Abstract
We compared acceptability, adherence and efficacy of trans-dermal nicotine patches and cognitive behavioral therapy (Group 1) to cognitive behavioral therapy alone (Group 2) in minority pregnant smokers. This is a randomized controlled trial. 52 women were recruited during pregnancy with a mean gestational age 18.5 ± 5.0 weeks and followed through delivery. Randomization was by site and initial cotinine levels. Interventionists and interviewers were blinded to group assignment. Two different nicotine replacement therapy dosing regiments were administered according to the baseline salivary cotinine level. A process evaluation model summarized patient adherence. The main outcome measure was self-report of cessation since last visit, confirmed by exhaled carbon monoxide. Analyses of categorical and continuous measures were conducted as well as linear trend tests of salivary cotinine levels. Women lost to follow-up were considered treatment failures. Participants were on average 27.5 ± 5.4 years old, 81 % were single, 69 % unemployed and 96 % were Medicaid eligible. A process evaluation indicated patients in both groups were adherent to scheduled program procedures through Visit 4, but not for Visits 5 and 6. Confirmed quit rates were: at visit 3, 23 (Group 1) and 0 % (Group 2) (p = 0.02); at visits 4 and 5, no difference; at visit 6, 19 (Group 1) and 0 % (Group 2) (p = 0.05). Group 1 delivered infants with a mean gestational age of 39.4 weeks versus 38.4 weeks in Group 2 (p = 0.02). 73 % (52/71) of the eligible smokers agreed to participate and 65 % (17/26) of Group 1 completed the protocol (i.e. attended 6 visits). A comparison of Group 1 and 2 quit rates confirmed a non-significant difference.
Keywords: Pregnancy, African-Americans, Smoking, Nicotine replacement therapy
Introduction
Despite the large body of evidence about the adverse impact of smoking on maternal and infant health and available effective treatments [1-3], between 9 and 13 % of women in the United States smoked during pregnancy [4]. Behavioral interventions currently offered during prenatal care have only modest success. Evidenced-based counseling typically results in an additional 6–8 % of women quitting over spontaneous cessation [5-8].
Nicotine replacement therapy (NRT) is safe and effective for non-pregnant smokers. When combined with counseling, it typically doubles cessation rates [5]. Windsor et al. [9] reviewed the risks and benefits of behavioral and pharmacological treatment for pregnant women. They reported that when used appropriately (patient does not smoke), NRT has the potential to reduce maternal and fetal cotinine levels and eliminate fetal exposure to carbon monoxide (CO) and hundreds of other chemicals and carcinogens. Other reports have indicated that when behavioral methods have failed for pregnant women smoking ≥10 cigarettes per day (CPD), the benefits of NRT may outweigh the risks [10, 11]. The current ACOG recommendations continue to emphasize that pregnant patients should try to quit smoking without pharmacological agents and that evidence of efficacy for smoking cessation medications is inconclusive [12].
Few studies have evaluated the safety and efficacy of NRT during pregnancy [7, 9-11, 13-16]. Wisborg et al. [16] conducted a study of women who smoked ≥10 CPD after their 1st trimester. The authors compared a behavioral program to a behavioral program plus nicotine patches, with CO-confirmed cessation rates of 19 versus 21 %, respectively. This difference was not statistically significant. The study did report a significant increase in mean infant birthweight (186 g) of women on NRT.
Two randomized controlled trials from the U.S. tested the efficacy of NRT during pregnancy. Oncken et al. conducted a double-blind, placebo-controlled trial to evaluate the efficacy of nicotine gum [14]. All patients received counseling after being randomly assigned to a 2 mg nicotine gum or a placebo gum group. No significant difference in biochemically-validated cessation rates was observed between groups. Pollak et al. [15] conducted an evaluation of patients between 13 and 25 weeks gestation who smoked ≥5 CPD. There were two groups: a cognitive behavioral therapy (CBT) control group and a CBT plus NRT (choice: patch, gum or lozenges) group. All patients received counseling. Cotinine-validated cessation rates were significantly higher for the experimental compared to the control group at 38 weeks (18 vs. 7 %, p = 0.03), but not at 3 months postpartum (20 vs. 14 %, p = 0.55). Recruitment was suspended by an independent data and safety monitoring board after determining a higher rate of poor birth outcomes in the NRT group. Later analyses did not corroborate the association [17].
Our paper reports the results of a randomized controlled trial (RCT) evaluating the acceptability, use and efficacy of trans-dermal patch combined with CBT compared to CBT alone among minority women in Washington, D.C. The behavioral outcome was a biologically-confirmed self-report of cessation. No previous study tested the efficacy of NRT in a minority population. We selected the patch because it was the only NRT with a controlled predictable rate of administration approved by the FDA for use during pregnancy [18].
Materials and Methods
Study Population
Eligible mothers were: English speaking, D.C. metropolitan area residents, self-identified as an ethnic minority, ≥18 years, <30 weeks pregnant and a smoker with a desire to quit. Women were eligible if their CO levels were ≥8 ppm, salivary cotinine levels were ≥20 ng/ml, or urinary cotinine levels were ≥100 ng/ml. Women under treatment for psychiatric illness, alcoholism or drug addiction were excluded.
Following IRB approval, women were recruited from three prenatal care sites from July 2006 to December 2009, and followed until May 2010. After consent women were screened using an audio-computer assisted self interview [19]. Initial consent was for eligibility screening, for participation in the initial visit activities and baseline telephone interview. There was a maximum of six clinical visits. Distribution of NRT patches occurred at visits 2–5.
