Abstract
The molecular mechanism regulating the cardiomyocyte response to energy stress has been a hot topic in cardiac research in recent years, since this mechanism could be targeted for treatment of patients with ischemic heart disease. We have shown recently that the activity of RAS homolog enriched in brain (RHEB), a small GTP binding protein, is inhibited in response to glucose deprivation (GD) in cardiomyocytes and ischemia in the mouse heart. This is a physiological adaptation, since it inhibits complex 1 of the mechanistic target of rapamycin (MTORC1) and activates autophagy, thereby promoting cell survival during GD and prolonged ischemia. Importantly, the physiological inhibition of RHEB-MTORC1 signaling during myocardial ischemia is impaired in the presence of obesity and metabolic syndrome caused by high-fat diet (HFD) feeding, leading to a dramatic increase in ischemic injury. Although MTORC1 and autophagy can be regulated through RHEB-independent mechanisms, such as the AMPK-dependent phosphorylation of RPTOR and ULK1, RHEB appears to be critical in the regulation of MTORC1 and autophagy during ischemia in cardiomyocytes, and its dysregulation is relevant to human disease. Here we discuss the biological relevance of the dysregulation of RHEB-MTORC1 signaling and the suppression of autophagy in obesity and metabolic syndrome.
Keywords: MTOR, Rheb, autophagy, ischemia, metabolic syndrome, obesity
Increasing lines of evidence suggest that MTORC1 is activated and autophagy is suppressed in many organs in mouse models of obesity and metabolic syndrome. In theory, multiple mechanisms can mediate the activation of MTORC1 in these conditions. For example, increases in energy intake and in circulating levels of lipids, amino acids, insulin and cytokines are associated with activation of MTORC1. However, the detailed signaling mechanisms mediating this event are not fully understood. Our study suggests that RHEB is activated in the heart after HFD feeding and that activation of RHEB is required for MTORC1 activation and consequent suppression of autophagy in this condition. Thus, although MTORC1 can be regulated by multiple signaling mechanisms, MTORC1 activation by HFD seems to be mediated through RHEB in cardiomyocytes. Whether RHEB regulates autophagy exclusively through MTORC1 or through other mechanisms as well needs further investigation.
Of note, when mice are fed with a HFD, RHEB-MTORC1 signaling is activated at baseline. More strikingly, however, it also fails to be inhibited during myocardial ischemia. Since the suppression of MTORC1 is an adaptive mechanism essential to the stimulation of catabolism during myocardial ischemia, some signaling mechanisms inadvertently preventing the suppression of RHEB may exist in the presence of HFD feeding. However, the identity of such a mechanism has yet to be elucidated. Since AMP-activated protein kinase (AMPK), a negative regulator of the MTORC1 signaling pathway, is inhibited in the hearts of obese mice during ischemia, it is possible that this is the mechanism responsible for maintained RHEB activation during ischemia. Nevertheless, the presence of other mechanisms cannot be excluded.
Other reports show that expression of SIRT1, a member of the sirtuin family, is reduced in the heart or liver in mice with obesity and metabolic syndrome. Since SIRT1 promotes autophagy through FOXO1 deacetylation/activation, SIRT1 inhibition may be an alternative mechanism through which autophagy is inhibited in obesity/metabolic syndrome. Since autophagy is completely restored by inhibition of MTORC1 in obesity/metabolic syndrome, SIRT1-FOXO1 signaling and RHEB-MTORC1 signaling could act on the same pathway to regulate autophagy. Recent evidence suggests, however, that FOXO1 is activated by long-term feeding of mice with HFD. Thus, the role of the SIRT1-FOXO1 pathway in regulating autophagy in the setting of obesity/metabolic syndrome appears to be more complex than that of the RHEB-MTORC1 pathway.
In obese mice, several autophagic genes are downregulated. These include Atg7, Becn1, Ulk1 and Atg5. Interestingly, it has been shown that restoration of Atg7 normalizes the level of autophagy in the livers of obese mice. We also demonstrated that activation of RHEB-MTORC1 markedly downregulates Atg7 and that restoration of Atg7 rescues autophagy. Thus, MTORC1 inhibits autophagy not only through post-translational mechanisms, such as the phosphorylation of ULK1, but also through transcriptional/translational mechanisms. For example, MTORC1 significantly suppresses the translational activity of TP73/p73, which may promote autophagy through upregulation of autophagic proteins, such as ATG7.
Why are the inadvertent activation of MTORC1 and the consequent inhibition of autophagy detrimental in the presence of myocardial ischemia? Aside from the importance of MTORC1 inhibition and autophagy in mediating the preservation of energy during ischemia, these mechanisms would reduce accumulation of misfolded proteins, which occurs during stress. Forced activation of RHEB-MTORC1, mimicking the conditions in mice fed with HFD, increases expression of ER stress markers, including the pro-apoptotic factor DDIT3/CHOP, during ischemia. A recent paper from Lerman’s group confirmed our results, demonstrating that MTOR is activated, autophagy is inhibited, and DDIT3 accumulates in the heart in the presence of metabolic syndrome. Hotamisligil also showed that autophagy is inhibited in the liver in the presence of obesity, and that this directly causes ER stress.
