versus those transferred with naïve T cells from 1d old mice (53.5%) (Fig.1C). This indicates that naïve T cells from 8d old mice are less susceptible to IL-13Rα1 up-regulation than naïve T cells from 1d old mice. When the cultures (comprising APCs, Th1 and Th2 primary cells) were stimulated with OVA peptide and death of Th1 cells was measured, there was significant apoptosis (89%) of Th1 cells in the hosts recipient of naïve T cells from 1d old mice (Fig.1D), most likely due to IL-4 from Th2 cells triggering their death through the IL-4Rα/IL-13Rα1 heteroreceptor (9). However, apoptosis was lower in hosts recipient of naïve T cells from 8- or 60d old mice (Fig. 1D), likely due to the diminished IL-13Rα1 expression on primary Th1 cells (Fig.1C). These findings indicate that day 8 after birth represents a transitional time point in which naïve T cells are no longer susceptible to Ag induced IL-13Rα1 up-regulation and become able to develop Th1 recall and secondary responses.
Figure 1. At day 8 after birth naïve CD4 T cells lose susceptibility to IL-13Rα1 up-regulation.
Newborn (1 d old) Balb/c mice were given 1 × 105 naïve CD4 T cells from 1 to 10 day-old DO11.10 (A-B) or IL-13Rα1-GFP DO11.10 (C-D) mice and the hosts were injected with 100 μg Ig-OVA. (A) Hosts were challenged with OVAp in CFA at 60d of age and their SP and LN secondary IFNγ responses were analyzed by both ELISPOT and ELISA. (B-D) The SP cells were harvested on day 14 after exposure to Ig-OVA and in vitro recall IFNγ responses were analyzed by both ELISPOT and ELISA (B). Each bar represents the mean ± SD of triplicate wells. (C) The SP cells were stained for TCROVA (with KJ1-26 antibody), CD4, and intracellular IFNγ after a rapid stimulation with OVAp (ex vivo conditions). The KJ1-26+ CD4+ T cells were analyzed for IFNγ production (upper panel) and those cells positive for KJ1-26, CD4 and IFNγ (KJ1-26+CD4+ IFNγ+) were assessed for TCROVA (KJ1-26) and GFP (IL-13Rα1) expression (lower panel). (D) The SP cells were stimulated with OVAp for 24h (to allow for IL-4 secretion by Th2 cells) and stained for TCROVA, CD4, intracellular IFNγ, and Annexin V. The KJ1-26+CD4+ IFNγ+ cells were analyzed for Annexin V binding. Quadrants in dot and density plots are drawn relative to isotype controls. This experiment was repeated 3 times with 4 mice each.
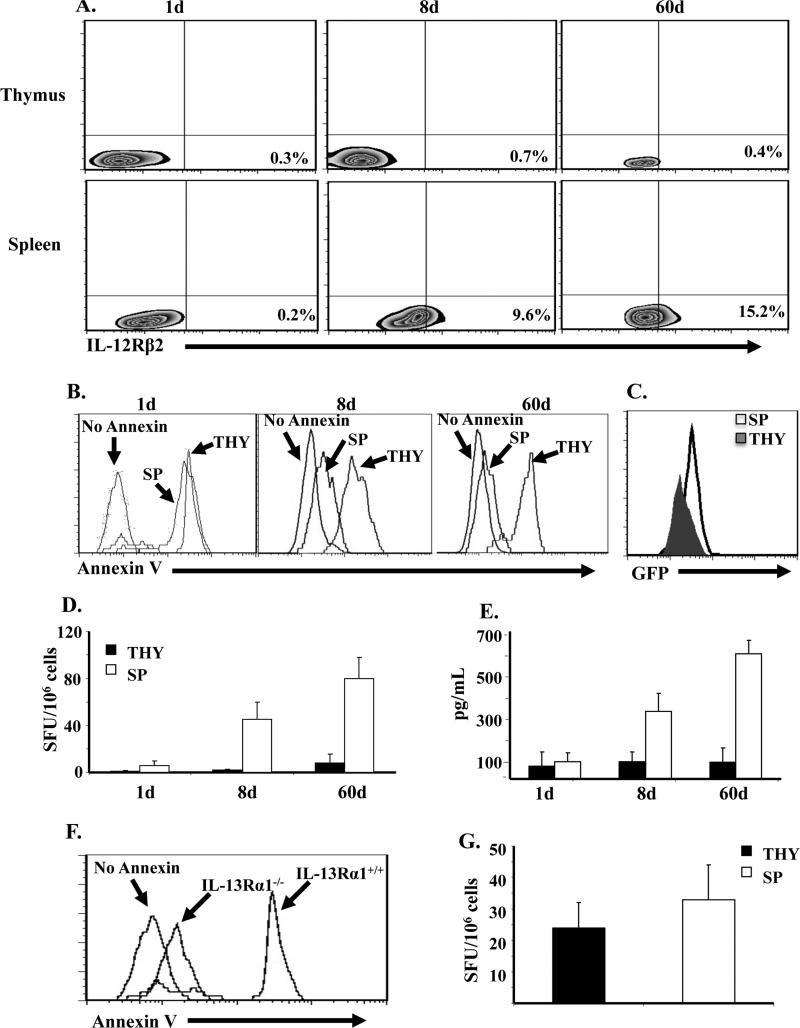
Spontaneous up-regulation of IL-12Rβ2 by naïve neonatal T cells reaches an optimal level by day 8 after birth
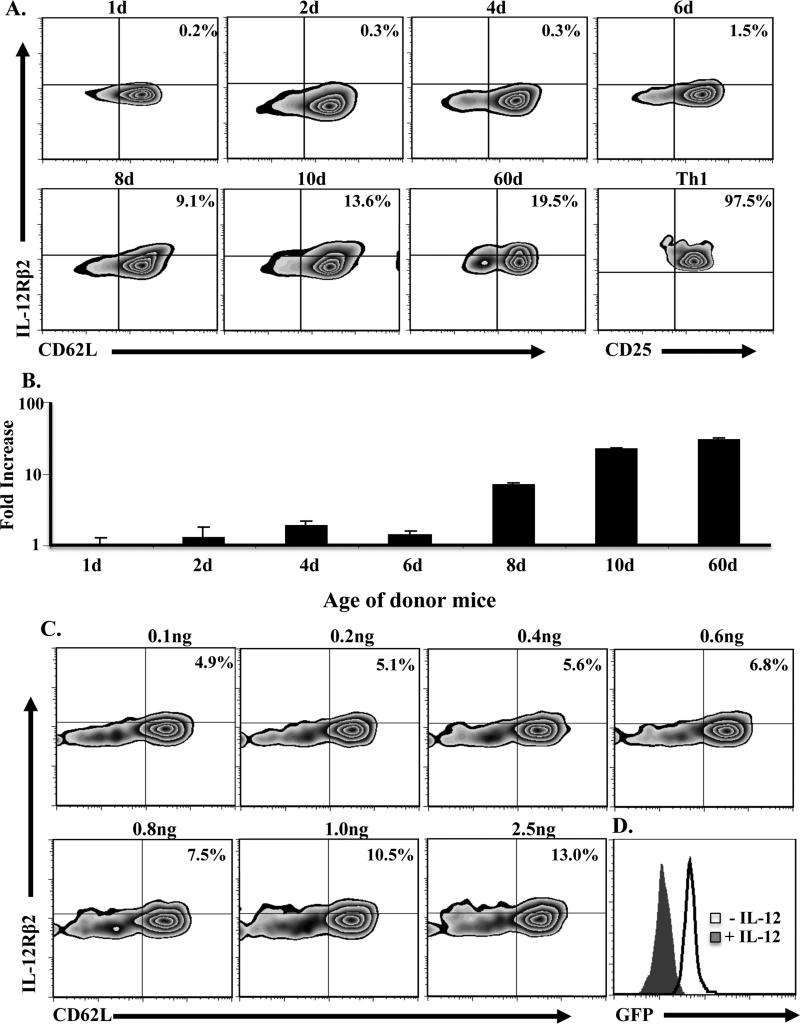
