Abstract
The coiled-coil domain of BECN1 serves as a protein interaction platform to recruit two major autophagy regulators ATG14 and UVRAG. Our crystal structure of the BECN1 coiled-coil domain reveals a homodimer with an imperfect dimer interface. This “imperfect” feature favors the formation of a stable BECN1-ATG14 or BECN1-UVRAG heterodimer over a metastable BECN1 homodimer to promote autophagy and/or endocytic pathways.
Keywords: autophagy, BECN1, ATG14, UVRAG, coiled-coil domain
The mammalian BECN1-PIK3C3/VPS34 complex is an essential component of the molecular machinery for macroautophagy (herein referred to as autophagy). PIK3C3 is the class III phosphatidylinositol 3-kinase (PtdIns3K) that specifically produces the membrane lipid phosphatidylinositol 3-phosphate (PtdIns3P). PIK3C3 activity is critical for autophagy because autophagosomes, the vacuolar structures that are synthesized during the autophagic process and a hallmark feature of autophagy, are particularly enriched in PtdIns3Ps.
Our study indicates that BECN1 is a scaffolding protein that facilitates the formation of functionally distinct BECN1-PIK3C3 subcomplexes by recruiting various regulators of PIK3C3 activity. Two major such regulators are ATG14 and UVRAG, which bind BECN1 in a mutually exclusive manner to form ATG14- or UVRAG-containing subcomplexes. While the ATG14-BECN1-PIK3C3 complex is required for autophagosome biogenesis during early-stage autophagy, the UVRAG-BECN1-PIK3C3 complex is important for endocytic trafficking, phagocytosis and autophagy.
The BECN1-ATG14 or BECN1-UVRAG interactions rely critically on the coiled-coil domains found in these three proteins. The coiled coil is a common protein structural motif in which two or more strands of protein α helices, i.e., coils, tightly wrap around each other as driven by the hydrophobic residues at their interface. It was previously shown that BECN1 forms a hetero-oligomeric coiled-coil complex with ATG14 and UVRAG as mediated by their respective coiled-coil domains. Yet the structural basis underlying the specific interaction of BECN1 with ATG14 or UVRAG to regulate PIK3C3-related activities remains unknown.
We have recently solved the structure of the BECN1 coiled-coil domain by X-ray crystallography. The structure reveals a dimeric anti-parallel coiled coil, with two BECN1 molecules wrapped around each other in head-to-tail fashion. A notable feature about the structure is its “imperfect” dimer interface (Fig. 1). That is, a significant number of polar or charged residues occupy the otherwise hydrophobic interface and render the BECN1 homodimer metastable. Such metastability was confirmed by our in vitro biochemical exchange assay, which indicated that the BECN1 coiled-coil domain homodimer readily dissociates and reforms in a temperature-dependent thermodynamic fashion. Thermo-denaturing experiments indicated a “melting” temperature of the homodimer at ~40°C. All these data confirm our structural finding that the BECN1 homodimer is metastable at physiological conditions.
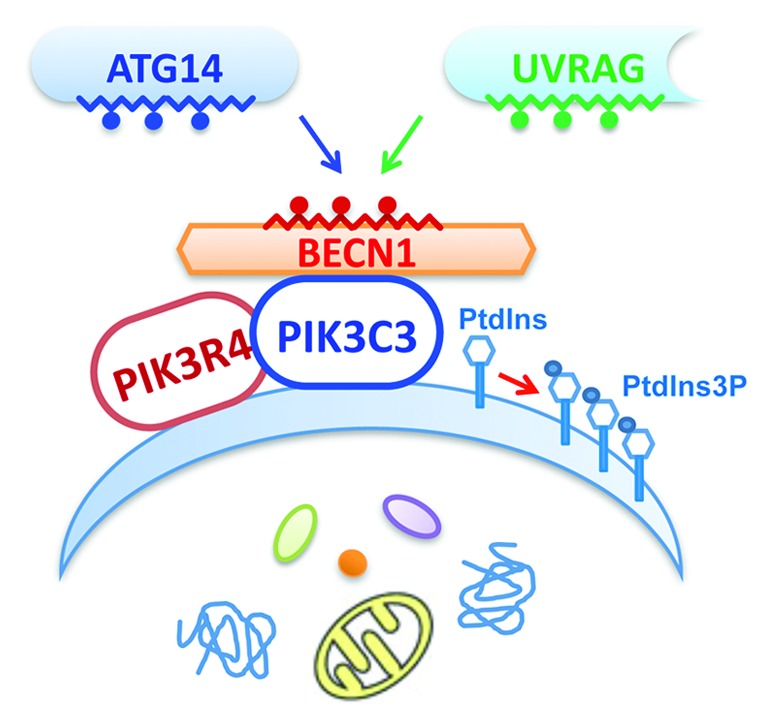
Figure 1. The assembly of ATG14- and UVRAG-containing BECN1-PIK3C3 subcomplexes as mediated by the “imperfect” dimer interface of the BECN1 coiled-coil domain. The BECN1-PIK3C3 complex core unit, consisting of PIK3C3, PIK3R4/VPS15 and BECN1, is recruited onto phagophores or other vesicles to convert PtdIns to PtdIns3P. The coiled-coil domains in BECN1, ATG14 and UVRAG are represented by the zigzag lines. The red-colored spheres on BECN1 represent the “imperfect” residues at its homodimer interface. The blue and green spheres on ATG14 and UVRAG represent the distinct sets of residues at their respective coiled-coil domains that either overcome or positively utilize the “imperfect” feature of BECN1 to form stable ATG14- or UVRAG-containing BECN1-PIK3C3 subcomplexes.
