Abstract
Aims
To compare the effects of two TNF-α antagonists, etanercept and infliximab, on post-cardiac arrest haemodynamics and global left ventricular function (LV) in a swine model following ventricular fibrillation (VF).
Methods
Domestic swine (n=30) were placed under general anaesthesia and instrumented before VF was induced electrically. After 7 minutes of VF, standard ACLS resuscitation was performed. Animals achieving return of spontaneous circulation (ROSC) were randomized to immediately receive infliximab (5 mg/kg, n=10) or etanercept (0.3 mg/kg [4 mg/m2], n=10) or vehicle (250 mL normal saline [NS], n=10) and LV function and haemodynamics were monitored for 3 hours.
Results
Following ROSC, mean arterial pressure (MAP), stroke work (SW), and LV dP/dt fell from prearrest values in all groups. However, at the 30 minute nadir, infliximab-treated animals had higher MAP than either the NS group (difference 14.4 mm Hg, 95% confidence interval [CI] 4.2–24.7) or the etanercept group (19.2 mm Hg, 95% CI 9.0–29.5), higher SW than the NS group (10.3 gm-m, 95% CI 5.1–15.5) or the etanercept group (8.9 gm-m, 95% CI 4.0–14.4) and greater LV dP/dt than the NS group (282.9 mm Hg/sec, 95% CI 169.6–386.1 higher with infliximab) or the etanercep group (228.9 mm Hg/sec, 95% CI 115.6–342.2 higher with infliximab).
Conclusions
Only infliximab demonstrated a beneficial effect on post cardiac arrest haemodynamics and LV function in this swine model. Etanercept was no better in this regard than saline.
Keywords: cardiopulmonary resuscitation, ventricular fibrillation, post-resuscitation period, inflammatory response, infliximab, etancercept
INTRODUCTION
The post cardiac arrest syndrome is a frequently observed pathophysiological state following the return of spontaneous circulation from cardiac arrest and is characterized by brain injury, myocardial dysfunction, a systemic ischaemia/reperfusion response, and the presence of persistent precipitating pathology, such as coronary ischaemia.1 Inpatient mortality rates following return of spontaneous circulation are as high as 70–80%. The early post cardiac period shares characteristics with the sepsis syndrome.2–4
Inflammatory cytokines appear to play an important role in the pathophysiology of the post cardiac arrest syndrome and have been implicated in the myocardial dysfunction and brain injury observed in the early post arrest period.5–8 The pro-inflammatory cytokine, TNF-α, a master regulator of the inflammatory response, is synthesized and released both locally and systemically following cardiac arrest in response to ischaemia, α-adrenergic stimulation, and lactic acidosis.9 We have previously demonstrated that infliximab, a monoclonal antibody directed against both soluble and transmembrane bound forms of TNF-α, reduces myocardial dysfunction in the early post cardiac arrest period in a swine model of ventricular fibrillation (VF) when administered immediately following the return of spontaneous circulation.10
The purpose of this study was to compare the effects of infliximab on post cardiac arrest myocardial function with those of etanercept, a soluble TNF-α antagonist that acts as a decoy receptor for the cytokine, in a swine model of VF. We hypothesized that etanercept would demonstrate comparable effects to infliximab.
MATERIALS AND METHODS
This study conforms to the American Physiologic Society’s Guiding Principals in the Care and Use of Animals and was approved by the LABioMed Institutional Animal Care and Use Committee.
Thirty male, mixed breed domestic swine (Yorkshire and Yorkshire/Hampshire crossbreed) weighing 39 ± 3 kg were used for this study. Animals were fasted the night before the experiment with ad libitum access to water. Animals were premedicated with intramuscular ketamine (20 mg/kg) and xylazine (2 mg/kg) after which Isoflurane was then administered via nosecone to induce general anaesthesia and animals were then intubated with a standard endotracheal tube. Following endotracheal intubation, anaesthesia was maintained with a combination of inhaled isoflurane (MAC 1.0–2.5%) and nitrous oxide in a 1:1 mixture with oxygen. End-tidal CO2 was monitored via side-stream capnography attached to the endotracheal tube and minute ventilation was adjusted to maintain an end-tidal CO2 at 35–45 mm Hg. Standard lead II of the surface ECG was also monitored during instrumentation and throughout the study protocol.