The first visit included CO testing, saliva and urine collection for cotinine measurement, and delivery of an evidenced-based intervention: the Smoking Cessation or Reduction in Pregnancy Treatment (SCRIPT) Program [5, 7, 8, 20]. At visit 2, women were considered to have quit if their self-report were confirmed by CO ≤ 8 ppm. Those who continued to smoke were invited to participate in the RCT after signing a second consent.
Using a 1:1 ratio, women were randomized to either the NRT patch and continued CBT (Group 1) or CBT only (Group 2). Randomization was stratified by site and initial salivary cotinine levels: 30–99 ng/ml and ≥100 ng/ml. The web-based database management system was programmed to randomize after entering the necessary data to verify eligibility and administration of the baseline survey. Telephone interviewers were blinded to group assignment.
Data and Biological Sample Collection
Socio-demographic and behavioral risk data were collected on all visit 1 participants during a telephone interview within 10 days after visit 1. Depressive symptoms were assessed using the Beck Depression Inventory Fast Screen [21]. Smoking behavior (number of cigarettes smoked in the last day, in the last 2 weeks, cigarettes smoked within 30 min of waking, amount of cigarette usually smoked, and how deeply they inhaled) was collected through a self-administered form, completed and sealed by the participant at the end of visits 2–6 and only available to researchers at the end of the study.
Data on pregnancy outcomes were abstracted from medical records at the delivery hospital by master’s level trained abstractors using standardized forms. Mothers signed a separate consent for release of medical records.
Saliva was collected at visits 1–6 using cotton salivettes (Sarstedt, Inc., Newton, NC), placed in the mouth for 3–5 min and then placed in capped vials. Urine aliquots were collected during visit 1, using standard methods. Urine and saliva samples were refrigerated for analysis within 5 days. Prior to analysis, samples were centrifuged for 5 min at 1,000×g to remove particulates. Cotinine analysis was performed with an Immulite 1000 system (Siemens Medical Solutions Diagnostics) using the Nicotine Metabolite Assay kit. This assay has a calibration range of 10–500 ng/ml: absolute sensitivity = 2 ng/ml. Each sample was tested in triplicate, using 20–100 μl of sample per assay. Samples with values >500 ng/ml were diluted and re-tested.
Interventions
Cognitive Behavioral Intervention
The SCRIPT program, an evidence-based health education intervention, applies the 5 A’s Model: Ask, Advise, Assess, Assist, and Arrange. It was derived from eight systematic evaluation studies that documented its effectiveness [5, 7, 8, 10]. In addition to counseling, women were given A Pregnant Woman’s Guide to Quit Smoking, a manual to assist patients with problem-solving and coping skills, written at a 6th grade reading level [20, 22]. Women who continued after visit 1 received reinforcement and behavioral methods at Visits 2–5.
Nicotine Replacement Therapy
We chose the trans-dermal nicotine patch, because it is FDA approved for use during pregnancy (after failing CBT), and its ease of application and controlled and steady dosing. None of the other forms of nicotine replacement (gum, inhaler, lozenge) have a controlled predictable rate of administration and are FDA approved during pregnancy [18]. We chose the standard 10-week NRT regimen, spanning Visits 2–5.
In order to minimize the chance of higher cotinine levels on NRT than prior to treatment, women randomized to the patch plus CBT were classified into two dosing groups based on baseline salivary cotinine levels (SCL). Women with a SCL ≥ 100 ng/ml received 21 mg patches for 2 weeks, 14 mg patches for 4 weeks, and 7 mg patches for 4 weeks. Women with a baseline SCL ≥ 20 and ≤100 ng/ml received 14 mg patches for 6 weeks and 7 mg patches for 4 weeks. Women received the first batch of patches at visit 2. Participants were given clear verbal and written instructions on patch use. They were advised never to smoke while using the patch, to remove the patch before going to sleep and never to use other NRT method(s). Actual use of the NRT patch was reported by the participant at the time of the next visit.
The intervention specialists were blinded to group assignment. Frequency of contact and total time with each patient was standardized to control for differential treatment exposure. The intervention was delivered by the same provider during a normal care visit in a private room by master’s trained intervention specialists who had received a multi-component training course from a doctoral level health behavior specialist.
Women were tested for CO at each visit before receiving the next batch. If CO values were ≥8 ppm, they were given 1 week’s supply of NRT and asked to return within 1 week for CO retesting (probationary period). If their CO values were still high, NRT treatment was discontinued and CBT continued. A process evaluation documenting patient adherence to the protocol was performed for both groups [22].
Sample Size Estimation
Initial sample size estimates were based on an expected quit rate of 5 % for the CBT Group and a quit rate of 20 % for the CBT plus NRT Group. The 5 % quit rate for the CBT Group was based on results for heavy smokers [20]. Given these rates, an alpha of 0.05 and 80 % power, ≥88 patients would need to be randomized to each treatment group to detect a significant difference in quit rates. With an anticipated 20 % attrition rate, the original goal was to enroll >100 women/group.
