Introduction
Lumbar degenerative spondylolisthesis (DS) is typically characterized by the forward slippage of a vertebra and is a common spine pathological condition in elderly individuals[1, 2]. Due to the slippage of superior vertebrae, significant morphological changes can occur to the spine canal and intervertebral foramen, which frequently cause spinal stenosis and induce low back pain, radicular lower limb pain and neurogenic claudication[3]. The clinical symptoms may worsen during standing or walking, but may be relieved in supine or flexion positioning. Many studies have shown that the available space within the lumbar spine canal decreases with extension and increases with forward flexion[4–9].
However, most of the previous studies focused on the measurements of cross-sectional area and diameter of the canal in a 2D axial plane using planar CT, MRI or X-ray images[5–9]. Measurements using simple radial line or area may be inaccurate depending on the section angle and/or thickness of the scans[10, 11]. Furthermore, canal shape can be complicated due to the vertebral slippage. Therefore, determination of the canal volume may be a more accurate way to evaluate the morphologic changes of the spine canal. However, due to the limitation in technology, only one reported on spinal canal volume in cadavers[4] and none on lumbar spine canal volume was reported in literature.
The objective of this study is to measure the volumes of the lumbar spine canal in DS patients at various functional postures and compare the data with those of asymptomatic subjects using a previously validated 3D imaging technique for analysis of the spinal motion. In addition, different spinal parameters such as disc height, canal dimensions in 2D, disc angle, and vertebrae slippage were measured. The data was interpreted regarding the changes of canal volume to explore the potential factors that may affect the DS patients.
Materials and methods
Study populations
Approval of the experimental design by the authors' Institutional Review Board was obtained prior to the initiation of the study. Nine symptomatic subjects with diagnosis of L4-5 DS (3 males and 6 females) were recruited from a single academic center. All patients had intermittent claudication and varying levels of low back pain, and two patients also had leg pain. The patients had a mean age of 73.6 years (range, fifty-two to eighty-seven years). All patients vertebrae slippage was graded I by Meyerding classification method[12]. Nine asymptomatic subjects with an age ranging from 50 to 60 years (4 males and 5 females) were recruited for this study (mean age 54.4 years). A written consent was obtained from each subject prior to participation into the study.
Three-dimensional CT/MRI-based model
Two DS patients and nine normal subjects were scanned using a Siemens Medical Solutions MAGNETOM Trio 3-T MRI scanner with a spine surface coil and a T2-weighted fat-suppressed 3-D spoiled gradient recalled sequence. Seven DS patients were scanned in a General Electric Light-Speed Pro16 CT scanner. All subjects were scanned in a supine, relaxed position. Parallel digital images with thickness of 1.5 mm and resolution of 512×512 pixels were obtained from the MRI scans; axial plane images with thickness of 0.625 mm and resolution of 512×512 pixels were obtained from the CT scans. The images of the spinal segments were then imported into a modeling software (Rhinoceros® Robert McNeel & Associates, Seattle, Washington) to construct 3-D anatomical vertebrae models of the L4 and L5 (Fig. 1). CT scans were required for the 7 patients for routine clinical purposes. 3D lumbar models were constructed from the CT images semi-automatically with minimal manual modification. In the other 2 patients, MRI scans were performed to minimize radiation risk to subjects. However, there was no difference in analysis of the spinal motion using models base on CT or MRI scanning[13].
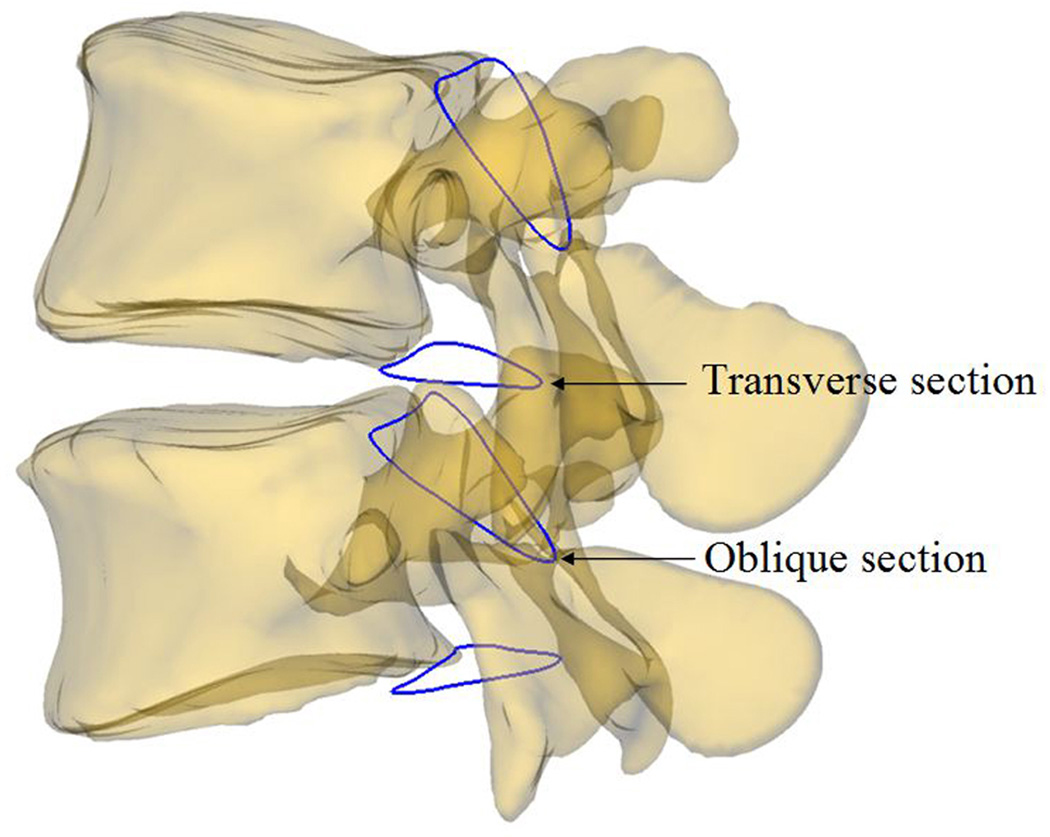
Fig. 1.