Animals were placed in the supine position and high fidelity, micro-manometer tipped catheters (Millar Instruments, Houston, TX) were maneuvered into the ascending aorta and left ventricle (LV) via the femoral arteries and into the right atrium (RA) via a jugular vein under fluoroscopic guidance. A thermistor-tipped catheter (Edwards Lifesciences, Irvine, CA) was flow-directed into a branch of the pulmonary artery for thermodilution cardiac output (CO) determinations. A standard pacing catheter was placed in contact with the right ventricular endocardium. Commercially available, standard adhesive defibrillation electrode patches were applied to the left and right lateral aspects of the shaved thorax. A tetrapolar constant current impedance measuring system (THRIM®, Morro Bay, CA) was used to measure transthoracic impedance, after which a small value non-inductive resistor (30Ω) was placed in series with a biphasic defibrillator (LifePak 12, Medtronic Emergency Response Systems, Redmond, WA).
With instrumentation complete, heart rate, aortic and LV pressure, RA pressure, LV dP/dt, and cardiac output (CO) were recorded and arterial blood was analyzed (I-Stat CG8+, I-Stat Corp, Princeton, NJ).
To induce VF, a 1 second pulse of 60 Hz alternating current was passed through the pacing catheter, after which the catheter was withdrawn. Animals were then observed in untreated VF for 7 minutes. After 7 min of untreated VF, mechanical closed-chest compressions (Thumper®, Michigan Instruments, Grand Rapids, MI) were begun with the animal in the supine position and were administered at a rate of approximately 100/min with force sufficient to depress the sternum 1.5 to 2.0 inches. Compression depth was confirmed visually. After one minute of chest compressions, a 200 J transthoracic biphasic shock was delivered. Positive pressure ventilations (FiO2=1.00) were initiated following the first shock at a rate of 8 ventilations/min. For the purpose of these experiments, successful defibrillation was defined as termination of VF, regardless of the postshock cardiac rhythm or haemodynamic outcome, e.g., spontaneous QRS complexes with or without associated arterial pressure pulses, determined 5 sec after a defibrillation shock. If VF persisted or recurred, additional shocks in an escalating energy sequence (300, 360J) were administered with interposed chest compressions. Adrenaline 1 mg was administered if VF persisted after the first three shocks and CPR continued for one to three minutes between repeating shocks at 360 J. Adrenaline was repeated every 3–5 minutes as needed for persistent or recurrent VF or if shocks resulted in pulseless electrical activity (PEA) or asystole. After 30 minutes, animals remaining in VF, PEA, or asystole were considered resuscitation failures and efforts terminated.
For the purposes of these experiments, return of spontaneous circulation (ROSC) was defined as an arterial systolic blood pressure (SBP) of at least 60 mm Hg for > 20 minutes. Animals achieving ROSC immediately received either infliximab, an anti-TNF-alpha monoclonal antibody (5 mg/kg in 250 mL of normal saline, n = 10), etanercept (0.3 mg/kg [4 mg/m2] in 250 mL of NS, n = 10) or control solution (250 mL of NS, n =10), infused through the RA catheter over 30 minutes with assignment to treatment group determined via permuted block design. Rescuers were blinded to treatment allocation. Haemodynamics were monitored throughout the post-ROSC period and blood gas measurements were made at intervals for 3 hours.
Arterial blood was sampled at baseline and at intervals following ROSC and samples placed in sterile, chilled (0° C), heparinized tubes, and centrifuged at 5000 rpm for 10 min. Plasma was separated and refrigerated at −80° C until analysis. TNF-α concentrations were measured using a quantitative sandwich ELISA from commercially available kits specific for this porcine cytokine (R&D Systems, Inc., Minneapolis, MN).
Data Analysis
Data were entered into an Excel Spreadsheet (v. 12.0, Microsoft Corp, Redmond, WA) and imported into SAS statistical software (v. 9.2, SAS Institute, Cary, NC) for analysis. Summary measures are reported as means and standard deviations (SD) or medians and interquartile ranges (IQR), according to the normality of the underlying distribution. Means of prearrest variables were compared using one-way ANOVA and the Mann-Whitney U test was performed to compare distributions of non-normally distributed variables. Generalized linear mixed models were developed to compare indices of LV function and haemodynamics between groups over time during the post-ROSC period. The SAS procedure PROC MIXED was employed for this purpose. Time was entered into the model as a random effect and the treatment assignment as a fixed effect with the assumption of an unstructured covariance matrix. All p values are two sided. When comparing post-ROSC values to controls values within treatment assignments, p values were adjusted using the simulate option in the LSMEANS statement. Due to a right-skewed distribution, TNF-α values were natural log transformed prior to analysis.