From the outset, it became clear enrollment would be difficult. The primary obstacles were low rates of demographically eligible women who admitted to smoking during pregnancy, restrictive gestational age criteria, and the multi-step enrollment process. Attempts were made to increase enrollment by relaxing the gestational age criteria, from ≤24 weeks to ≤30 weeks in February 2009, and by re-contacting women who said they had quit smoking after the CBT to see if they had relapsed and were eligible for the RCT. In March 2009, after 40 participants had enrolled, an additional sample size calculation was made. This calculation, based on a pilot study, assumed a 5 % quit rate in the CBT Group, a 25 % quit rate in the CBT plus NRT Group, and an alpha of 0.05 and 80 % power. The intention was to add a new recruitment site and extend recruitment 12 months to recruit 50 women/group. Women were followed until May 2010, but we were unable to add a new site.
Data Management
Screening, process indicator and biomarker data were entered into a database management system with enforced data quality-control checks. Data from questionnaires and medical records were keyed separately, examined for quality, and merged with the management system data.
Statistical Analysis
Baseline measures of demographics, pregnancy characteristics, biomarkers and smoking behaviors were compared for: (1) patients who quit after the CBT, versus eligible smokers; (2) patients randomized versus women eligible but refusing randomization; (3) participants randomized to NRT and CBT versus those randomized to CBT; (4) randomized participants who completed the trial versus discontinued patients; and (5) biologically confirmed quitters versus smokers.
The primary outcome was self-reports of cessation since the last visit via private questionnaire, confirmed by CO < 8 ppm. Confirmed cessation was calculated at Visits 3–6 using the “Intent-to-Treat” (ITT) principle, which assesses the effect of therapy comparable to a clinical practice. In the ITT analysis, women who did not attend a visit for any reason were treatment failures. Women delivering prior to an expected visit were removed from the denominator for those visit(s).
Analyses of categorical measures were performed using Fisher exact tests; continuous measures were analyzed using t tests. Continuous measures that were highly skewed (e.g., salivary cotinine) were transformed to approximate normality prior to analysis. Linear trend tests of SCL across the study period were conducted using Generalized Estimating Equation modeling. SAS v.9.1 was used for all analyses (SAS Institute Inc., Cary, NC).
Results
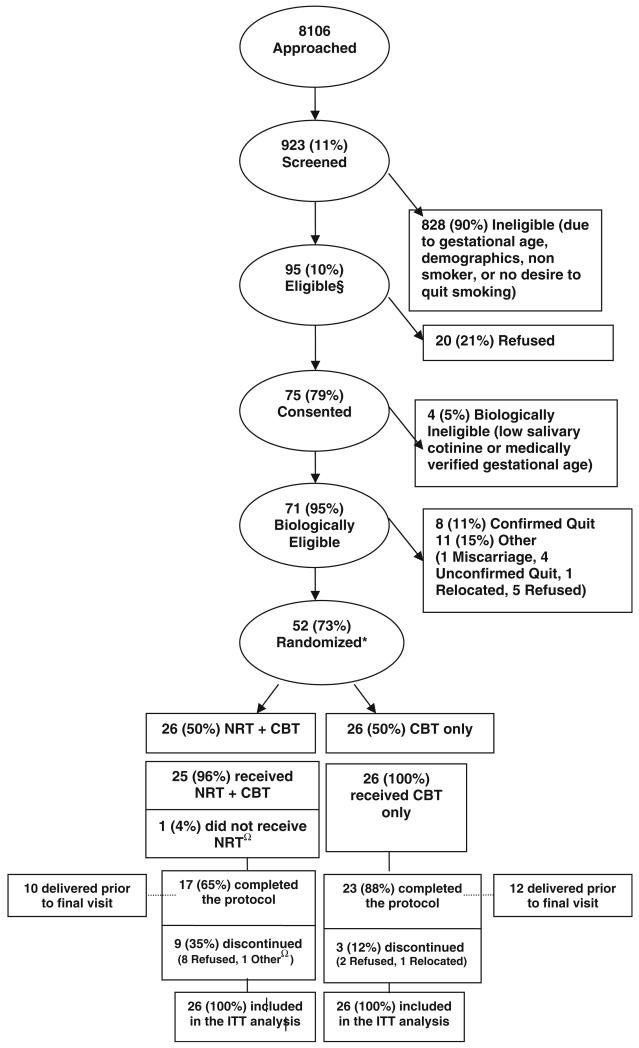
As presented in Fig. 1, a total of 8,106 women were approached: 923 consented and were screened. Of these, 95 were eligible: 75 consented in Phase 1. This included biological screening and receiving the first CBT session [20]. Of the 75, 71 were eligible and completed the baseline interview. At visit 2, 8/71 participants (11.3 %) were cotinine confirmed quitters. The significant differences between women who quit after visit 1 and smokers were: average CPD’s in the last week, (2.1 vs. 5.9, p = 0.04); number of cigarettes smoked ≤24 h (1.6 vs. 4.3, p = 0.01); and always smoking the first cigarette ≤30 min of rising: (12.5 vs. 57.4 % p = 0.02). Differences in baseline SCL approached statistical significance (64.4 vs. 157.6 ng, p = 0.06).
Fig. 1.