Spontaneous and gradual up-regulation of IL-12R on naïve T cells may serve as a compensatory mechanism to counter up-regulation of IL-13Rα1 expression under limited IL-12 conditions. To test this premise, naïve CD4 T cells were harvested from neonates at different time points after birth and analyzed for expression of the inducible IL-12Rβ2 chain (β1 is constitutively expressed ) (18). The results show that IL-12Rβ2 expression on CD62L+ (naïve) T cells reached a significant level between 8 and 10 days after birth relative to naïve T cells from adult mice (60d old) (Fig. 2A). Indeed, the level of IL-12Rβ2 expression on naïve T cells from 1 to 6d old mice represents only 0.5-5% relative to T cells from 60d old mice. In contrast, naïve T cells from 8d to 10 old mice had 48-64% IL-12Rβ2 expression relative to T cells from adult mice. These IL-12Rβ2 levels on 8d naïve T cells, although small relative to Ag stimulated Th1 cells (97.5% stained positive for IL-12Rβ2), they are significant because they represent half the levels observed with naïve T cells from 60d old mice which do not up-regulate IL-13Rα1 upon exposure to Ag even under limited IL-12 conditions. Similar results were observed at the mRNA level as naïve T cells from 8d old mice, like those from adult mice, had a significant mRNA increase relative to T cells from younger animals (Fig. 2B). These results indicate that spontaneous up-regulation of IL-12Rβ2 expression reaches an optimal level by day 8 of age. This likely compensates for limited IL-12 to counter IL-13Rα1-up-regulation, confers resistance to apoptosis, and sustains development of recall and secondary Th1 responses. To test this premise, we needed to first determine the amount of IL-12 that could up-regulate IL-12Rβ2 on 1d old naïve T cells to the level found on the 8d T cells. Accordingly, the cells were incubated with various concentrations of rIL-12 and IL-12Rβ2 expression was analyzed. As shown in Fig. 2C, 1ng of rIL-12 is needed to induce IL-12Rβ2 expression that is comparable to levels observed on naïve CD4 T cells from 8d old mice. Subsequently, when naïve CD4 T cells from 1d old mice were incubated with 1 ng rIL-12 and stimulated with anti-CD3 and anti-CD28, they had diminished IL-13Rα1 expression relative to those stimulated in the absence of rIL-12 (Fig. 2D). These findings indicate that by day 8 of age spontaneous up-regulation of IL-12Rβ2 expression reaches an optimal level able to counter up-regulation of IL-13Rα1 during Ag stimulation.
Figure 2. Increase in IL-12Rβ2 chain expression on naïve CD4 T cells occurs spontaneously and reaches a transitional level by day 8 after birth.