RESULTS
Ten animals were assigned to each group (saline, infliximab, etanercept). Prearrest haemodynamic variables were similar between groups (Table 1). No statistically significant differences in important resuscitation variables were found between groups (Table 2).
Table 1.
Pre-arrest variables for the study groups
| Saline (n = 10) | Infliximab (n = 10) | Etanercept (n = 10) | |
|---|---|---|---|
| MAP* (mm Hg) | 85 (11) | 84 (13) | 77.4 (7) |
| LV EDP** (mm Hg) | 4.3 (2.4) | 3.8 (1.1) | 3.2 (2.4) |
| Stroke Work (gm-m) | 49.8 (12.8) | 46.7 (8.1) | 40.7 (9.7) |
| Systolic dp/dt | 1052 (189) | 1222 (250) | 989 (202) |
| Tau (msec) | 32 (5) | 30 (5) | 31 (4) |
| Tumor Necrosis Factor-α (pg/mL) | 55.0 (24–124) | 69.5 (54–108) | 73 (50–137) |
| Interleukin-1β (pg/mL) | 0 (0–0) | 0 (0–0) | 0 (0–103) |
| Interleukin-6 (pg/mL) | 0.5 (0–60) | 13.5 (0–60) | 0 (0–263) |
Values are mean (Standard Deviation) or median (Interquartile Range).
Mean Arterial Pressure
Left Ventricular End-Diastolic Pressure
Table 2.
Resuscitation variables for the study groups
| Saline | Infliximab | Etanercept | |
|---|---|---|---|
| Countershocks to Defibrillate | 1 (1–2) | 2 (1– 2) | 1 (1–2) |
| Ohms | 71 (5) | 71 (6) | 71 (2) |
| Time to ROSC (s) | 129 (44) | 152 (80) | 99 (38) |
Reported as mean (Standard Deviation) or median (Interquartile Range)
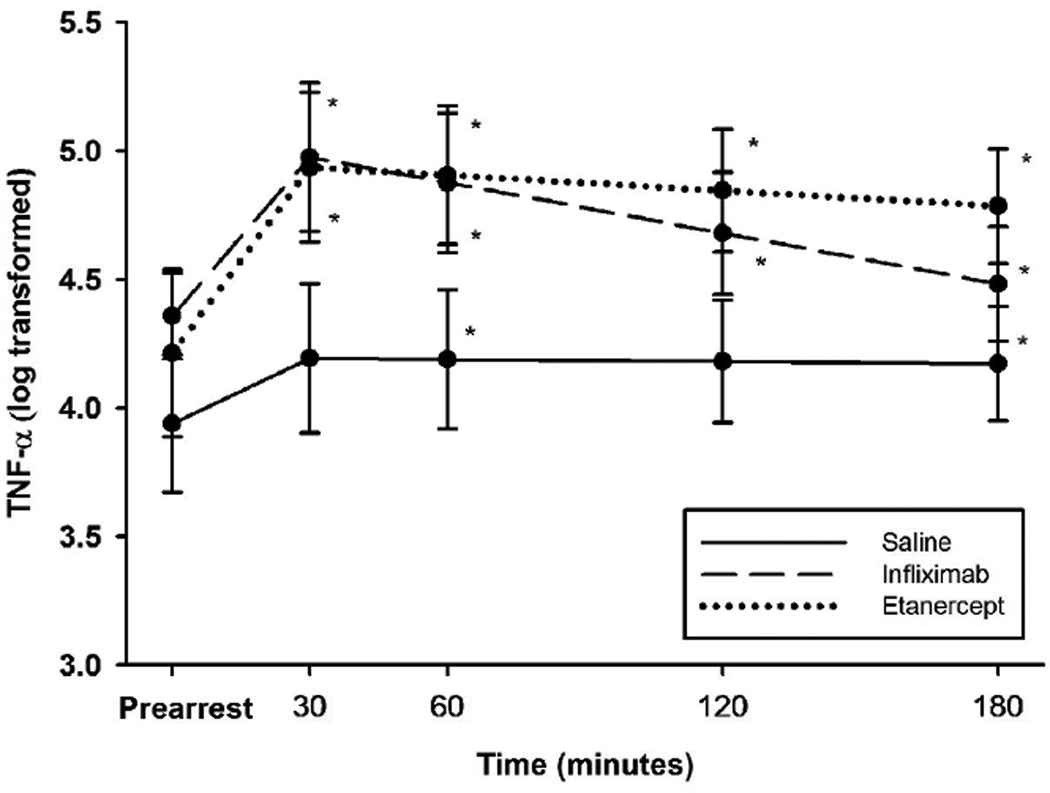
TNF-α levels rose in all groups in the post-ROSC period (Figure 1). There was no statistically significant difference in TNF-α levels between groups at any time point.