NRT study flowchart. §Met sociodemographic, pregnancy, and gestational age criteria, reported smoking during pregnancy, and wanted to quit. *Two women were each screened and randomized into the study twice. Each pregnancy was counted as a separate study participation. ΩOne participant was randomized to NRT, but was subsequently admitted into a rehabilitation facility and was discontinued. She was never administered NRT. Neither woman quit smoking at any time during the pregnancy
Eleven of the 63 biologically eligible women (17.4 %) did not give consent to participate in the RCT. The difference between those randomized (n = 52) and refusals (n = 11) was women consenting to randomization reported higher rates of depressive symptoms (60 vs. 18 %, p = 0.02). Baseline characteristics presented in Table 1, confirmed no significant differences between groups. The mean interval between visit (V) 1 and V2 was 4.2 weeks, for V2 and V3 was 3.7 weeks, for V3 and V4 was 4.1 weeks, for V4 and V5 was 4.7 weeks, and V5 and V6 was 3.0 weeks. The mean estimated gestational age (EGA) of mothers participating in V1 was 18.5 weeks, in V2 was 22.7 weeks, in V3 was 25.9 weeks, in V4 was 30.0 weeks, in V5 was 32.9 weeks, and in V6 was 33.8 weeks. The median EGA of mothers participating in V1 was 18.0 weeks, in V2 was 23.4 weeks, in V3 was 26.9 weeks, in V4 was 30.6 weeks, in V5 was 33.9 weeks, and in V6 was 34.3 weeks.
Table 1.
Baseline characteristics of participants by group
| Characteristics | Group 1: NRT + SCRIPT (n = 26) | Group 2: SCRIPT (n = 26) | p valuea | Total (n = 52) |
|---|---|---|---|---|
| Maternal age: mean ± SD, years | 27.5 ± 5.0 | 27.6 ± 5.9 | 0.94 | 27.5 ± 5.4 |
| Gestational age: mean ± SD week | 19.6 ± 5.1 | 17.5 ± 4.7 | 0.13 | 18.5 ± 5.0 |
| Pregnancies (+current), mean ± SD | 5.5 ± 3.7 | 5.7 ± 3.6 | 0.85 | 5.6 ± 3.6 |
| Number of live births, mean ± SD | 2.4 ± 1.6 | 2.5 ± 2.3 | 0.89 | 2.4 ± 2.0 |
| Gestational age: mean SD week | 8.9 ± 4.0 | 9.5 ± 5.8 | 0.67 | 9.2 ± 4.9 |
| Relationship status: baseline interview | ||||
| Married or living with partner, n (%) | 5 (19 %) | 1 (4 %) | 0.28 | 6 (12 %) |
| Single/never married, n (%) | 19 (73 %) | 23 (88 %) | 42 (81 %) | |
| Education level | ||||
| <High school, n (%) | 7 (27 %) | 10 (38 %) | 0.58 | 17 (33 %) |
| HS graduate/GED, n (%) | 15 (58 %) | 11 (42 %) | 26 (50 %) | |
| At least some college, n (%) | 4 (15 %) | 5 (19 %) | 9 (17 %) | |
| Employment status | ||||
| Worked part-time, n (%) | 5 (19 %) | 2 (8 %) | 0.41 | 7 (13 %) |
| Worked full-time, n (%) | 5 (19 %) | 4 (15 %) | 9 (17 %) | |
| Not employed, n (%) | 16 (62 %) | 20 (77 %) | 36 (69 %) | |
| Medicaid, n (%) | 25 (96 %) | 23 (96 %) | 1 | 48 (96 %) |
| Alcohol use during pregnancy | 8 (31 %) | 8 (31 %) | 1 | 16 (31 %) |
| Any depressive symptoms | 14 (54 %) | 17 (65 %) | 0.58 | 31 (60 %) |
| Marijuana during pregnancy | 4 (15 %) | 8 (31 %) | 0.32 | 12 (23 %) |
| # CPD < 7 days, mean ± SD | 7.0 ± 7.4 | 5.1 ± 3.3 | 0.63 | 6.0 ± 5.7 |
| Amount of cigarette usually smoked | ||||
| All, n (%) | 7 (27 %) | 12 (46 %) | 0.33 | 19 (37 %) |
| Most, n (%) | 3 (12 %) | 3 (12 %) | 6 (12 %) | |
| Half, n (%) | 15 (58 %) | 10 (38 %) | 25 (48 %) | |
| How deeply inhale smoke | ||||
| Slightly | 8 (31 %) | 9 (35 %) | 0.49 | 17 (33 %) |
| Moderately | 10 (38 %) | 13 (50 %) | 23 (44 %) | |
| Deeply or very deeply | 8 (31 %) | 4 (15 %) | 12 (23 %) | |
| 1st cigarette <30 min of rising | 17 (65 %) | 15 (58 %) | 0.84 | 32 (62 %) |
| Exhaled CO, visit 1, mean ± SDb | 8.8 ± 6.1 | 9.0 ± 6.9 | 0.89 | 8.9 ± 6.5 |
| Salivary cotinine, visit 1, mean ± SDb | 171 ± 143 | 158 ± 109 | 0.85 | 165 ± 126 |
CO and Cotinine were square-root transformed prior to analysis; CPD were transformed prior to analysis by adding a constant (1) to all values and then taking the natural log
Comparing NRT patch + behavioral intervention to behavioral intervention alone
Data are from visit 1 rather than baseline
Twelve participants were discontinued after randomization: six between V2 and V3 (5 in Group 1, 2 in Group 2). Comparing patients who discontinued post randomization (n = 12) and those who completed the protocol (n = 40), the significant differences were: initial SCL (233 vs. 144 ng/ml; p = 0.03) and participation in the food stamps program (45 vs. 80 %, p = 0.05). Of the 12 women who dropped out, 11 consented for a release of their own and their newborn’s medical record. The one woman who did not consent for release of medical records was in Group 2; she completed the protocol but miscarried. The number of women in the NRT group who exhaled CO > 8 ppm were one woman at V3, one at V4 and one at both V3 and V4.