Naïve CD4 T cells were purified from DO11.10 mice at different time points after birth and assessed for IL-12Rβ2 expression at the protein (A) and mRNA (B) level by flow cytometry and real-time PCR, respectively. (A) The Zebra plots show IL-12Rβ2 protein and CD62L expression on KJ1-26+CD4+ T cells. For comparison purposes, IL-12Rβ2 expression was analyzed on CD4+CD11c- IFNγ+ differentiated neonatal Th1 cells. (B) The bars represent the mean ± SD fold mRNA increase from 3 experiments based on the comparative threshold cycle (CT). (C) Naïve CD4 T cells were isolated from 1d old DO11.10 mice and incubated with graded amounts of rIL-12 for 24h. The cells were then stained with KJ1-26, anti-CD4, anti-CD62L and anti-IL-12Rβ2 antibodies. The percentages of IL-12Rβ2+CD62L+ among total KJ1-26+-CD4+ T cells are indicated in the upper right corner of each quadrant. (D) Naïve CD4 T cells from 1d old IL-13Rα1-GFP reporter mice were stimulated with anti-CD3 (10 μg/mL), and anti-CD28 (1 μg/mL) for 5 days in the absence or presence of 1 ng/ml IL-12 and stained for CD4, CD11c, and intracellular IFNγ. The CD4+CD11c-IFNγ+ Th1 cells were analyzed for IL-13Rα1 expression by GFP.
Spontaneous increase in IL-12Rβ2 expression on naïve T cells is necessary for prevention of IL-13Rα1 up-regulation on differentiating Th1 cells
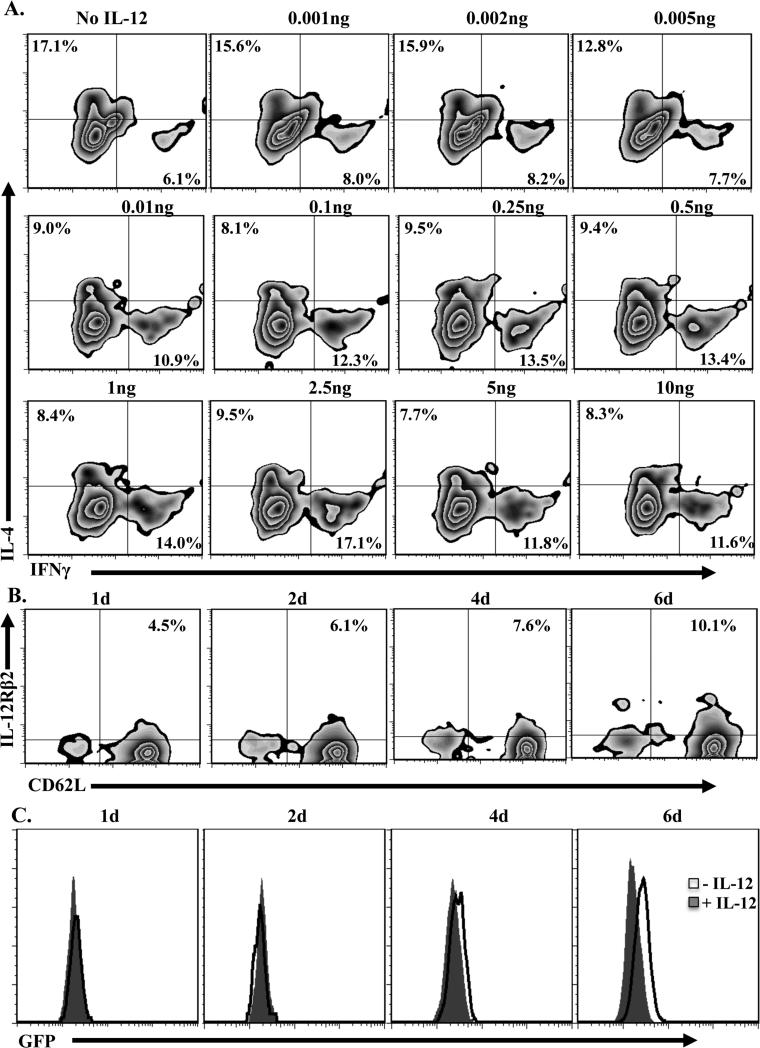
Because naïve T cells from 8d old mice transferred into 1d old hosts are able to develop into primary Th1 cells that resist apoptosis and develop secondary responses upon re-exposure to antigen, it is likely that IL-12Rβ2 countered the up-regulation of IL-13Rα1 and nullified the formation of the IL-4Rα/IL-13Rα1 death heteroreceptor. To further define the contribution of IL-12Rβ2 to the control of IL-13Rα1 expression, we first needed to determine the minimal amount of IL-12 that drives differentiation of Th1 cells from adult mice. Figure 3A shows that as little as 0.1 ng of IL-12 can drive optimal differentiation of naïve T cells into Th1 cells. We then used this concentration of IL-12, along with naïve neonatal T cells from 1 to 6 day old mice, to ascertain the contribution of IL-12Rβ2 to the control of IL-13Rα1 expression during differentiation from naïve to Th1 phenotype. The results show that with the minimum IL-12, IL-12Rβ2 up-regulation occurs at all time points (Fig. 3B), but only the T cells from 6 day old mice, which had 10.1% IL-12Rβ2, were able to counter up-regulation of IL-13Rα1 (Fig. 3C). Together these results indicate that the minimal IL-12 is able to drive differentiation, but optimal levels of IL-12Rβ2 expression are required to counter IL-13Rα1 up-regulation.
Figure 3. Developmental increase in IL-12Rβ2 expression on naïve T cells is required for counter regulation of IL-13Rα1 during differentiation into Th1 cells.