Figure 1.
Mean natural log transformed values for TNF-alpha levels between animals administered infliximab, etanercept, or saline in a swine model of ischemic VF (original scale in pg/mL). *Adjusted p<0.05 for comparison with pre-arrest values.
In the post-ROSC period, MAP fell from prearrest values in all groups (Figure 2). However, at the 30 minute nadir, infliximab treated animals demonstrated higher MAP than either the saline group (14.4 mm Hg higher, 95% confidence interval [CI] 4.2–24.7 mm Hg, p=0.0065) or the etanercept group (19.2 mm Hg higher, 95% CI 9.0–29.5 mm Hg, p=0.0004). By 180 minutes, MAP in the infliximab group had largely recovered and was statistically similar to prearrest values (difference 8.7 mm Hg, 95% CI −2 to 19 mm Hg, adjusted p=0.11). However, in the saline and etanercept groups, MAP remained lower than prearrest values.
Figure 2.
Mean arterial pressure (MAP) measured during the first three hours post-ROSC in animals administered infliximab, etanercept, or placebo. a) p<0.05 for infliximab/saline comparison, b) p<0.05 for infliximab/etanercept comparison
SW also fell from prearrest values in the post-ROSC period in all groups (Figure 3). In infliximab-treated animals this decline was blunted, however, remaining 10.3 gm-m higher than saline-treated animals (95% CI 5.1–15.5 gm-m, p=0.0002) and 8.9 gm-m higher than animals in the etanercept group (95% CI 4.0–14.4 gm-m, p=0.0012).
Figure 3.
Mean Left Ventricular Stroke Work (SW) as measured during the first three hours post-ROSC in animals administered infliximab, etanercept, or placebo. a) p<0.05 for infliximab/saline comparison, b) p<0.05 for infliximab/etanercept comparison
LV dP/dt declined less in infliximab-treated animals than in those treated with saline or etanercept post-ROSC (30 min nadir 282.9 mm Hg/sec higher than saline group [95% CI 169.6–386.1 mm Hg/sec, p=0.0002] and 228.9 mm Hg/sec higher than the etanercept group [95% CI 115.6–342.2 mm Hg/sec, p=0.0024]).
Tau, the time constant for LV isovolumic relaxation, rose in all groups post-ROSC (Figure 4). However, the rise in tau was reduced in infliximab-treated animals and was lower than that observed in saline-treated animals by, on average, 27.3 msec (95% CI 6.4–48.2 msec, p=0.01), but was statistically similar to etanercept (12.0 msec lower in infliximab group, 95% CI −4.3 to 28.3 msec, p=0.12). Differences between etanercept and saline groups were not statistically significant.
Figure 4.
Tau measured during the first three hours post-ROSC in animals administered infliximab, etanercept, or placebo. *p<0.05 for differences between infliximab and saline.
DISCUSSION
The results of this study suggest that infliximab is superior to etanercept in ameliorating post cardiac arrest myocardial dysfunction during the early post-ROSC period. The effects observed in swine treated with infliximab was consistent with our prior published experience.10 However, we found no evidence of a significant effect of etanercept on post cardiac arrest haemodynamics or indices of LV function. This suggests that as immune modulators for the treatment of post cardiac arrest myocardial dysfunction, etanercept and infliximab are not exchangeable, at least at the dosing scheme employed.