A 3D L4/L5 models and two sections created to define the boundary of the canal volume
Reproduction of lumbar spine kinematics under in vivo weight-bearing
Two fluoroscopes (BV Pulsera; Philips, Bothell, Washington) were positioned with their intensifiers orthogonal to each other to simultaneously scan the lumbar positions[14]. The subjects were asked to stand and position their lumbar spines within the views of both fluoroscopes and actively move to different postures: standing upright, maximum lumbar flexion, maximal lumbar extension. For each posture, the subject was asked to hold for about 1 second and imaged by the two fluoroscopes simultaneously from two orthogonal directions. The geometry of the dual-orthogonal fluoroscopic system was created in the solid modeling software[13–16]. After calibration, the pair of fluoroscopic images was imported into the software and placed on the virtual orthogonal planes to simulate the positions of the intensifiers. The lumbar vertebrae models were introduced into the virtual system and were independently moved and rotated until their silhouettes matched those captured on the two orthogonal fluoroscopic images. Thus the positions of the lumbar vertebrae during the in vivo weight-bearing activities were reproduced.
Modeling of the lumbar spine canal
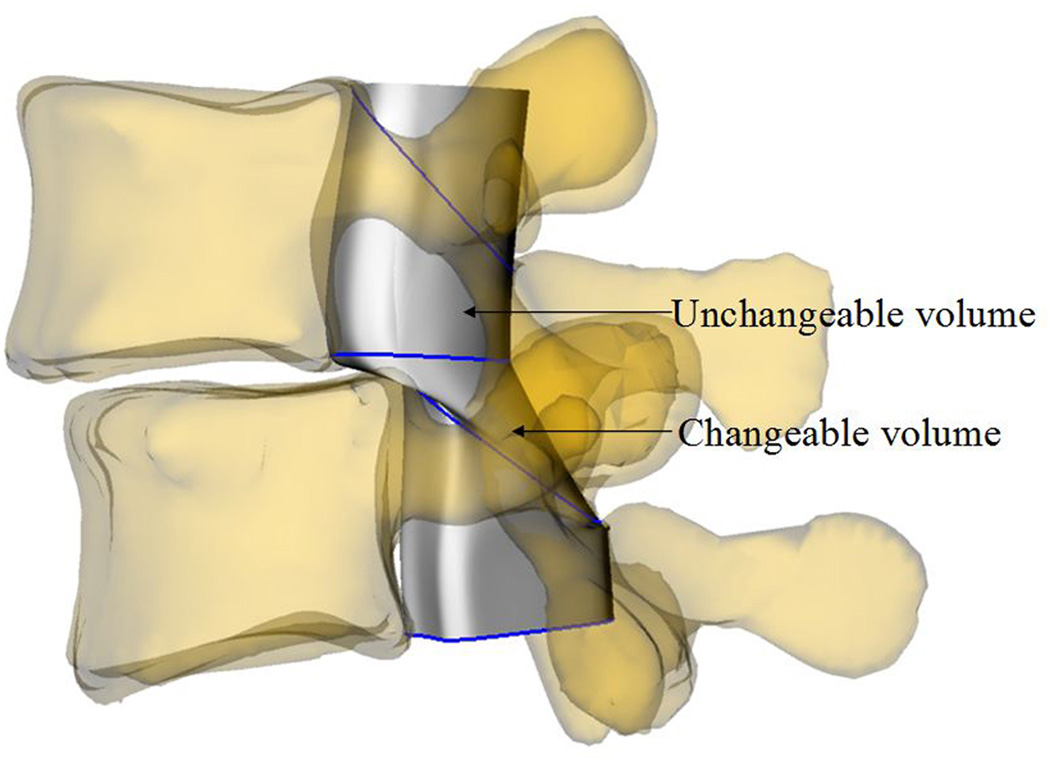
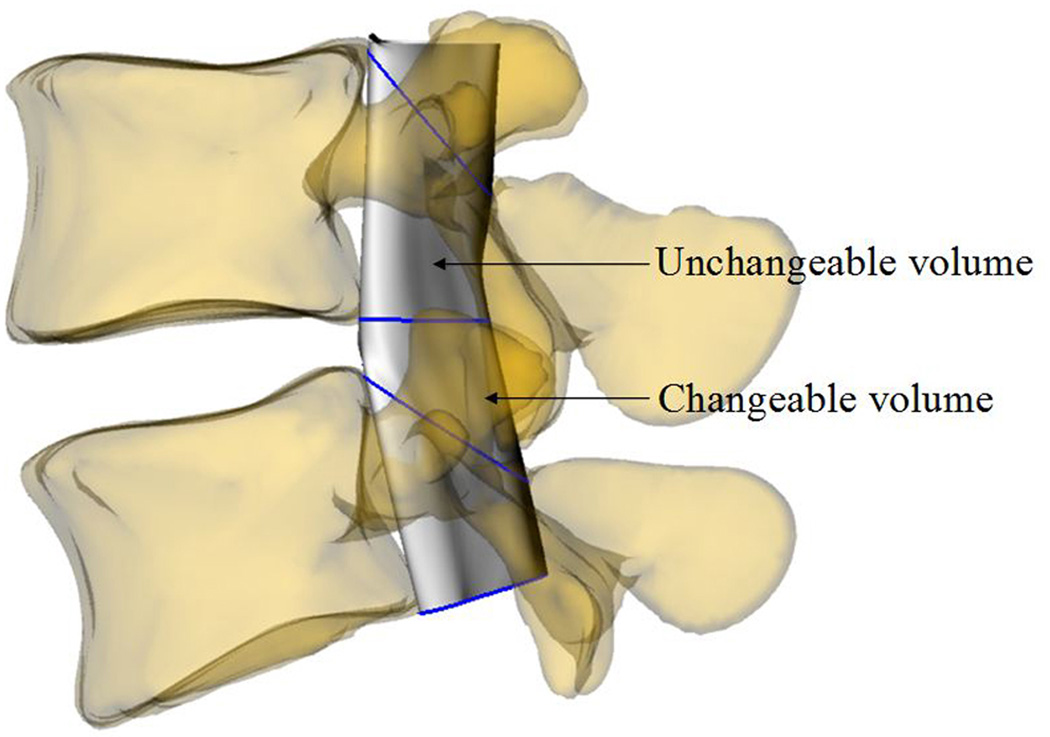
For each L4/L5 vertebral motion segment unit, the volume of the spine canal was sub- divided into changeable and unchangeable portions (Figs. 1 and 2). The changeable volume is defined between any two adjacent vertebrae, which is enclosed by soft component (anterior intervertebral disc and posterior ligamentum flavum). The unchangeable volume is enclosed by the bony structures of the spine (anterior vertebral body, bilateral pedicle and posterior lamina) which changed negligibly and was not included in the calculation.