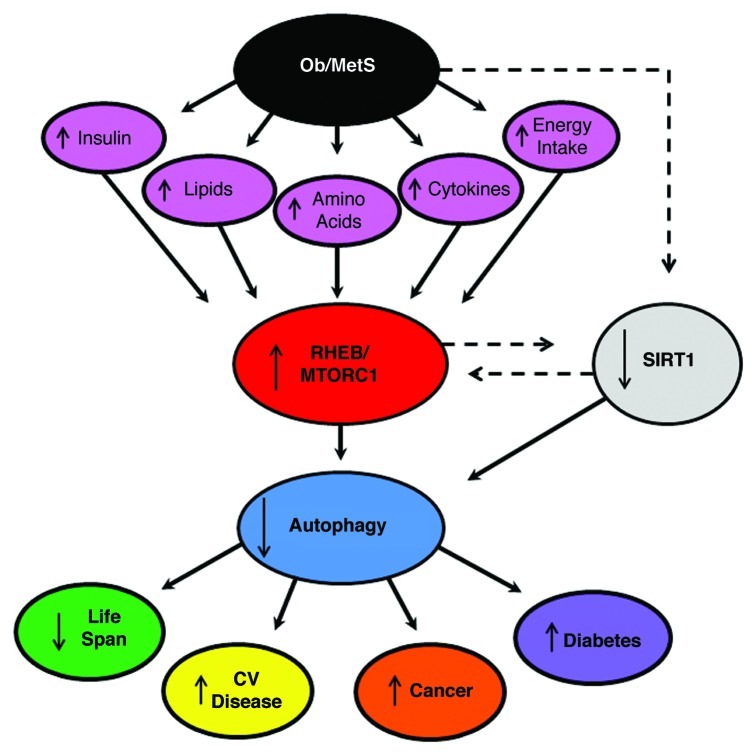
Obesity reduces life span, and promotes the development of cancer, cardiovascular diseases and diabetes. Activation of RHEB-MTORC1 and suppression of autophagy may play a significant role in mediating these events (Fig. 1). Autophagy activation promotes longevity in lower organisms. Autophagy activation also reduces the development of cancer, as does MTORC1 inhibition. Activation of autophagy stabilizes atherosclerotic plaque, thereby preventing its rupture, maintains cardiac function during mechanical overload, and prevents cell death during ischemia. Autophagy is required to preserve the function of pancreatic β-cells, thereby preventing the transition from obesity to diabetes mellitus. Metabolic syndrome is characterized by an increase in ROS production and a reduction in insulin sensitivity. Autophagy mediates the clearance of damaged mitochondria, which in turn reduces ROS. In addition, reactivation of autophagy in the livers of obese animals restores insulin sensitivity. All of these clearly support the tight relationship between the impairment of autophagy and the complications observed in obesity.
Figure 1. Schematic representation of the hypothesis that suppression of autophagy by RHEB-MTORC1 activation promotes the development of complications of obesity and metabolic syndrome (Ob/MetS), namely cardiovascular (CV) disease, cancer, diabetes and reduction in life expectancy.
What is the best treatment to reactivate autophagy in the presence of metabolic syndrome? Obviously, MTORC1 inhibitors, including rapamycin, should be effective in treating obese subjects with acute coronary syndrome. These treatments may also reduce the incidence of cancer in obese subjects and increase their life expectancy. Alternatively, more specific interventions targeting RHEB could be considered, since they may target only the detrimental activation of MTORC1 by RHEB. Because MTORC1 is involved in many cellular functions, chronic inhibition of the entire MTORC1 complex may not necessarily be ideal. Long-term treatment with rapamycin can lead to insulin resistance through MTORC2 disruption, which could be detrimental in obese patients. Alternatively, AMPK and SIRT1 activators may be considered as a way to reactivate autophagy in obese patients. Specific therapies directly modulating the autophagic machinery may also be appropriate. Finally, exercise should be considered for all patients with metabolic syndrome, based upon a recent paper from Levine’s group showing that physical exercise protects obese mice and ameliorates glucose intolerance through activation of autophagy.
Further investigations are warranted to investigate the signaling mechanism through which the RHEB-MTOR pathway is inadvertently activated in animals with obesity and metabolic syndrome and to identify the most effective way to normalize autophagy and prevent ischemic damage in these conditions. It should be noted, however, that attention should also be given to the role of autophagy in frank diabetes (type I and type II), which, according to recent reports, appears to be more complex than metabolic syndrome alone.
Acknowledgment
This work was supported in part by U.S. Public Health Service Grants HL102738, HL67724, HL69020, HL91469, AG23039 and AG27211. This work was also supported by the Foundation Leducq Transatlantic Networks of Excellence. S.S. is supported by a Postdoctoral Fellowship from the Founders Affiliate, American Heart Association. We thank Daniela Zablocki for critical reading of the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/20670