TNF-α is known to be an important mediator in the progression of contractile dysfunction in the setting of congestive heart failure (CHF) through several mechanisms, including disordered mitochondrial respiration 11, decreased β-adrenergic receptor responsiveness12, impaired calcium homeostasis13, and increased nitric oxide production.14 The precise mechanisms involved in the pathophysiology of acute myocardial dysfunction following resuscitation from cardiac arrest have not been completely elucidated, but likely involve similar derangements. It should be noted, however, that while the post-arrest decline in MAP and LV function is temporally correlated with the observed rise in TNF-α in this and other studies, at least a partial recovery occurs regardless of treatment and despite persistently elevated TNF-α levels, suggesting that TNF-α is not the sole cause of this post-arrest pathology or that a complex counter-regulatory mechanism is also at play.
Early preclinical models and human studies of CHF suggested a beneficial effect of TNF-α antagonists for patients with chronic heart failure that wasn’t observed in subsequent randomized trials.15,16 The reasons for the disparity between trial outcomes and earlier evidence remain the subject of speculation. There are likely significant differences between chronically failing hearts that have undergone adaptive remodeling and those that are acutely decompensated following resuscitation from cardiac arrest. The post-resuscitation TNF-α concentrations in our study greatly exceed values reported in trials of targeted anti-TNF-α therapies in chronic CHF suggesting a greater severity of illness. A review of sepsis therapies suggests that the severity of illness is an important determinant of beneficial response to cytokine directed therapy.17
The etanercept group showed a delayed recovery in MAP, even when compared to saline and was, by almost all other measures, no better than saline. The reason for a lack of observed effect in the etanercept group in our study is uncertain, but may be due to the relatively lower potency of this particular immune modulator. The effective stoichiometry of these agents is such that one or two etanercept monomers bind to three soluble TNF-α (sTNFα) molecules while infliximab forms very large, complex multimers.18 The etanercept/sTNFα complex is also relatively unstable when compared to infliximab and is more likely to dissociate, leaving more free, biologically active sTNF available. Low levels of TNF-α appear to provide protection against acute ischaemia/reperfusion injury, while high levels contribute to contractile dysfunction.19 Thus, differences in effective dose and avidity may explain much of the differences in effect seen. The dose of etanercept used in our study is based upon early work demonstrating efficacy of a 4–5 mg/m2 dose in clinical chronic CHF trials.20,21
If not due to ineffective dosing of etanercept, the differences observed may also be related to differences in blockade of intracellular signal transduction between the two biologics. Infliximab is a significantly more potent inhibitor of the transmembrane form of TNF-α (tmTNFα) than etanercept.22 This transmembrane form participates in cell-to-cell signaling and can also be released by proteolytic cleavage to become sTNFα for paracrine and endocrine signaling. Of the two TNF-α receptors, TNFR1 and TNFR2, TNFR2 is activated primarily by tmTNFα.9 Infliximab would be expected to more effectively reduce both effective sTNFα levels and myocyte TNFR2 signaling than etanercept. However, without information concerning relative pathway signaling in this model, such reasoning is purely speculative at this time. While we have observed consistent, albeit modest, effects with infliximab treatment in our laboratory, the poor response to etanercept suggests that more study is needed to determine the potential contribution of TNF-α to the post-arrest syndrome and the role of anti-TNF-α therapy, if any, in post-arrest care.
While TNF-α inhibition has failed to produce benefits in human studies of ambulatory congestive heart failure patients and in patients hospitalized with severe sepsis, these studies suffer from administration timing that is likely too late in the course of illness to benefit patients. Unlike cardiac arrest, these illnesses have a variable period of latency prior to clinical appearance making them less amenable to early inflammatory response modification.
The results of our study must be interpreted within the context of several limitations. The swine employed in this study lacked any of the significant comorbidities that often co-existent in victims of human cardiac arrest. VF was induced electrically, a model that produces a milder form of post-ROSC myocardial dysfunction and haemodynamic compromise and a smaller rise in inflammatory cytokines than induction of VF under conditions of focal myocardial ischaemia.23,24 It is estimated that focal ischaemia is a persistent precipitating pathology in approximately 50% of patients.25 Male swine were employed in this study due to gender differences in the cytokine response to cardiac arrest observed in this species.26 It is uncertain whether similar differences exist in human populations. We did not induce hypothermia during the early post-arrest period modeled in this investigation. Some evidence suggests therapeutic hypothermia reduces the inflammatory cytokine response27,28 and the interaction between hypothermia and TNF-α blockade is a subject worthy of future study.