Fig. 2.
Definition of the changeable and unchangeable volumes: (a) DS patient; (b) healthy subject
In the solid modeling software, two spine canal sections were created based on the definition of changeable volume and the anatomic features of each vertebra. The superior oblique section was created from the projection of the canal on the plane through the posterior rim of the superior endplate and superior rim of the lamina. The inferior transverse section was created from the projection of the canal on the plane of the inferior endplate (Figs. 1 and 2). The sections were obtained at supine MRI/CT position, and were fixed to L4 or L5, respectively, to ensure the consistency in the measurement of the canal volume under all four tested postures. The volume between the transverse section of superior vertebra and oblique section of inferior vertebra was approximated and automatically calculated by using lines connecting the equally divided points on the two section outlines in the software (Figs. 1 and 2).
Measurement of lumbar parameters
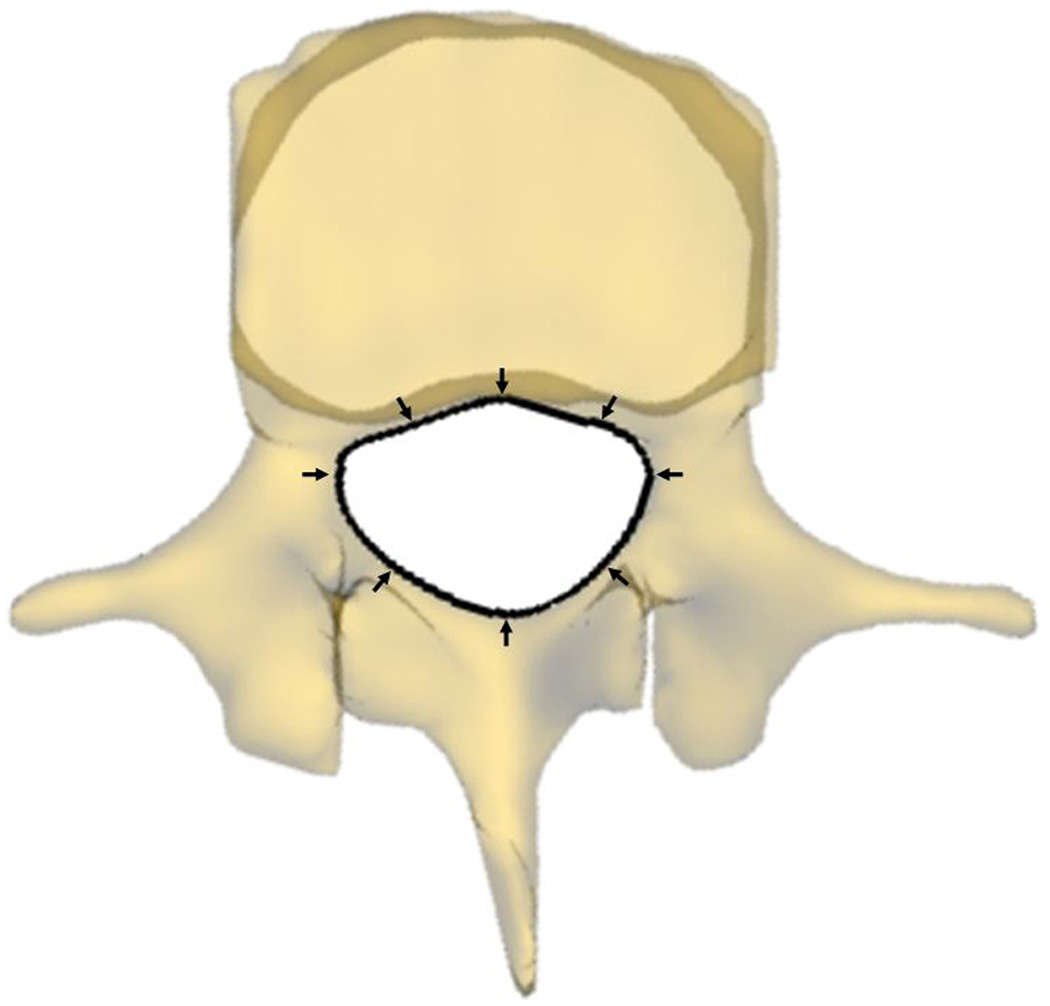
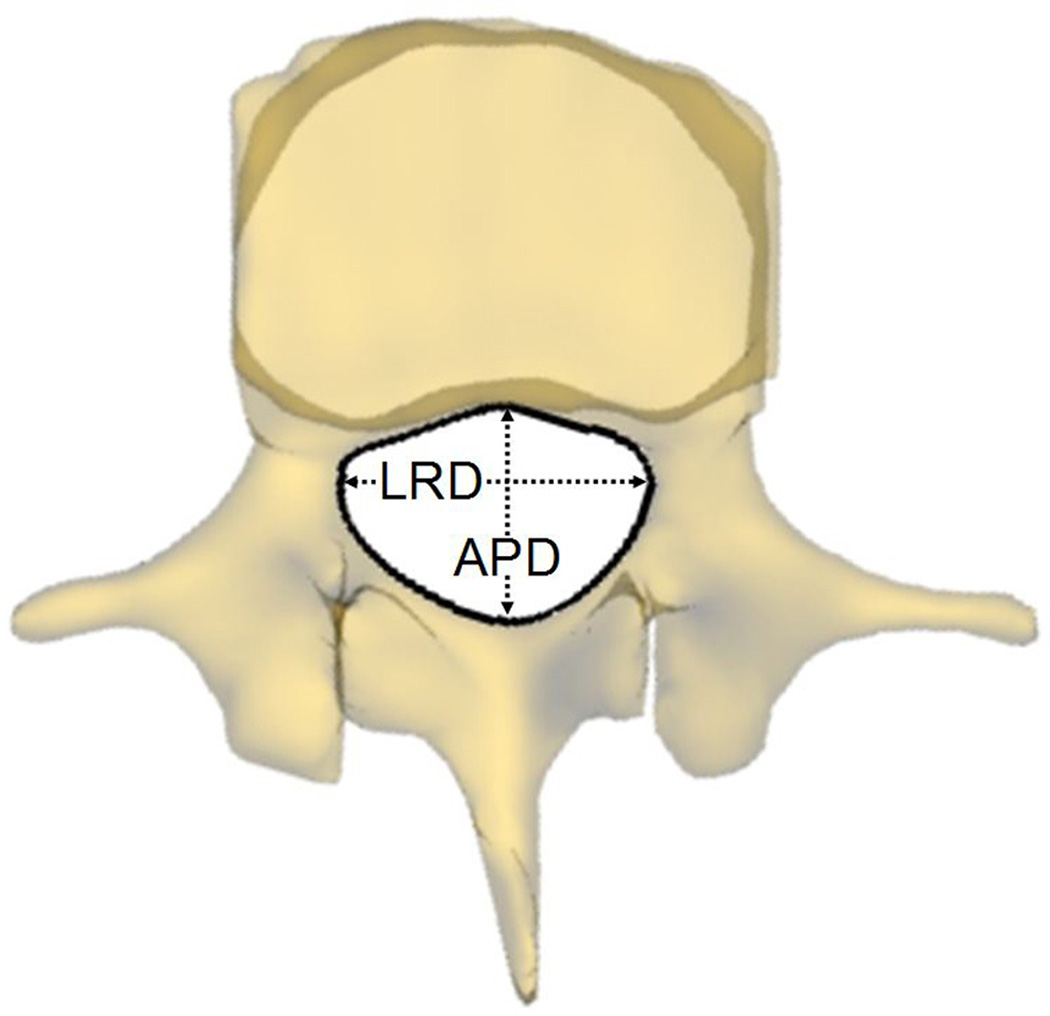
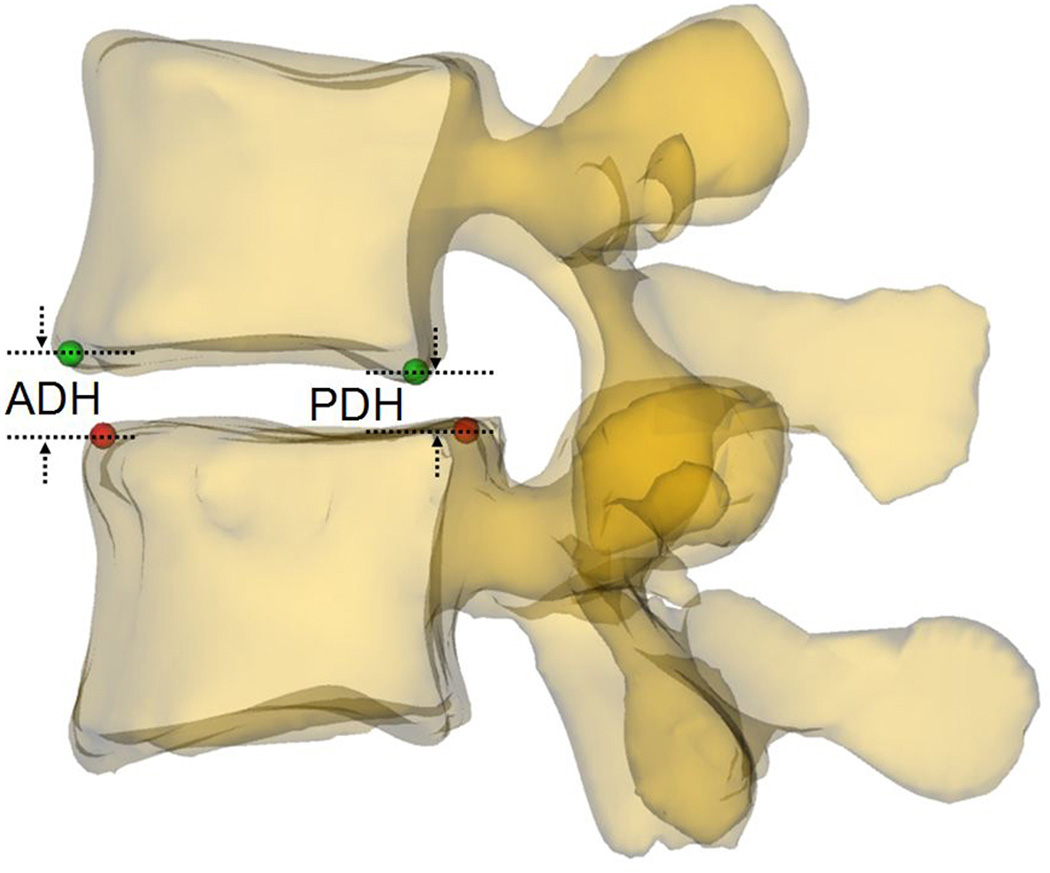
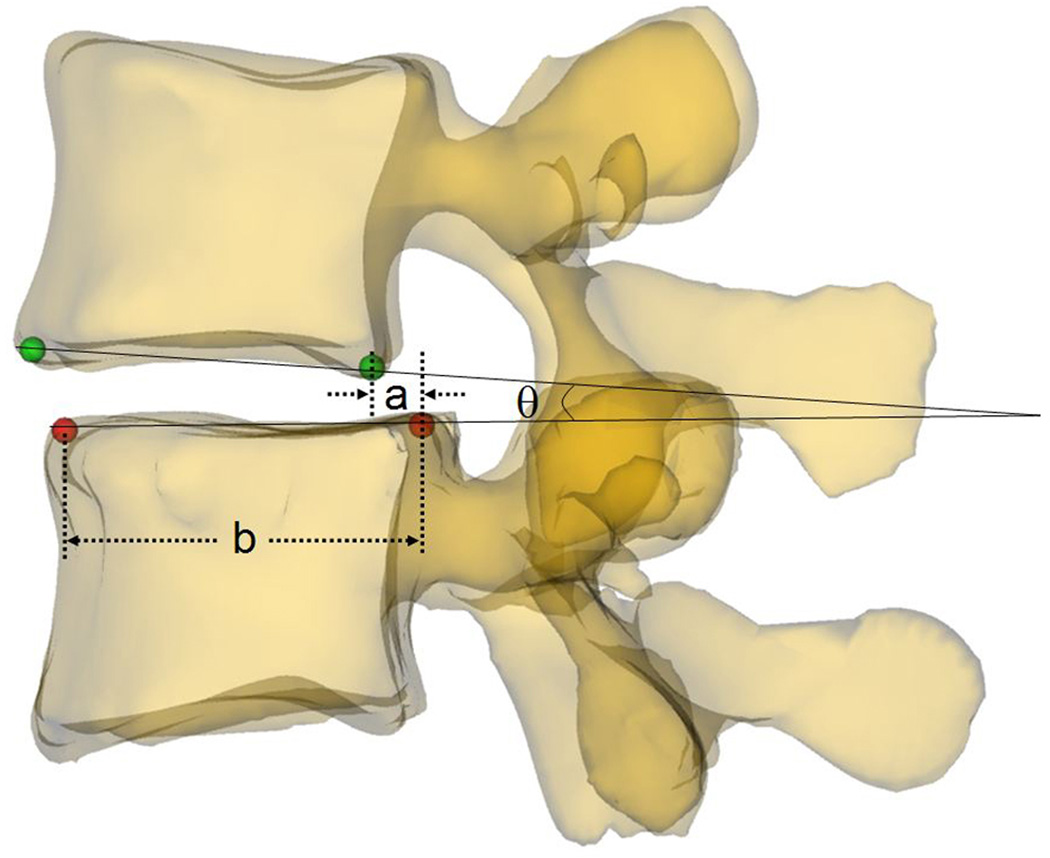
Parameters that may potentially affect changeable volume of spine canal were measured at the four postures. Spine canal cross-sectional area (CSA L4 and L5) was measured as the area of the projection of the canal on the plane of the inferior endplate (Fig. 3a). Anterior-posterior diameter (APD L4 and L5) was measured as the length of between anterior and posterior margin of spine canal projection on the plane of the inferior endplate (Fig. 3b). Left-right diameter (LRD L4 and L5) was measured as the distance of left and right margin of spine canal projection on