(A) Purified naïve CD4 T cells from adult IL-13Rα1+/+ mice were stimulated with anti-CD3 (10μg/mL), anti-CD28 (1μg/mL), and graded amounts of rIL-12 for five days. Cells were then tested for differentiation by measuring intracellular IFNγ or IL-4 control. Zebra plots show intracellular cytokine staining on CD4+CD11c- gated cells. Cells that were not given rIL-12 were included for control purposes. (B) Naïve CD4 T cells from 1, 2, 4, and 6d old IL-13Rα1+/+ mice were cultured with or without 0.1ng/ml rIL-12 for 24h and the naïve CD4+CD62L+CD11c- cells were analyzed for IL-12Rβ2 expression. (C) Naïve CD4 T cells from 1, 2, 4, and 6d old IL-13Rα1+/+-GFP mice were stimulated with anti-CD3 and anti-CD28 antibodies in the absence or presence 0.1ng/ml rIL-12 and the differentiated CD4+CD11c-IFNγ+ Th1 cells were analyzed for IL-13Rα1 expression by GFP. Cells that were not given rIL-12 were used for control purposes. This is representative of at least 5 experments
Spontaneous up-regulation of IL-12Rβ2 on naïve neonatal CD4 T cells is dependent on environmental IL-12
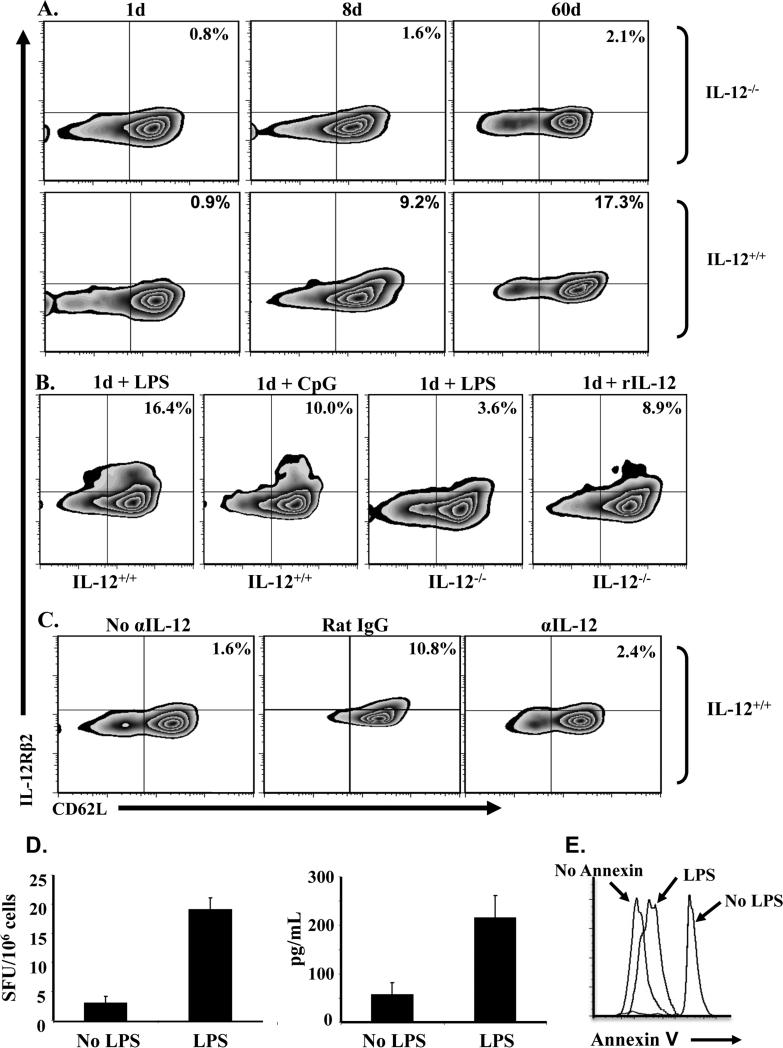
The fact that CD4 T cells gradually up-regulate IL-12Rβ2 expression over time in the absence of any immunization suggests an association with progressive neonatal colonization by commensals and symbiotic organisms. Since IL-12Rβ2 is inducible, rather than constitutively expressed on the cells, it is logical to envision an involvement of environmental IL-12 produced by innate immune cells as a consequence of interaction with commensals (19, 20). To test this premise, we began by analyzing the dependence of spontaneous IL-12Rβ2 expression on environmental IL-12. Accordingly, naïve T cells from IL-12-/- mice were harvested on day 1, 8 and 60 after birth and IL-12Rβ2 expression was compared with naïve T cells from IL-12+/+ mice. As indicated in Fig. 4A, naïve CD4 T cells from IL-12-/- mice of all ages had minimal IL-12Rβ2 expression while those from IL-12+/+ mice had a gradual increase in receptor expression that reached a maximum level by 60d of age. Given that commensals trigger the production of IL-12 by dendritic cells (19), it is possible that the spontaneous expression of IL-12Rβ2 over time is related to a progressive increase in environmental IL-12 due to colonization by commensals (21). To test this premise, we mimicked the effects of commensals (which are non-infectious) by injecting 1d old mice with the major bacterial compounds LPS and CpG and analyzed IL-12Rβ2 expression on CD62L+ naïve CD4 T cells. Figure 4B shows that IL-12+/+ mice recipient of LPS or CpG had an increased expression of IL-12Rβ2 on their naïve T cells while those from IL-12-/- mice remained at a basal level. The up-regulation of IL-12Rβ2 in the IL-12+/+ mice is related to environmental IL-12 because exogenous IL-12 given to the deficient mice triggers IL-12Rβ2 up-regulation on the naïve T cells. Furthermore, neutralization of environmental IL-12 by injection of anti-IL-12 antibody beginning at day 6 after birth suppresses the up-regulation of IL-12Rβ2, and the naïve T cells had minimal receptor expression on day 8 that was much lower than mice recipient of rat IgG isotype control (Fig. 4C). In fact, only 2.4% receptor expression was observed, which is comparable to the level usually seen on day 6 before injection of IL-12. The 1d old naïve T cells that were induced to up-regulate IL-12Rβ2 by LPS were able to overcome apoptosis and develop IFNγ recall responses like naïve T cells that up-regulated the receptor spontaneously over a period of 8 days (Fig. 4D and E). Indeed, when naive CD4 T cells isolated from 1d old LPS-treated mice were transferred to 1d old BALB/c mice, and the hosts were exposed to Ig-OVA, their SP T cells were able to develop IFNγ responses 2 weeks later upon recall with OVAp in vitro (Fig 4D) and did not undergo apoptosis (Fig 4E). Transfer of naïve CD4 T cells that were not exposed to LPS did not yield IFNγ responses.