We have also investigated the functional significance of an “imperfect” and metastable BECN1 coiled-coil domain in mediating the BECN1-ATG14 or BECN1-UVRAG interactions. As ATG14- and UVRAG-containing BECN1-PIK3C3 complexes both exist in cells and play critical roles in autophagy and endosomal trafficking, the BECN1-ATG14 and BECN1-UVRAG interactions are expected to be strong. How would ATG14 and UVRAG form a stable complex with BECN1, an “imperfect” partner for coiled-coil assembly?
To address this question we first analyzed the stoichiometry of BECN1-ATG14 and BECN1-UVRAG complexes by analytical ultracentrifugation. The results show that both BECN1:ATG14 and BECN1:UVRAG are stable heterodimers with 1:1 ratio. The association affinities of BECN1- ATG14 and BECN1-UVRAG complexes are 1–2 orders of magnitude stronger than that of the BECN1 homodimer as measured with isothermal titration calorimetry. Interestingly, in both in vitro assays and cultured cells, UVRAG appears to be a stronger binding partner of BECN1 compared with ATG14 and easily competes off ATG14 in binding BECN1. The results indicate that the BECN1-UVRAG subcomplex is a dominant species compared with the BECN1-ATG14 subcomplex under normal conditions. The biophysical and biochemical results from our study confirm that the “imperfect” feature of the BECN1 coiled-coil domain, though rendering the BECN1 homodimer unstable, does not impose a problem for BECN1-ATG14 or BECN1-UVRAG interaction. On the contrary, UVRAG and ATG14 interact strongly with BECN1 in spite of its “imperfect” feature.
To delineate the molecular mechanism for the strong BECN1-ATG14 or BECN1-UVRAG interaction we generated two groups of BECN1 mutants. One group is termed MutM because these mutants have a few “perfect” hydrophobic leucine residues at the homodimer interface modified (changed to alanine) and become mostly monomeric. The second group of mutants is termed MutStab because these mutants have their “imperfect” charged residues at the otherwise hydrophobic dimer interface modified to leucine to stabilize the BECN1 homodimer. Through protein-protein binding assays and co-immunoprecipitation we confirmed that the MutM constructs interact with ATG14 and UVRAG in a manner similar to that of wild type. For MutStab mutants their interaction with ATG14 is almost completely lost, yet, in contrast, their interaction with UVRAG is retained, albeit reduced. Cell imaging studies demonstrate that MutM, but not MutStab mutants, colocalize with ATG14 and autophagosome markers. These studies with the BECN1 coiled-coil mutants indicate that the “imperfect” residues, rather than the “perfect” residues, are critical for mediating the strong BECN1-ATG14 and BECN1-UVRAG interaction. How these “imperfect” BECN1 residues are either overcome or positively utilized by UVRAG or ATG14 to form stable BECN1-ATG14 and BECN1-UVRAG heterodimeric coiled-coil assemblies awaits further studies such as solving the structures of the various protein complexes.
While BECN1 forms stable complex with ATG14 and UVRAG, BECN1 can self-associate when overexpressed in cultured cells. The metastable BECN1 homodimer, incompatible with an ATG14 or UVRAG interaction, could be important in autophagy regulation by mediating the formation of various BECN1-PIK3C3 subcomplexes. Therefore, the central role of BECN1 can be viewed as a protein interaction platform that recruits different PIK3C3 activity modulators. Indeed, multiple BECN1-PIK3C3 subcomplexes exist in the cells, each containing a distinct set of accessory factors and serve specific cellular functions. The homeostatic levels of all subcomplexes may rapidly change in response to cellular metabolic demand. We speculate that the metastable BECN1 homodimers are functionally inactive due to lack of interaction with ATG14 or UVRAG. However, the BECN1 homodimer may serve as a readily available reserve pool to facilitate the formation of new BECN1-PIK3C3 subcomplexes without disrupting the existing BECN1 heterodimer subcomplexes.
One important question is how ATG14 or UVRAG binding to BECN1 regulates PIK3C3 activity that promotes autophagy and other PIK3C3-related processes such as endosomal trafficking and phagocytosis. Future experiments should also investigate whether many previously identified BECN1-binding proteins regulate PIK3C3 activity via ATG14 and UVRAG interactions. Biochemical and structural analysis has begun to tease apart these dynamic and complex protein interactions centralized at BECN1-PIK3C3, which will offer new insight to the understanding of the multiple roles of PIK3C3-related activities and their distinct regulatory mechanisms.
Acknowledgment
This work was supported by Hong Kong Research Grants Council grants PolyU5641/08M (Y.Z.), PolyU5640/11M (Y.Z.), HKUST6/CRF/10 (Y.Z. and M.Z.), CA07/08.SC01 (Y.Z. and M.Z.) and the National Institute of Health Grants R01NS060123–03 NIH/NINDS (Z.Y.) and U54RR022220 NIH (Z.Y.). The authors declare no competing financial interests.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/20750