the plane of the inferior endplate (Fig. 3b). Anterior disc height (ADH) and posterior disc height (PDH) was the perpendicular distance between the two opposite points of the endplate rims (Fig. 4a). Disc angle (DA) was measured as the angulation between the inferior endplate of L4 and superior endplate of L5 in the sagittal plane (Fig. 4b). Slippage percentage (SP) was defined as the percentage of the offset distance between the posterior edges of the adjacent vertebral bodies over the total length of the superior endplate of inferior vertebral body in the sagittal plane (Fig. 4b). In these definitions, CSA, APD, and LRD were fixed during different positions while ADH, PDH, DA, and SP varied with the lumbar posture changing.
Fig. 3.
(a) Measurement of the cross-sectional area of the spine canal (CSA); (b) Measurement of the anterior-posterior diameter (APD) and left-right diameter (LRD) of the spine canal
Fig. 4.
(a) Measurement of the anterior disc height (ADH) and posterior disc height (PDH); (b) Measurement of the slippage percentage (SP) and disc angle (DA): SP= (a/b)×100%, DA=θ
Statistical analysis
The changeable volume of spine canal was measured as mean±SD and two-way ANOVA test was used to examine the between-group differences. Multiple linear regression analysis was performed to determine the contribution of the parameters to the changeable volumes of the spine canal of L4-5 in DS patients. Positive relationships exist and were defined as Pearson correlation coefficient γb: ≥ 0.7 very strong, ≥ 0.4 strong, ≥ 0.3 moderate, ≥ 0.2 weak correlation and otherwise negligible relationship. Similar analysis was performed for negative values of γb[17]. All analyses were performed using the Statistical Package for the Social Sciences (SPSS for Windows, release 19.0, IBM) and the significant level was defined as p < 0.05.