Figure 4. Up-regulation of IL-12Rβ2 expression on naïve T cells is dependent upon the availability of environmental IL-12.
(A) Naïve CD4 T cells were isolated from both IL-12+/+ and IL-12-/- Balb/c mice on day 1 (1d), 8 (8d) or 60 (60d) after birth and analyzed for IL-12Rβ2 expression by flow cytometry. The plots show IL-12Rβ2 protein expression on CD3+CD4+CD62L+ naïve T cells. (B) IL-12+/+ and IL-12-/- Balb/c newborn mice ( 3 to 5 per group depending on the litter) were given on the day of birth 10 μg LPS, 20 μg CpG or 100 ng rIL-12. Twenty-four hours later the CD4 T cells were purified and analyzed for IL-12Rβ2 expression. The plots show IL-12Rβ2 protein expression on CD3+CD4+CD62L+ naïve T cells. (C) DO11.10 mice were given an injection of 50 μg anti-IL-12 antibody or control rat IgG on day 6, 7 and 8 after birth and the naïve CD4 T cells were purified and assessed for IL-12Rβ2 expression 24h after the last injection. A group of mice that was not given any injection (No αIL-12) was sacrificed on d6 and included for control purpose. The plots show IL-12Rβ2 protein expression on KJ1-26+CD4+CD62L+ naïve T cells. (D) Naïve CD4 T cells isolated from LPS-treated (LPS) or untreated (No LPS) DO11.10 newborns were transferred (105 cells/mouse) into 1-day-old Balb/c mice and the hosts were given 100 μg Ig-OVA. Two weeks later the SP cells (106 cells/well) were stimulated with 10 μM OVA peptide in vitro and IFNγ production was measured by ELISPOT (left panel) and ELISA (right panel). Each bar represents the mean ± SD of triplicate wells. (E) The SP cells were also analyzed for apoptosis by Annexin V binding upon stimulation with OVA peptide. The histograms show Annexin V binding to cells gated on CD4+KJ1-26+IFNγ+.
Furthermore, only LPS experienced T cells had reduced Annexin V binding upon exposure to Ig-OVA, indicating resistance to apoptosis (Fig. 4D, right panel). Overall, these results indicate that up-regulation of the IL-12Rβ2 chain on naïve CD4 T cells reaches optimal levels on day 8 after birth, most likely due to progressive colonization by commensals and the consequent accumulation of environmental IL-12.
Naïve CD4 T cells from a sterile thymic environment do not up-regulate IL-12Rβ2 and cannot sustain IFNγ recall responses
The lapse of time after birth required for accumulation of environmental IL-12 and up-regulation of IL-12Rβ2 expression on naïve CD4 T cells may be related to the gradual nature of colonization by commensals. If this indeed is the case, naïve T cells that originate from a sterile environment will not display significant IL-12Rβ2 expression even after an extended time lapse. To test this premise, we analyzed IL-12Rβ2 expression by mature naïve T cells from the thymus, a sterile environment whose blood-thymus barrier prevents accessibility to microbes (11), and compared the findings with T cells from the spleen, a non sterile environment that is accessible to microbes and their products. Indeed, naïve CD4 T cells purified from the thymus 8 days after birth did not up-regulate the IL-12Rβ2 chain as compared to CD4 T cells from the spleen of the same mice (Fig. 5A). Even when CD4 T cells were purified from the thymus of 60d old mice, IL-12Rβ2 expression remained insignificant like thymic and splenic CD4 T cells from 1d old mice. Interestingly, when the thymic CD4 T cells from 8d old mice were transferred into Balb/c mice and exposed to Ig-OVA, they gave rise to Th1 cells that displayed up-regulation of IL-13Rα1 expression while the splenic counterparts did not (Fig. 5C). Furthermore, these Th1 cells displayed increased apoptosis upon recall with OVA peptide while the splenic counterparts lost susceptibility to apoptosis (Fig. 5B). In fact, even thymic T cells from 60 day old mice had increased apoptosis like the control thymic and splenic cells from 1d old mice, indicating that the thymic environment remains sterile perhaps throughout the life span of the mouse. The up-regulation of IL-13Rα1 and the consequent apoptosis resulted in a lack of IFNγ recall responses when the naïve CD4 T cells originated from the thymus (Fig. 5D and E). However, when the donor CD4 T cells were from the spleen of 8 or 60d old mice, those IFNγ responses were restored. Annexin V binding by the Th1 cells emanating from the transfer of thymocytes and their inability to develop IFNγ response is due to IL-13Rα1 up-regulation rather than spontaneous apoptosis because transfer of naïve CD4 T cells from the thymus of 1d old IL-13Rα1-/- mice did not lead to elevated Annexin V (Fig. 5F) and the Th1 cells were able to develop recall IFNγ responses equivalent to Th1 cells emanating from the transfer of splenic naïve CD4 T cells from IL-13Rα1-/- mice (Fig. 5G). Together these results indicate that a sterile environment non-permissive to microbes and their products does not support IL-12Rβ2 up-regulation on naïve CD4 T cells leading to the inability of these cells to counter IL-13Rα1 up-regulation during differentiation into Th1 cells.
Figure 5. Splenic but not thymic naive CD4 T cells from 8d old mice up-regulate IL-12Rβ2 expression and develop recall Th1 responses upon re-exposure to Ag.