Process Evaluation Results
The percent of patients who agreed to participate (73 %) was comparable to the two reported U.S. trials, 78 [14] and 70 % [23]. Sixty-five percent of patients completed the NRT protocol. A process evaluation model [23] documenting participation and exposure levels to the core protocol procedures for Group 1 and 2 is presented in Table 2, including a Protocol Implementation Index (PII). The core procedures were delivered with a very good level of fidelity by staff indicating a high PII for SCRIPT. Group 2 patients were adherent to the scheduled counseling and assessment visits. Although adherence levels in Group 1 and 2 were comparable for Visit 1 to 4, the NRT Implementation Index was much lower for Visit 5 and 6. The PII and data for each group provide empirical insight for future NRT Trials. A comparison of these process evaluation results to other SCRIPT implementation results reveals that the Group 2 PII of 0.89 was comparable to a published report (PII = 0.95) that used the SCRIPT Program [20, 24].
Table 2.
Process evaluation of RCT patient exposure to core procedures
| Procedures (P) | NRT + SCRIPT group 1 |
SCRIPT group 2 |
||||
|---|---|---|---|---|---|---|
| Eligible | Exposed | Rate | Eligible | Exposed | Rate | |
| Visit #1 | 26 | 26 | 1.00 | 26 | 26 | 1.00 |
| Visit #2 | 26 | 25 | 0.96 | 26 | 26 | 1.00 |
| Visit #3 | 26 | 21 | 0.81 | 26 | 24 | 0.92 |
| Visit #4 | 26 | 20 | 0.77 | 26 | 24 | 0.92 |
| Visit #5 | 26 | 13 | 0.50 | 26 | 19 | 0.73 |
| Visit #6 | 16a | 7 | 0.44 | 14b | 11 | 0.79 |
| Implementation index | PII = 0.75 | PII = 0.89 | ||||
10 delivered < V6
12 delivered < V6
Because the mean EGA at V1 was 18.5 weeks, the average number of weeks from V1 to V6 was 20 and 42 % (22/52) of the participants delivered prior to V6. These data strongly suggest that future RCT’s should reduce the time interval between V4–V5 to 2 weeks, and V5–V6 to 2 weeks. Group 1 data also indicated that the relaxation of the enrollment EGA from ≤24 to ≤30 weeks needs to be reconsidered. If a ≤ 24 week EGA had been maintained, the proportion of patients who delivered before V6 would be substantially reduced.
There were no significant differences noted between Group 1 and Group 2 in the prepregnancy BMI (28.1 ± 10.7 vs. 30.3 ± 6.6, p = 0.58), mean weight gain in kilograms (8.0 ± 15.1 vs. 6.9 ± 10.7, p = 0.87), previous preterm delivery (17 vs. 9 %, p = 0.67), diabetes during pregnancy [3/22 (14 %) vs. 2/23 (9 %), p = 0.67] and hypertension during pregnancy [1/22 (5 %) vs. 1/24 (4 %), p = 1.00].
Impact Evaluation Results
Table 3 compares the distribution of cotinine values between groups during the study. Figure 2 represents the mean cotinine values at each visit for both study groups. The trend of cotinine values over time was not significant for either group (Group 1, p = 0.07). The quit rates between groups is presented in Table 4. Using the ITT approach, women randomized, but not participating in the visit were considered treatment failures. Confirmed Group 1 quit rates at V3 were 23 (n = 6) and 0 % (n = 0) for Group 2 (p = 0.02). At V4 quit rates were 12 % (n = 3) for both groups. At V5 the quit rate was 12 % (n = 3) for Group 1 and 8 % (n = 2) for Group 2. At V6, the quit rates were significantly higher in Group 1, 19 (n = 5) vs. 0 % (n = 0) for Group 2 (p = 0.05).
Table 3.
Final analyses: salivary cotinine distributions by group and visit
| Visit | Group | n | Cotinine (%) |
p valuea | |||
|---|---|---|---|---|---|---|---|
| <50 ng/ml | 50–99 ng/ml | 100–199 ng/ml | 200 + ng/ml | ||||
| 1 | 1. NRT | 26 | 19.2 | 23.1 | 23.1 | 34.6 | 1.00 |
| 2. Behavioral intervention | 26 | 19.2 | 19.2 | 23.1 | 38.5 | ||
| 2 | 1. NRT | 25 | 32.0 | 8.0 | 28.0 | 32.0 | 1.00 |
| 2. Behavioral intervention | 26 | 31.0 | 11.5 | 23.1 | 34.6 | ||
| 3 | 1. NRT | 21 | 23.8 | 33.3 | 9.5 | 33.3 | 0.45 |
| 2. Behavioral intervention | 24 | 37.5 | 20.8 | 20.8 | 20.8 | ||
| 4 | 1. NRT | 20 | 40.0 | 10.0 | 25.0 | 25.0 | 0.77 |
| 2. Behavioral intervention | 24 | 33.0 | 4.2 | 25.0 | 37.5 | ||
| 5 | 1. NRT | 13 | 23.1 | 46.2 | 15.4 | 15.4 | 0.57 |
| 2. Behavioral intervention | 19 | 36.8 | 21.0 | 15.8 | 26.3 | ||
| 6 | 1. NRT | 7 | 71.4 | 14.3 | 14.3 | 0.0 | 0.65 |
| 2. Behavioral intervention | 11 | 45.4 | 9.1 | 27.3 | 18.2 | ||
Difference between groups by Fisher exact test
Fig. 2.