Results
Spine canal volumes of the DS patients (mean 802.9 to 1072.0 mm3) were significantly lower than that of normal subjects (mean 2699.4 to 2952.9 mm3) under all the four postures (Fig. 5, p < 0.05). In both the DS and the normal group, same trends were observed on spine canal volume changes with postures. On average, the canal volume was relative bigger at supine (2952.9±1090.4 mm3 in normal, 1072.0±612.6 mm3 in DS) and flexion postures (2925.4±1183.1 mm3 in normal, 953.4±575.9 mm3 in DS) than at upright stand (2732.0±977.8 mm3 in normal, 880.11±667.7 mm3 in DS) and extension postures (2699.4±1168.6 mm3 in normal, 802.9±476.7 mm3 in DS).
Fig. 5.
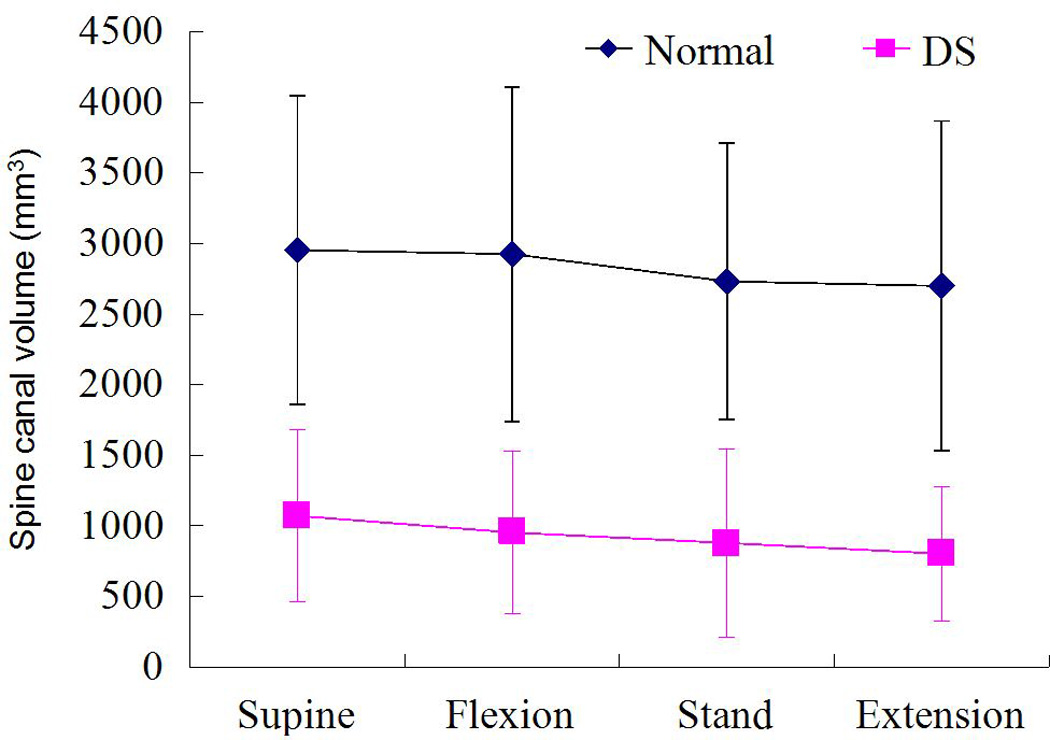
Comparison of the spine canal volumes between normal and DS groups at the various postures (Mean±SD, n=9 in each group)
The various parameters that may affect spine canal volume were measured in the two groups (Table. 1 and 2). The changeable spine canal volume was very strongly affected by the posterior disc height (γb = 0.822, p = 0.000) and the cross-sectional area of L4 (γb = 0.735, p = 0.000); strongly affected by the left-right diameter of L4 (γb = 0.6520, p = 0.000), the anterior-posterior diameter of L4 (γb = 0.644, p = 0.000), the slippage percentage (γb = −0.593, p= 0.000), the cross-sectional area of L5 (γb = 0.536, p = 0.000) and the anterior-posterior diameter of L5 (γb = 0.459, p = 0.000); moderately affected by the anterior disc height (γb = 0.300, p = 0.005); and weakly affected by the disc angle (γb = −0.237, p = 0.023) (Fig. 6). The left-right diameter of L5 (γb = 0.18, p = 0.065) and posture (γb = −0.09, p = 0.227) had no effect on the changeable spine canal volume (Fig. 6).