(A) Naïve CD4 T cells were purified from the spleen (SP) and thymus (THY) of DO11.10 mice on day 1 (1d), 8 (8d) and 60 (d60) after birth and assessed for IL-12Rβ2 expression by flow cytometry. The plots show IL-12Rβ2 expression on KJ1-26+CD4+CD62L+ cells. (B-D) Purified SP and THY CD4 T cells from 1, 8, and 60 day-old IL-13Rα1-GFP DO11.10 mice were transferred (105 cells per mouse) into 1d old Balb/c mice and the hosts were given 100 μg Ig-OVA. Two weeks later the SP cells (1× 106 cells/well) were stimulated with 10 μM OVA peptide in vitro and analyzed for Annexin V binding (B), IL-13Rα1 expression (C), and IFNγ production by both ELISPOT (D) and ELISA (E). The histograms show Annexin V binding (B) and GFP expression (C) by cells gated on CD4+KJ1-26+IFNγ+. (D and E) Bars represent the mean ± SD of triplicate wells. (F and G) Purified SP and THY CD4 T cells from 1d DO11.10 IL-13Rα1-/- or IL-13Rα1+/+ control mice were transferred (105 cells per mouse) into 1d old Balb/c newborns and the hosts were given 100 μg Ig-OVA. Annexin V binding (F) and recall IFNγ responses (G) were measured by ELISPOT as described in B and D.
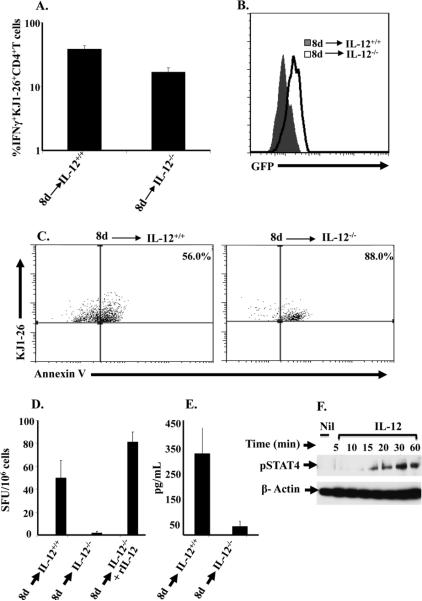
Naïve T cells expressing optimal IL-12Rβ2 differentiate into Th1 cells independent of IL-12 but require the cytokine to counteract IL-13Rα1 up-regulation
Thus far, we have found that neonatal T cells from 8d old mice express an optimal level of IL-12Rβ2 which is able to counter up-regulation of IL-13Rα1. The time lapse for IL-12Rβ2 expression is likely due to gradual colonization by commensals, which trigger IL-12 production by cells of the innate immune system, and progressive accumulation of the cytokine in the neonatal environment. We have previously shown that re-stimulation of primary neonatal Th1 cells with Ag leads to apoptosis of the cells, but addition of IL-12 to the culture nullifies cell death (9, 10). Since Th1 cells usually have abundant IL-12R (IL-12Rβ1/B2) (22), it is possible that the cytokine (IL-12) signals through the IL-12R to counteract the function of IL-13Rα1. In light of these observations, it is possible that upon optimal expression of IL-12Rβ2, naïve T cells are able to differentiate into Th1 cells but require IL-12 to signal through the IL-12R to counter up-regulation of IL-13Rα1. To test this premise, CD4 T cells were purified from 8d old DO11.10/Rag2-/- mice and transferred to a 1d old IL-12-/- Balb/c host and assessed for IFNγ production and IL-13Rα1 expression. Indeed, CD4 T cells transferred into IL-12-/- Balb/c hosts were able to differentiate into Th1 cells, though to a lesser extent than those transferred into IL-12+/+ Balb/c recipients (Fig. 6A). However, the Th1 cells were unable to counteract IL-13Rα1 up-regulation as compared to those transferred into IL-12+/+ hosts (Fig. 6B). Furthermore, these cells displayed increased Annexin V binding upon recall with OVA peptide (Fig. 6C). Consistent with these results, the recall IFNγ responses were decreased when the transfer was made with T cells from 8d old mice into IL-12-/- hosts as compared to their IL-12+/+ counterparts (Fig. 6D and E). IL-12Rβ2 on naïve T cells from 8d old mice is functional because it drives phosphorylation of STAT4 upon incubation with IL-12 cytokine without any antigen stimulation (Fig. 6F). Together these results indicate that naïve T cells expressing optimal IL-12Rβ2 are able to differentiate into Th1 cells in an IL-12-independent manner, but require the cytokine to counter up-regulation of IL-13Rα1 expression.
Figure 6. Naïve T cells expressing optimal IL-12Rβ2 differentiate into Th1 cells but require IL-12 cytokine to counteract IL-13Rα1 up-regulation.
(A) Newborn (1d old) IL-12-/- and IL-12+/+ control Balb/c mice were given 1 × 105 naïve CD4 T cells from 8d DO11.10 IL-13Rα1-GFP reporter mice and the hosts were injected with 100 μg Ig-OVA. SP cells were harvested 14 days after transfer, stained for surface TCROVA (with KJ1-26 antibody), CD4 and intracellular IFNγ after a rapid stimulation with OVAp (ex vivo conditions). The KJ1-26+CD4+ T cells were analyzed for intracellular IFNγ production. The bars represent the percent IFNγ+ among KJ1-26+CD4+ double positive cells. (B) Those cells positive for IFNγ (KJ1-26+CD4+ IFNγ+) were assessed for IL-13Rα1 expression by GFP. (C) The SP cells were stimulated with OVAp for 24h and stained for TCROVA, CD4, intracellular IFNγ, Annexin V and the KJ1-26+CD4+IFNγ+ cells were analyzed for Annexin V binding. (D-E) the SP cells stimulated with OVAp were analyzed for IFNγ production by both ELISPOT (D) and ELISA (E). Each bar represents the mean ± SD of triplicate wells. This experiment was repeated 3 times. (F) Naïve CD4 T cells (1 × 104 cells/well) were purified from 8d old DO11.10 mice and incubated with 2.5 ng rIL-12 for the indicated time points. Cells were then lysed and pSTAT4 levels were analyzed by Western blot. β actin levels were also analyzed for control purposes.