Mean cotinine level by care group
Table 4.
Comparison of confirmed quit rates across groups
| Visit | Quit rates: intent to treat analysis |
Quit rates: pregnant patients |
||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | p value | Group 1 | Group 2 | p value | |
| Visit 3 | 6/26 (23 %) | 0/26 (0 %) | 0.02 | 6/26 (23 %) | 0/25 (0 %) | 0.02 |
| Visit 4 | 3/26 (12 %) | 3/26 (12 %) | 1.00 | 3/26 (12 %) | 3/25 (12 %) | 1.00 |
| Visit 5 | 3/26 (12 %) | 2/26 (8 %) | 1.00 | 3/21 (14 %) | 2/22 (9 %) | 0.66 |
| Visit 6 | 5/26 (19 %) | 0/26 (0 %) | 0.05 | 5/16 (31 %) | 0/14 (0 %) | 0.04 |
Participants in Group 1 delivered infants with higher gestational age compared to Group 2, 39.4 versus 38.4 weeks (p = 0.02) respectively. There was no difference in the prematurity rate, for Group 1 (4 %, n = 1) versus Group 2 (8 %, n = 2). No participants delivered an extremely premature infant. There were no differences in rates of hypertension or of diabetes. Participants in Group 1 delivered babies with higher mean birthweights compared to Group 2, 3,203 versus 2,997 g, respectively (p = 0.18). There were no differences in low birthweight (LBW) rates for Group 1 (12 %, n = 3) versus Group 2 (16 %, n = 4). No participants delivered extremely LBW infants.
When comparing confirmed quitters at any time during pregnancy (n = 12) with smokers (n = 40), quitters were significantly younger (24.3 vs. 28.4 years, p = 0.04), had fewer pregnancies (3.2 vs. 6.4, p = 0.0001), and were less likely to report depression symptoms (33 vs. 68 %, p = 0.048). Quitters smoked fewer CPD over the past week (2.2 vs. 7.2, p = 0.0001); smoked fewer cigarettes ≤24 h (1.8 vs. 5.3, p = 0.005); were less likely to smoke the whole cigarette (0 vs. 48 %, p = 0.002); and were less likely to always smoke their first cigarette within 30 min of waking up (33 vs. 70 %, p = 0.04). One Group 2 subject, who had reported quitting, verified by CO, delivered a LBW infant. Her salivary cotinine remained high (219 ng/ml). LBW rates for quitters were 8.3 and 15.8 % for smokers (p > 0.99). Mothers who quit delivered infants with a significantly higher gestational age compared to smokers (39.7 vs. 38.6 weeks, p = 0.03). No significant differences were noted when comparing prematurity rates: 0 % for quitters versus 7.9 % for smokers, p > 0.99. While participants who quit at any time delivered babies with higher birthweights (+96 g) compared to smokers, the difference was not significant (3,173 vs. 3,077 g, p = 0.60).
Discussion
Recent pharmaco-dynamic studies have shown cotinine levels in pregnant smokers to drop by up to 48 % when using the patch [13]. This level of impact could not be replicated in this study. This may have been due to genetically mediated differences of nicotine metabolism in African-American smokers [25]. Although the sample size of our study precluded drawing any generalizable conclusions, the results were informative. Only one participant in Group 1 was discontinued due to side effects (nausea). Women on the patch showed no significant increase in mean cotinine level compared to women not on the patch. Average cotinine levels in the patch group gradually declined from visit 1 to 6. Comparisons of quitters versus smokers suggested that women who were younger could have been more motivated to quit, and women with fewer depressive symptoms were more able to quit successfully. It is not surprising that women who were able to quit could have been less addicted as measured by the number of cigarettes smoked and the percentage smoking their first cigarette within 30 min of waking.
Similar to previously published studies [5, 7, 8] women receiving the behavioral intervention alone were only modestly successful in quitting. Women receiving both the NRT and CBT had rates higher than women receiving CBT only. While a report in the literature shows a doubling of the rate [5] our sample size was too small to reach conclusive results. At the end of the study women in the NRT and CBT group did have a significantly higher quit rate. The cessation pattern in the NRT plus CBT group showed the percentage of women with a validated cessation rate significantly higher than the CBT group during the initial study phase. This rate difference was not sustained at the 8 week evaluation. It is worthy of note, that the NRT plus CBT group demonstrated a significantly higher quit rate at the end of the study. Unresolved issues in studies addressing this important intervention include whether a placebo patch is necessary and whether standardized doses used in non-pregnant adults are applicable to the pregnant population. Furthermore emphasizing the role that cultural and sociodemographic differences could play in different levels of efficacy for NRT should be emphasized. Using culturally sensitive and ethnically diverse interventionists may have a significant effect on enhancing efficacy as well.