Table 1.
Mean values of ADH, PDH, DA, and SP at different postures (n=9 in each group).
| Supine | Standing | Extension | Flexion | |||||
|---|---|---|---|---|---|---|---|---|
| Normal | DS | Normal | DS | Normal | DS | Normal | DS | |
| ADH (mm) | 10.7 | 8.7 | 10.3 | 6.9 | 10.9 | 8.4 | 9.2 | 6.5 |
| PDH (mm) | 6.4 | 4.0 | 6.1 | 3.3 | 5.9 | 3.0 | 6.4 | 3.7 |
| DA (°) | 8.2 | 4.8 | 8.0 | 4.8 | 9.3 | 9.2 | 5.4 | 4.8 |
| SP (%) | −3.65 | 12.44 | −0.21 | 15.52 | −1.42 | 13.75 | −0.03 | 16.66 |
ADH: Anterior Disc Height, PDH: Posterior Disc Height, DA: Disc Angle, SP: Slippage Percentage
Table 2.
Mean values of CSA, LRD, and APD of L4 and L5 (n=9 in each group)
| Normal | DS | |
|---|---|---|
| CSA L4 (mm2) | 306 | 228 |
| CSA L5 (mm2) | 315 | 273 |
| LRD L4 (mm) | 25.5 | 23.5 |
| LRD L5 (mm) | 27.7 | 27.8 |
| APD L4 (mm) | 16.4 | 14.3 |
| APD L5 (mm) | 16.5 | 14.9 |
CSA: Cross-sectional area, LRD: Left-right diameter, APD: Anterior-posterior diameter
Fig. 6.
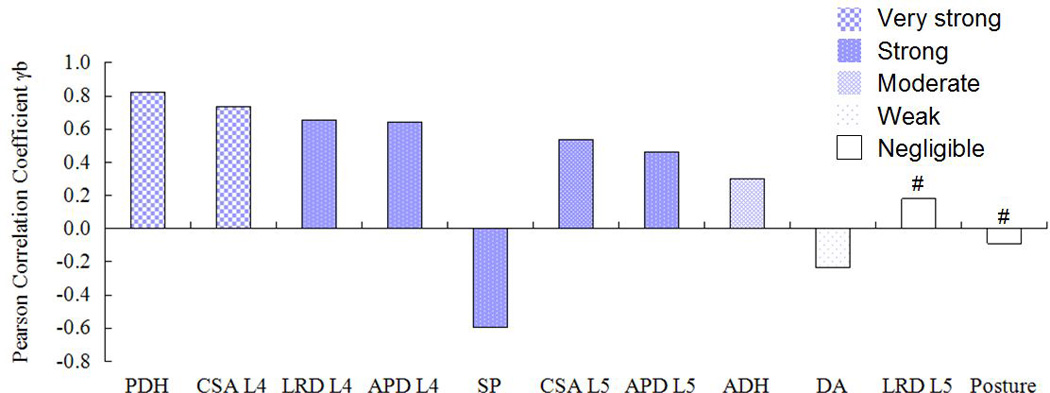
Correlations between the spine canal volume and the affecting factors (from strong to weak). PDH: Posterior Disc Height, CSA: Cross-sectional area, LRD: Left-right diameter, APD: Anterior-posterior diameter, SP: Slippage Percentage, ADH: Anterior Disc Height, DA: Disc Angle. #: not significant, p>0.05
Discussion
DS is a common condition in the elderly population and can affect a patient's life quality. However, no data has been reported on the in vivo volume changes of the lumbar spine canal in patients with DS. Using a combined dual fluoroscopic and MR/CT imaging technique, this study examined the in vivo volume of the lumbar spine canal in DS and normal subjects at various functional postures. The data indicated that patients with DS had significantly smaller spine canal volume than the asymptomatic subjects. Spine canal volume increased at supine and flexion postures compared with those measured at upright and extension postures. Spine canal volume had significant correlations with both the canal dimensions such as CSA (L4 and L5), APD (L4 and L5) and LRD (L4), and other anatomic parameters such as PDH, SP, DA and ADH.
In a cadaver lumbar spine flexion-extension study, Dai et al. found that a larger capacity of the dural sac in flexion than in extension[4]. Mauch et al. studied the dural sac dimensions in lumbar spine of athletes using open MRI and found that the sagittal dural sac diameter was significantly smaller in true standing position than in supine position[6]. Madsen et al. found that the dural sac cross-sectional area was reduced at all 5 lumbar levels after extension and extension was the dominant cause rather than compression in reducing dural sac cross-sectional area[7]. The reported data indicated that the dimension of spine canal is affected by lumbar posture. The variation trends of the in vivo L4-5 spine canal volume in both DS and normal subjects observed in current study were consistent with those reported in literature. The spine canal volume increased when changing postures from upright and extension to supine and flexion.