Discussion
In recent years it has become clear that cells of the innate immune system, particularly dendritic cells, play a major role in the outcome of T cell immunity in the newborn (10, 23, 24). Even maturation of DCs dictates the time during which Ag exposure leads to balanced immunity in neonates (10, 25). On the other hand, if naïve T cells from adult mice are placed in the neonatal environment, the innate cells no longer restrict immunity (9), suggesting that factors intrinsic to the T cell contribute to the balance of immunity in the newborn. This study examined this issue and shows that by day 8 after birth, naïve T cells are no longer susceptible to up-regulation of IL-13Rα1 mRNA or protein. Indeed, naïve T cells taken from neonates of different ages and primed with Ag in 1d old newborns develop functional Th1 cells alongside Th2 cells and are devoid of IL-13Rα1 expression only when the donor is 8 days of age or older. These Th1 cells were in fact able to resist IL-4-mediated apoptosis and develop secondary responses that were otherwise absent in animals recipients of naïve T cells from donors younger than 8 days of age. The question that begs for an answer then is what happens during the first 8 days of life for naïve T cells to acquire resistance to up-regulation of IL-13Rα1 during antigen stimulation and differentiation to Th1 cells? It is well known that up-regulation of IL-12Rβ2 during Ag stimulation programs naïve T cells to differentiate into Th1 cells (22). This is likely mediated by IL-12 produced by the cells presenting Ag to the T cells. Given that naïve T cells from 8d old mice are able to differentiate within the neonatal environment, which contains very little IL-12, and develop secondary responses without undergoing apoptosis, we suspected that IL-12Rβ2 provides a compensatory mechanism to counter up-regulation of IL-13Rα1 expression. This was indeed the case as IL-12Rβ2 expression on naïve T cells underwent progressive up-regulation over time and by day 8 reached an optimal level that was able to counter IL-13Rα1 up-regulation under low neonatal levels of IL-12. In fact, T cells from mice younger than 8 days had lower levels of IL-12Rβ2 and even at day 6 exogenous IL-12 was required to counter IL-13Rα1 up-regulation (Figure 3). Also, the up-regulation of IL-12Rβ2 on naïve T cells seems to be dependent on environmental IL-12 because 8 day old T cells from IL-12-deficient mice had lower IL-12Rβ2 expression and were unable to resist apoptosis or develop recall responses. The finding that Th1 cells from neonatal mice express high levels of IL-12Rβ2 like those from adult mice suggest that, the reduced IL-12 in the neonatal environment is able to stimulate up-regulation of IL-12Rβ2 but is not sufficient to counter IL-13Rα1 up-regulation during differentiation. IL-12Rβ2 up-regulation on naïve T cells serves as a compensatory mechanism to overcome IL-13Rα1 up-regulation. Moreover, the progressive up-regulation of IL-12Rβ2 on naïve T cells can be explained by the lapse of time required for optimal accumulation of IL-12 in the environment. IL-12 is produced by cells of the innate immune system upon stimulation with TLR ligands (12, 13). However, the 8 d old mice from which the T cells were harvested were naïve animals maintained under specific pathogen free barrier conditions and the accumulation of IL-12 needed for IL-12Rβ2 expression may be related to progressive colonization by commensals (19, 20). This may provide TLR ligation leading to IL-12 production (21). The experiments with thymic CD4 T cells provide support to this postulate as the thymus is a privileged site that is inaccessible to microbes. In fact, naïve T cells from the thymus of 8 or even 60 d old mice had low levels of IL-12Rβ2 despite optimal up-regulation of the chain by their splenic counterparts. The cells were not able to counter up-regulation of IL-13Rα1, prevent apoptosis, or support recall of primary Th1 responses. The observation that thymocytes from IL-13Rα1-deficient mice resist apoptosis and produce IFNγ upon stimulation with Ag under limited IL-12 emphasizes the compensatory mechanism of IL-12Rβ2 in the counter regulation of IL-13Rα1. The findings also indicate that IL-12 needs to signal through the up-regulated and functional IL-12Rβ2 in order to counter IL-13Rα1 up-regulation. This conclusion is drawn from the observation that the naïve T cells expressing optimal IL-12Rβ2 were able to differentiate into Th1 cells upon transfer into IL-12-/- hosts but could not counter IL-13Rα1 expression. The differentiation into Th1 cells in the absence of IL-12 may be related to an IL-12-independent mechanism as was observed previously (26-28). On the other hand, the IL-12 signaling through IL-12Rβ2 to counter IL-13Rα1 expression and nullify apoptosis may provide an explanation as to the rescue of neonatal secondary Th1 responses by IL-12 (5). Overall, the findings provide insights as to how IL-12 and its receptor IL-12Rβ2 function to connect cells of the innate and adaptive immune system and shape neonatal immunity. In this regard, neonatal dendritic cells produce little IL-12 which is sufficient to drive differentiation of Th1 cells but not enough to counter up-regulation of IL-13Rα1 on these lymphocytes. However, overtime the DCs are exposed to environmental stimuli including commensals and produce more IL-12 cytokine leading to up-regulation of IL-12Rβ2 on naïve neonatal T cells. This serves to provide a compensatory mechanism by which the naïve T cells refrain from up-regulation of IL-13Rα1during priming with antigen. Ultimately, IL-4Rα/IL-13Rα1 formation is limited and the Th1 cells will not undergo apoptosis upon re-challenge with Ag even when Th2 cells are producing excessive IL-4 cytokine.