While our sample size was too small to evaluate intervention impact, a significant increase in gestational age of 1 week was observed for the NRT plus CBT group. Wisborg et al. [16] found a significant increase in mean birthweight (186 gms) amongst the NRT participants. While women receiving NRT in our study did deliver infants with a mean birthweight 206 gms larger than women receiving only CBT, this difference did not reach statistical significance. In the aggregate, this study served as a formative evaluation, providing useful data about a variety of issues including, recruitment, retention, exposure to core protocol procedures for each group for the six visits. It also produced data on side effects. A larger randomized clinical trial is needed to compare the efficacy of various dosing regimens across different addiction levels for African-American women.
Two large observational studies reported on nicotine replacement during pregnancy [26, 27]. The first used the 2004 Pregnancy Risk Assessment Monitoring System (PRAMS) and reported that women using NRT (here defined as nicotine spray, inhaler, pill, patch or gum), after adjusting for age, marital status, education and race/ethnicity, were twice as likely to have a low birthweight infant as a nonsmoker, which was higher than that reported in smokers during pregnancy (1.3 times as likely as non-smokers) [26]. The data from this study were collected retrospectively. The dose exposure to NRT was neither monitored nor reported by the users. Furthermore there is no guarantee that the women did not smoke while using NRT. These results are neither comparable nor applicable to our study. The second study by Lassen et al. [26], used the Danish National Birth Cohort, and analyzed the association between the use of NRT (gum, patches or inhalers) and infant birthweight. They found no significant association between duration of use and birthweight. The researchers adjusted for gestational age, smoking (self and partner), parity, pre-pregnancy body mass index, maternal height, alcohol use, coffee consumption, physical exercise, infant gender, socio-economic status, weight loss, eating disorder, fertility problems, vaginal bleeding, nausea, and hypertension. In this population, the majority of women who used NRT also smoked. In addition, the researchers did not monitor whether participants used the NRT as recommended or prescribed. In our study we only selected the patch as a method of nicotine replacement due to the predictability of the dose administered. Furthermore we monitored the mother’s use of the patch and tested them routinely for active smoking while on the patch. Those that continued to actively smoke were eliminated from the patch treatment group and were considered to be intervention failures.
These important differences in our study design and implementation could explain the important difference in our results.
Studies evaluating interventions for smokers during pregnancy frequently encounter recruitment difficulties. Our study was no exception, especially since both the desire to quit and failing the behavioral intervention were amongst our eligibility criteria. Future studies evaluating the efficacy of interventions in similar populations should endeavor to include multiple sites serving this high risk population in order to reach recruitment targets. Furthermore, due to relatively lower adherence rates amongst high risk populations, recruitment targets should exceed projected sample sizes to show significant differences, especially when using an intent-to-treat model for analysis.
Acknowledgments
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U10 HD036104 and U18 HD031206-07). This research was supported, in part, by the intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Clinical Trial Registration: ClinicalTrials.gov, www.clinicaltrials.gov, NCT00341432.
Conflict of interest None of the authors have any competing interests to declare.
Contributor Information
Ayman A. E. El-Mohandes, College of Public Health, University of Nebraska Medical Center, Omaha, NE, USA
Richard Windsor, School of Public Health, George Washington University, Washington, DC, USA.
Sylvia Tan, RTI International, Rockville, MD, USA.
David C. Perry, Department of Pharmacology and Physiology, George Washington University, Washington, DC, USA
Marie G. Gantz, RTI International, Rockville, MD, USA
Michele Kiely, Epidemiology Branch, Division of Epidemiology, Statistics and Prevention Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, 6100 Executive Blvd, Rm. 7B-05, Rockville, MD 20852-7510, USA.
References
- 1.Simpson WJ. A preliminary report on cigarette smoking and the incidence of prematurity. American Journal of Obstetrics and Gynecology. 1957;73:807–815. [PubMed] [Google Scholar]
- 2.Mohsin M, Jalaludin B. Influence of previous pregnancy outcomes and continued smoking on subsequent pregnancy outcomes: an exploratory study in Australia. BJOG: An International Journal of Obstetrics & Gynaecology. 2008;115:1428–1435. doi: 10.1111/j.1471-0528.2008.01864.x. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services . How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and health Promotion, Office on Smoking and Health; Atlanta, GA: 2010. Retrieved from http://www.surgeongeneral.gov/library/tobaccosmoke/report/full_report.pdf. [Google Scholar]
- 4.National Center for Health Statistics . Health, United States, 2009: With Special Feature on Medical Technology. Hyattsville, MD: 2010. Retreived from http://www.cdc.gov/nchs/data/hus/hus09.pdf. [PubMed] [Google Scholar]
- 5.Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER. Treating tobacco use and dependence: Clinical practice guideline. US-DHHS, Public Health Service; Rockville, MD: 2000. [Google Scholar]
- 6.Fiore MC, Jaén CR. A clinical blueprint to accelerate the elimination of tobacco use. Journal of the American Medical Association. 2008;299(17):2083–2085. doi: 10.1001/jama.299.17.2083. [DOI] [PubMed] [Google Scholar]
- 7.Windsor R. Behavioral treatment methods for pregnant smokers: The evidence-base for prenatal care programs and professional practice. In: Handler A, Kennedy J, Peacock N, editors. Reducing racial/ethnic disparities in reproductive and perinatal outcomes: The evidence from population-based interventions. Springer; New York: 2010. pp. 239–264. [Google Scholar]
- 8.Windsor R, Boyd N, Orleans C. A meta-evaluation of smoking cessation intervention research among pregnant women: Improving the science and art. Health Education Research. 1998;13:419–438. doi: 10.1093/her/13.3.419. [DOI] [PubMed] [Google Scholar]
- 9.Windsor R, Oncken C, Henningfield J, Hartmann K, Edwards N. Behavioral and pharmacological treatment methods for pregnant smokers: Issues for clinical practice. Journal of American Medical Women’s Association. 2005;55:304–310. [PubMed] [Google Scholar]
- 10.Benowitz NL. Nicotine replacement therapy during pregnancy. Journal of the American Medical Association. 1991;266:3174–3177. [PubMed] [Google Scholar]
- 11.Benowitz NL, Dempsey D. Pharmacotherapy for smoking cessation during pregnancy. Nicotine & Tobacco Research. 2004;6(Suppl 2):S189–S202. doi: 10.1080/14622200410001669169. [DOI] [PubMed] [Google Scholar]
- 12.American College of Obstetricians and Gynecologists Smoking Cessation during Pregnancy. A Clinician’s Guide to Helping Pregnant Women Quit Smoking. 2011 2011 Self-instructional Guide and Tool Kit. An Educational Program from the American College of Obstetricians and Gynecologists. Retrieved from http://www.acog.org/departments/healthIssues/scdp/files/scdp.pdf.