The etiologies of change of spine canal volume are multi-factorial in DS patients and it is important to detect critical factors affecting spine canal volume. From a biomechanical engineering point of view, slippage of the superior vertebrae can cause deformation and volume change of the spine canal. Compression of spine canal contents due to spine canal volume decrease has been thought to be a main reason for clinical symptoms of patients with DS[3]. We found that on average, the spine canal had larger volume at supine and flexion postures t han at standing and extension postures. This posture-dependent spine canal volume variation may help to explain why a patient's clinical symptoms are relieved in supine and flexion postures. Correlation analysis showed that the intervertebral disc angle had a significant negative correlation with spine canal volume. Spine canal volume increases as disc angle decreased, which implies slight intervertebral flexion can effectively increase spine canal volume. This suggests why interspinous implant devices might be a possible option for the elderly patients with DS. Interspinous devices are known to limit extension and keep intervertebral slight flexion, thus induce enlargement of spine canal volume and intervertebral foreman[18, 19].
Decompression is a clinical routine for patients with DS and can increase spinal canal volume. In addition to decompression, reduction of the slipped vertebrae as a part of surgical approach is still a controversial issue in spine surgery[20, 21]. In a randomized and double blinded study of symptomatic patients with Meyerding grade I and II isthmic spondylolisthesis, Audat et al. concluded that the outcome is almost similar in patients who underwent surgical fixation and decompression with or without reduction of the slippage[20]. Many other authors proposed that reduction of the slippage should be considered to correct sagittal deformity and enhance the spinal biomechanics[22, 23]. By comparing sagittal alignment with a normal control population, Roussouly et al. found that patients with spondylolysis and low-grade spondylolisthesis demonstrated an increased pelvic incidence and increased lumbar lordosis, indicating that the disease may influence the spinal biomechanical environment[22]. From a biomechanical point of view, the results of this study showed that the slippage of vertebra has strong negative correlation with the spine canal volume. Theoretically, surgical reduction to correct spine canal morphology may need to be considered to effectively increase spine canal volume in addition to decompression, especially for patients with severe slippage.
Intervertebral disc height can obviously affect spine canal volume. Spinal stenosis was believed to be indirectly decompressed by restoration of disc height[24]. Similar clinical outcomes have been observed indicating that stenosis symptoms of DS patients could be effectively alleviated by both anterior lumbar interbody fusion and posterolateral fusion with decompressive laminectomies[25–27]. Vamvanij et al. found that spine canal volume was markedly increased with anterior distraction (19.92%) and lateral distraction (21.96%) in an in-vitro cadaveric lumbar spine test[24]. Infusa et al. reported that the posterior disc height, foraminal height, and foraminal area increased with posterior distraction of pedicle screws[28]. In the current study, the intervertebral disc height was shown to positively correlate to the spine canal volume, especially the PDH which has the strongest influence on the spine canal volume among all the factors studied. Disc height change implies longitudinal size changes of the spine canal. Appropriate distraction of intervertebral space during anterior or posterior operation may effectively increase spine canal volume.
The data of this study also showed that the spine canal volume was strongly correlated to the spine canal cross-sectional area and diameter, anterior-posterior and left-right diameter of L4, and to a lesser extent, of L5. The data strongly supports surgical decompression to enlarge spine canal as a surgical treatment for lumbar DS patients with spinal stenosis, to relieve radicular symptoms and neurogenic claudication[3, 29]. Decompressive laminectomy should include both L4 and L5[30].
There are certain simplifications and assumptions in the modeling of the canal that may need to be improved in future investigations. The canal volume was calculated using straight lines connecting the two canal cross sections on the superior and inferior vertebrae. This method did not include the effect of disc budging and ligament folding on canal volume measurements, thus might result in over estimation of the canal volume. We only investigated patients with DS at L4-L5 level. Future study might include patients with DS at other levels and patients with DS at multiple levels. In the future, we plan to recruit more normal and symptomatic patients with DS. With a larger sample size, we would expect to find the critical value or range of the canal volume that may be useful to identify onset of symptoms and provide a basis for necessary volume improvement after treatment. Furthermore, the post-operative patients also will be examined to evaluate the effectiveness of surgical treatments.
In conclusion, this study describes a 3D in-vivo measurement of the volume of the lumbar spine canal of a group of patients with DS at various functional postures and compares the results with those of normal subjects. The canal volumes were significantly smaller in all postures in DS patients compared with those of normal subjects. The volume is affected by multiple factors, having significant correlation to both the canal dimensions such as CSA (L4 and L5), APD (L4 and L5) and LRD (L4), and other anatomic parameters such as PDH, SP, DA and ADH. Increased volume at supine and flexion positions may explain the relief of clinical symptoms found at these postures of DS patients. Therefore, in order to increase the canal volume in DS patients to relieve clinical symptoms, reduction of slipped vertebral body, decrease of disc angle, employing intervertebral distraction, as well as decompression, can all be effective, but need to be planned accordingly for each specific case during the surgical treatment.