This investigation lends further support to the notion that post cardiac arrest myocardial dysfunction is related to the rise in inflammatory cytokines in survivors of cardiac arrest and that selective blockade of TNF-α by infliximab, when administered immediately after ROSC, improves myocardial function and haemodynamics in the swine model. Etanercept failed to demonstrate a similar effect in this model.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors report no conflicts of interest.
REFERENCES
- 1.Neumar R, Nolan J, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Stroke Council. Circulation. 2008;118:2452–2535. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 2.Nadkarni VM, Larkin GL, Peberdy MA, et al. First Documented Rhythm and Clinical Outcome From In-Hospital Cardiac Arrest Among Children and Adults. JAMA. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 3.Nolan JP, Laver SR, Welch CA, Harrison DA, Gupta V, Rowan K. Outcome following admission to UK intensive care units after cardiac arrest: a secondary analysis of the ICNARC Case Mix Programme Database*. Anaesthesia. 2007;62:1207–1216. doi: 10.1111/j.1365-2044.2007.05232.x. [DOI] [PubMed] [Google Scholar]
- 4.Adrie C. Successful Cardiopulmonary Resuscitation After Cardiac Arrest as a "Sepsis-Like" Syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 5.Ito T, Saitoh D, Fukuzuka K, et al. Significance of elevated serum interleukin-8 in patients resuscitated after cardiopulmonary arrest. Resuscitation. 2001;51:47–100. doi: 10.1016/s0300-9572(01)00382-3. [DOI] [PubMed] [Google Scholar]
- 6.Kern K, Berg R, Hilwig R, Larson D, Gaballa M. Myocardial cytokine IL-8 and nitric oxide synthase activity during and after resuscitation: preliminary observations in regards to post-resuscitation myocardial dysfunction. Resuscitation. 2008;77:401–410. doi: 10.1016/j.resuscitation.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niemann J, Garner D. Tumor necrosis factor-α is associated with early postresuscitation myocardial dysfunction. Critical care medicine. 2004;32 doi: 10.1097/01.ccm.0000132899.15242.d3. [DOI] [PubMed] [Google Scholar]
- 8.Youngquist S, Niemann J, Heyming T, Rosborough J. The central nervous system cytokine response to global ischaemia following resuscitation from ventricular fibrillation in a porcine model. Resuscitation. 2009;80:249–301. doi: 10.1016/j.resuscitation.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Kleinbongard P, Schulz R, Heusch G. TNFα in myocardial ischaemia/reperfusion, remodeling and heart failure. Heart Fail Rev. 2011;16:49–69. doi: 10.1007/s10741-010-9180-8. [DOI] [PubMed] [Google Scholar]
- 10.Niemann J, Youngquist S, Rosborough J, Shah A, Phan Q, Filler S. Infliximab attenuates early myocardial dysfunction after resuscitation in a swine cardiac arrest model. Crit Care Med. 2010;38:1162–1169. doi: 10.1097/CCM.0b013e3181d44324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moe GW, Marin-Garcia J, Konig A, Goldenthal M, Lu X, Feng Q. In vivo TNF-α inhibition ameliorates cardiac mitochondrial dysfunction, oxidative stress, and apoptosis in experimental heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1813–H1820. doi: 10.1152/ajpheart.00036.2004. [DOI] [PubMed] [Google Scholar]
- 12.Gulick T, Chung MK, Pieper SJ, Lange LG, Schreiner GF. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc Natl Acad Sci U S A. 1989;86:6753–6757. doi: 10.1073/pnas.86.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao Y-H, Chen Y-C, Cheng C-C, Lee T-I, Chen Y-J, Chen S-A. Tumor necrosis factor-alpha decreases sarcoplasmic reticulum Ca2+-ATPase expressions via the promoter methylation in cardiomyocytes. Crit Care Med. 2010;38:217–239. doi: 10.1097/CCM.0b013e3181b4a854. [DOI] [PubMed] [Google Scholar]