Acknowledgments
Funding. This work was supported by grants RO1AI048541, and R21HD060089 (to H.Z.) from the National Institutes of Health. J.A.C. was supported by Life Sciences Fellowship from the University of Missouri, Columbia. C. M. H. was supported by a training grant GM008396 from NIGMS.
Footnotes
Abbreviations: DC, dendritic cell; IL-13Rα1, IL-13 receptor alpha 1; SFU, spot forming unit; SP, spleen; THY, thymus
References
- 1.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 2.Gammon G, Dunn K, Shastri N, Oki A, Wilbur S, Sercarz EE. Neonatal T-cell tolerance to minimal immunogenic peptides is caused by clonal inactivation. Nature. 1986;319:413–415. doi: 10.1038/319413a0. [DOI] [PubMed] [Google Scholar]
- 3.Powell TJ, Jr., Streilein JW. Neonatal tolerance induction by class II alloantigens activates IL-4-secreting, tolerogen-responsive T cells. J Immunol. 1990;144:854–859. [PubMed] [Google Scholar]
- 4.Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 5.Min B, Legge KL, Pack C, Zaghouani H. Neonatal exposure to a self-peptide-immunoglobulin chimera circumvents the use of adjuvant and confers resistance to autoimmune disease by a novel mechanism involving interleukin 4 lymph node deviation and interferon gamma-mediated splenic anergy. J Exp Med. 1998;188:2007–2017. doi: 10.1084/jem.188.11.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 7.Adkins B, Du RQ. Newborn mice develop balanced Th1/Th2 primary effector responses in vivo but are biased to Th2 secondary responses. J Immunol. 1998;160:4217–4224. [PubMed] [Google Scholar]
- 8.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, Zaghouani H. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004;20:429–440. doi: 10.1016/s1074-7613(04)00072-x. [DOI] [PubMed] [Google Scholar]
- 10.Lee HH, Hoeman CM, Hardaway JC, Guloglu FB, Ellis JS, Jain R, Divekar R, Tartar DM, Haymaker CL, Zaghouani H. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J Exp Med. 2008;205:2269–2280. doi: 10.1084/jem.20071371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerling IC, Serreze DV, Christianson SW, Leiter EH. Intrathymic islet cell transplantation reduces beta-cell autoimmunity and prevents diabetes in NOD/Lt mice. Diabetes. 1992;41:1672–1676. doi: 10.2337/diab.41.12.1672. [DOI] [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 13.Krummen M, Balkow S, Shen L, Heinz S, Loquai C, Probst HC, Grabbe S. Release of IL-12 by dendritic cells activated by TLR ligation is dependent on MyD88 signaling, whereas TRIF signaling is indispensable for TLR synergy. J Leukoc Biol. 88:189–199. doi: 10.1189/jlb.0408228. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen M, Leuridan E, Zhang T, De Wit D, Willems F, Van Damme P, Goldman M, Goriely S. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS One. 5:e10407. doi: 10.1371/journal.pone.0010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haymaker CL, Guloglu FB, Cascio JA, Hardaway JC, Dhakal M, Wan X, Hoeman CM, Zaghouani S, Rowland LM, Tartar DM, et al. Bone marrow-derived IL-13Ralpha1-positive thymic progenitors are restricted to the myeloid lineage. J Immunol. 188:3208–3216. doi: 10.4049/jimmunol.1103316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattner F, Magram J, Ferrante J, Launois P, Di Padova K, Behin R, Gately MK, Louis JA, Alber G. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur J Immunol. 1996;26:1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Legge KL, Min B, Bell JJ, Gregg R, Caprio J, Zaghouani H. Neonatal immunity develops in a transgenic TCR transfer model and reveals a requirement for elevated cell input to achieve organ-specific responses. J Immunol. 2001;167:2585–2594. doi: 10.4049/jimmunol.167.5.2585. [DOI] [PubMed] [Google Scholar]
- 18.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 19.Becker C, Wirtz S, Blessing M, Pirhonen J, Strand D, Bechthold O, Frick J, Galle PR, Autenrieth I, Neurath MF. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J Clin Invest. 2003;112:693–706. doi: 10.1172/JCI17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel RM, Lin PW. Developmental biology of gut-probiotic interaction. Gut Microbes. 1:186–195. doi: 10.4161/gmic.1.3.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168:171–178. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 22.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 24.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 25.Hoeman C, Dhakal M, Zaghouani H. Overcoming dendritic cell tardiness to triumph over IL-13 receptor: a strategy for the development of effective pediatric vaccines. Discov Med. 2010;9:554–559. [PubMed] [Google Scholar]
- 26.Zhou W, Zhang F, Aune TM. Either IL-2 or IL-12 is sufficient to direct Th1 differentiation by nonobese diabetic T cells. J Immunol. 2003;170:735–740. doi: 10.4049/jimmunol.170.2.735. [DOI] [PubMed] [Google Scholar]
- 27.Owaki T, Asakawa M, Morishima N, Hata K, Fukai F, Matsui M, Mizuguchi J, Yoshimoto T. A role for IL-27 in early regulation of Th1 differentiation. J Immunol. 2005;175:2191–2200. doi: 10.4049/jimmunol.175.4.2191. [DOI] [PubMed] [Google Scholar]
- 28.Skokos D, Nussenzweig MC. CD8- DCs induce IL-12-independent Th1 differentiation through Delta 4 Notch-like ligand in response to bacterial LPS. J Exp Med. 2007;204:1525–1531. doi: 10.1084/jem.20062305. [DOI] [PMC free article] [PubMed] [Google Scholar]