Acknowledgements
This study was supported by NIH (R21AR057989), NASS, SRS and China Scholarship Council (2009912008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Junghanns H. Spondylolisthesen ohne Spaltim Zwischergelenkstulcz (Pseudospondylisthen) Arch Orthop Unfallcbir. 1931;29:118–127. [Google Scholar]
- 2.Iguchi T, Wakami T, Kurihara A, Kasahara K, Yoshiya S, Nishida K. Lumbar multilevel degenerative spondylolisthesis: radiological evaluation and factors related to anterolisthesis and retrolisthesis. J Spinal Disord Tech. 2002;15:93–99. doi: 10.1097/00024720-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Sengupta DK, Herkowitz HN. Degenerative spondylolisthesis: review of current trends and controversies. Spine. 2005;30(6 Suppl):S71–S81. doi: 10.1097/01.brs.0000155579.88537.8e. [DOI] [PubMed] [Google Scholar]
- 4.Dai LY, Xu YK, Zhang WM, Zhou ZH. The effect of flexion-extension motion of the lumbar spine on the capacity of the spinal canal. An experimental study. Spine. 1989;14:523–525. [PubMed] [Google Scholar]
- 5.Willén J, Danielson B, Gaulitz A, Niklason T, Schönström N, Hansson T. Dynamic effects on the lumbar spinal canal: axially loaded CT-myelography and MRI in patients with sciatica and/or neurogenic claudication. Spine. 1997;22:2968–2976. doi: 10.1097/00007632-199712150-00021. [DOI] [PubMed] [Google Scholar]
- 6.Mauch F, Jung C, Huth J, Bauer G. Changes in the lumbar spine of athletes from supine to the true-standing position in magnetic resonance imaging. Spine. 2010;35:1002–1007. doi: 10.1097/BRS.0b013e3181bdb2d3. [DOI] [PubMed] [Google Scholar]
- 7.Madsen R, Jensen TS, Pope M, Sørensen JS, Bendix T. The effect of body position and axial load on spinal canal morphology: an MRI study of central spinal stenosis. Spine. 2008;33:61–67. doi: 10.1097/BRS.0b013e31815e395f. [DOI] [PubMed] [Google Scholar]
- 8.Danielson B, Willén J. Axially loaded magnetic resonance image of the lumbar spine in asymptomatic individuals. Spine. 2001;26:2601–2606. doi: 10.1097/00007632-200112010-00015. [DOI] [PubMed] [Google Scholar]
- 9.Hirasawa Y, Bashir WA, Smith FW, Magnusson ML, Pope MH, Takahashi K. Postural changes of the dural sac in the lumbar spines of asymptomatic individuals using positional stand-up magnetic resonance imaging. Spine. 2007;32:E136–E140. doi: 10.1097/01.brs.0000255202.94153.ca. [DOI] [PubMed] [Google Scholar]
- 10.Eubanks BA, Cann CE, Brant-Zawadzki M. CT measurement of the diameter of spinal and other bony canals: effects of section angle and thickness. Radiology. 1985;157:243–246. doi: 10.1148/radiology.157.1.4034968. [DOI] [PubMed] [Google Scholar]
- 11.Bayley JC, Kruger DM, Schlegel JM. Does the angle of the computed tomographic scan change spinal canal measurements? Spine. 1991;16(10Suppl):S526–S529. doi: 10.1097/00007632-199110001-00014. [DOI] [PubMed] [Google Scholar]
- 12.Ganju A. Isthmic spondylolisthesis. Neurosurg Focus. 2002;13:E1. doi: 10.3171/foc.2002.13.1.2. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Passias P, Li G, Li G, Wood K. Measurement of vertebral kinematics using noninvasive image matching method-validation and application. Spine. 2008;33:E355–E361. doi: 10.1097/BRS.0b013e3181715295. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Wang S, Passias P, Xia Q, Li G, Wood K. Segmental in vivo vertebral motion during functional human lumbar spine activities. Eur Spine J. 2009;18:1013–1021. doi: 10.1007/s00586-009-0936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passias PG, Wang S, Kozanek M, et al. Segmental lumbar rotation in patients with discogenic low back pain during functional weight-bearing activities. J Bone Joint Surg Am. 2011;93:29–37. doi: 10.2106/JBJS.I.01348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia Q, Wang S, Kozanek M, Passias P, Wood K, Li G. In-vivo motion characteristics of lumbar vertebrae in sagittal and transverse planes. J Biomech. 2010;43:1905–1909. doi: 10.1016/j.jbiomech.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Jackson RA. Interpretation of research data: selected statistical procedures. Am J Hosp Pharm. 1980;37:1673–1680. [PubMed] [Google Scholar]
- 18.Hong SW, Lee HY, Kim KH, Lee SH. Interspinous ligamentoplasty in the treatment of degenerative spondylolisthesis: midterm clinical results. J Neurosurg Spine. 2010;13:27–35. doi: 10.3171/2010.3.SPINE0957. [DOI] [PubMed] [Google Scholar]
- 19.Wan Z, Wang S, Kozánek M, et al. Biomechanical Evaluation of the X-Stop Device for Surgical Treatment of Lumbar Spinal Stenosis. J Spinal Disord Tech. 2011 doi: 10.1097/BSD.0b013e318227eb84. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Audat ZM, Darwish FT, Al Barbarawi MM, et al. Surgical management of low grade isthmic spondylolisthesis; a randomized controlled study of the surgical fixation with and without reduction. Scoliosis. 2011;6:14. doi: 10.1186/1748-7161-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehdian SM, Arun R, Jones A, Cole AA. Reduction of severe adolescent isthmic spondylolisthesis: a new technique. Spine. 2005;30:E579–E584. doi: 10.1097/01.brs.0000181051.60960.32. [DOI] [PubMed] [Google Scholar]
- 22.Roussouly P, Gollogly S, Berthonnaud E, Labelle H, Weidenbaum M. Sagittal alignment of the spine and pelvis in the presence of L5-s1 isthmic lysis and low-grade spondylolisthesis. Spine. 2006;31:2484–2490. doi: 10.1097/01.brs.0000239155.37261.69. [DOI] [PubMed] [Google Scholar]
- 23.Labelle H, Roussouly P, Chopin D, et al. Spino-pelvic alignment after surgical correction for developmental spondylolisthesis. Eur Spine J. 2008;17:1170–1176. doi: 10.1007/s00586-008-0713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vamvanij V, Ferrara LA, Hai Y, Zhao J, Kolata R, Yuan HA. Quantitative changes in spinal canal dimensions using interbody distraction for spondylolisthesis. Spine. 2001;26:E13–E18. doi: 10.1097/00007632-200102010-00004. [DOI] [PubMed] [Google Scholar]
- 25.Yan DL, Pei FX, Li J, Soo CL. Comparative study of PILF and TLIF treatment in adult degenerative spondylolisthesis. Eur Spine J. 2008;17:1311–1316. doi: 10.1007/s00586-008-0739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi K, Kitahara H, Yamagata M, et al. Long-term results of anterior interbody fusion for treatment of degenerative spondylolisthesis. Spine. 1990;15:1211–1215. doi: 10.1097/00007632-199011010-00022. [DOI] [PubMed] [Google Scholar]
- 27.McAfee PC, DeVine JG, Chaput CD, et al. The indications for interbody fusion cages in the treatment of spondylolisthesis: analysis of 120 cases. Spine. 2005;30(6 Suppl):S60–S65. doi: 10.1097/01.brs.0000155578.62680.dd. [DOI] [PubMed] [Google Scholar]
- 28.Infusa A, An HS, Glover JM, McGrady L, Lim TH, Riley LH., 3rd The ideal amount of lumbar foraminal distraction for pedicle screw instrumentation. Spine. 1996;21:2218–2223. doi: 10.1097/00007632-199610010-00008. [DOI] [PubMed] [Google Scholar]
- 29.Majid K, Fischgrund JS. Degenerative lumbar spondylolisthesis: trends in management. J Am Acad Orthop Surg. 2008;16:208–215. doi: 10.5435/00124635-200804000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Bassewitz H, Herkowitz H. Lumbar stenosis with spondylolisthesis: current concepts of surgical treatment. Clin Orthop Relat Res. 2001;384:54–60. [PubMed] [Google Scholar]