- 14.Song W, Lu X, Feng Q. Tumor necrosis factor-α induces apoptosis via inducible nitric oxide synthase in neonatal mouse cardiomyocytes. Cardiovasc Res. 2000;45:595–602. doi: 10.1016/s0008-6363(99)00395-8. [DOI] [PubMed] [Google Scholar]
- 15.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT Investigators ftA. Randomized, Double-Blind, Placebo-Controlled, Pilot Trial of Infliximab, a Chimeric Monoclonal Antibody to Tumor Necrosis Factor-α, in Patients With Moderate-to-Severe Heart Failure. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 16.Mann DL, McMurray JJV, Packer M, et al. Targeted Anticytokine Therapy in Patients With Chronic Heart Failure. Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 17.Kalil AC, LaRosa SP, Gogate J, Lynn M, Opal SM the Eritoran Sepsis Study Group. Influence of severity of illness on the effects of eritoran tetrasodium (E5564) and on other therapies for severe sepsis. Shock. 2011;36:331–331. doi: 10.1097/SHK.0b013e318227980e. [DOI] [PubMed] [Google Scholar]
- 18.Kohno T, Tam LT, Stevens SR, Louie JS. Binding characteristics of tumor necrosis factor receptor-Fc fusion proteins vs anti-tumor necrosis factor mAbs. J Investig Dermatol Symp Proc. 2007;12:5–8. doi: 10.1038/sj.jidsymp.5650034. [DOI] [PubMed] [Google Scholar]
- 19.Skyschally A, Gres P, Hoffmann S, et al. Bidirectional Role of Tumor Necrosis Factor-α in Coronary Microembolization. Circ Res. 2007;100:140–146. doi: 10.1161/01.RES.0000255031.15793.86. [DOI] [PubMed] [Google Scholar]
- 20.Deswal A, Bozkurt B, Seta Y, et al. Safety and efficacy of a soluble p75 tumor necrosis factor receptor (Enbrel, etanercept) in patients with advanced heart failure. Circulation. 1999;99:3224–3226. doi: 10.1161/01.cir.99.25.3224. [DOI] [PubMed] [Google Scholar]
- 21.Bozkurt B, Torre-Amione G, Warren MS, et al. Results of targeted anti-tumor necrosis factor therapy with etanercept (Enbrel) in patients with advanced heart failure. Circulation. 2001;103:1044–1047. doi: 10.1161/01.cir.103.8.1044. [DOI] [PubMed] [Google Scholar]
- 22.Scallon B, Cai A, Solowski N, et al. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther. 2002;301:418–444. doi: 10.1124/jpet.301.2.418. [DOI] [PubMed] [Google Scholar]
- 23.Niemann J, Rosborough J, Youngquist S, Lewis R, Phan Q, Filler S. The proinflammatory cytokine response following resuscitation in the swine model depends on the method of ventricular fibrillation induction. Acad Emerg Med. 2008;15:939–983. doi: 10.1111/j.1553-2712.2008.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niemann J, Rosborough J, Youngquist S, Thomas J, Lewis R. Is all ventricular fibrillation the same? A comparison of ischemically induced with electrically induced ventricular fibrillation in a porcine cardiac arrest and resuscitation model. Crit Care Med. 2007;35:1356–1417. doi: 10.1097/01.CCM.0000261882.47616.7D. [DOI] [PubMed] [Google Scholar]
- 25.Spaulding CM, Joly L-M, Rosenberg A, et al. Immediate Coronary Angiography in Survivors of Out-of-Hospital Cardiac Arrest. NEJM. 1997;336:1629–1633. doi: 10.1056/NEJM199706053362302. [DOI] [PubMed] [Google Scholar]
- 26.Niemann J, Rosborough J, Youngquist S. Is the tumour necrosis factor-alpha response following resuscitation gender dependent in the swine model? Resuscitation. 2008;77:258–321. doi: 10.1016/j.resuscitation.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meybohm P, Gruenewald M, Albrecht M, et al. Hypothermia and postconditioning after cardiopulmonary resuscitation reduce cardiac dysfunction by modulating inflammation, apoptosis and remodeling. PLoS One. 2009;4:e7588. doi: 10.1371/journal.pone.0007588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meybohm P, Gruenewald M, Zacharowski KD, et al. Mild hypothermia alone or in combination with anesthetic post-conditioning reduces expression of inflammatory cytokines in the cerebral cortex of pigs after cardiopulmonary resuscitation. Crit Care. 2010;14:r21. doi: 10.1186/cc8879. [DOI] [PMC free article] [PubMed] [Google Scholar]