- 13.Oncken C, Campbell W, Chan G, Hatsukami D, Kranzler HR. Effects of nicotine patch or nasal spray on nicotine and cotinine concentrations in pregnant smokers. Journal of Maternal-Fetal and Neonatal Medicine. 2009;22:751–758. doi: 10.3109/14767050902994515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oncken C, Dornelas E, Greene J, Sankey H, Glasmann A, Feinn R, et al. Nicotine gum for pregnant smokers: A randomized controlled trial. Obstetrics & Gynecology. 2008;112:859–867. doi: 10.1097/AOG.0b013e318187e1ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollak KI, Oncken CA, Lipkus IM, Lyna P, Swamy GK, Pletsch PK, et al. Nicotine replacement and behavioral therapy for smoking cessation in pregnancy. American Journal of Preventive Medicine. 2007;33:297–305. doi: 10.1016/j.amepre.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wisborg K, Henriksen TB, Jespersen LB, Secher NJ. Nicotine patches for pregnant smokers: A randomized controlled study. Obstetrics & Gynecology. 2000;96:967–971. doi: 10.1016/s0029-7844(00)01071-1. [DOI] [PubMed] [Google Scholar]
- 17.Swamy GK, Roelands JJ, Peterson BL, Fish LJ, Oncken CA, Pletsch PK, et al. Predictors of adverse events among pregnant smokers exposed in a nicotine replacement therapy trial. American Journal of Obstetrics and Gynecology. 2009;201(4):354e1–354e7. doi: 10.1016/j.ajog.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Physicians’ desk reference. Medical Economics Co.; Oradell, NJ: 2003. [Google Scholar]
- 19.Thornberry J, Bhaskar B, Krulewitch CJ, Wesley B, Hubbard ML, Das A, et al. Audio computerized self-report interview use in prenatal clinics: Audio computer-assisted self interview with touch screen to detect alcohol consumption in pregnant women: Application of a new technology to an old problem. Computers, Informatics, Nursing. 2002;20:46–52. doi: 10.1097/00024665-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Windsor RA, Woodby LL, Miller TM, Hardin JM, Crawford MA, DiClemente CC. Effectiveness of Agency for Health Care Policy and Research clinical practice guideline and patient education methods for pregnant smokers in Medicaid maternity care. American Journal of Obstetrics and Gynecology. 2000;182(1 Pt 1):68–75. doi: 10.1016/s0002-9378(00)70492-3. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA, Brown GK. BDI-II fast screen for medical patients’ manual. The Psychological Corporation; London: 2000. [Google Scholar]
- 22.Windsor R. A Pregnant woman’s guide to quit smoking. The Society for Public Health Education; SOPHE, 10 G St. Suite 605; Washington DC: 2000. 20002. [Google Scholar]
- 23.Windsor R, Whiteside HP, Jr., Solomon LJ, Prows SL, Donatelle RJ, Cinciripini PM, et al. A process evaluation model for patient education programs for pregnant smokers. Tobacco Control. 2000;9(S. 3):III29–35. doi: 10.1136/tc.9.suppl_3.iii29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Windsor R, Woodby L, Miller T, Hardin M. Effectiveness of the smoking cessation and reduction in pregnancy treatment (SCRIPT) program for a medicaid system of care: SCRIPT trial III. Health Education & Behavior. 2011;38:653–663. doi: 10.1177/1090198110382503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. Journal of the American Medical Association. 2001;287:195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 26.Gaither KH, Brunner Huber LR, Thompson ME, Huet-Hudson YM. Does the use of nicotine replacement therapy during pregnancy affect pregnancy outcomes? Maternal and Child Health Journal. 2009;13:497–504. doi: 10.1007/s10995-008-0361-1. [DOI] [PubMed] [Google Scholar]
- 27.Lassen TH, Madsen M, Skovgaard LT, Strandberg-Larsen K, Olsen J, Andersen A-MN. Naterbak use of nicotine replacement therapy during pregnancy and offspring birthweight: a study within the Danish National Birth Cohort. Paediatric and Perinatal Epidemiology. 2010;24:272–281. doi: 10.1111/j.1365-3016.2010.01104.x. [DOI] [PubMed] [Google Scholar]