Abstract
Mice are by far the most widely used species for scientific research including many studies involving biopotentials such as electroencephalogram (EEG) and electromyogram (EMG) signals for sleep analysis. Unfortunately, current methods for the analysis of these systems involve either tethered systems that are restrictive and heavy on the animal or wireless systems using transponders that are large relative to the animal and surgically invasive for implantation; thus natural behavior/activity is altered. Herein, we propose an inexpensive novel system for measuring electroencephalographic signals and other biopotentials in mice that allows for natural movement and evaluate it for the analysis of sleep architecture and EEG power during spontaneous sleep and sleep responses after sleep deprivation in mice. Vigilance states of non-rapid eye movement sleep (NREMS), rapid eye movement sleep (REMS), and wakefulness and EEG power and NREMS EEG delta power in the 0.5–4 Hz range (an indicator of sleep intensity) showed the typical diurnal rhythms found in mice using our new system and these values were similar to mice values using telemetry transponders. Mice that used the new system also demonstrated typical enhanced NREMS EEG delta power responses after sleep deprivation and few signal artifacts. Moreover, similar movement activity counts were found in the new system compared to a wireless system. This novel system for biopotential measuring can be used for polysomnography, infusion, microdialysate, and optogenetic studies providing a platform allowing reduced artifacts and a more natural moving environment for more accurate investigation of biological systems and pharmaceutical development.
Keywords: biopotentials, EEG, sleep, mice, transponders, sleep deprivation
1. Introduction
Many experimental studies require sampling biological signals, stimulating the brain or infusing test substances in freely moving mice. Although several forms of tethers are currently available for this purpose, these devices restrain activity in the mouse to some degree. The animal’s mobility is usually reduced even more when several connections are needed with the mouse [i.e., electroencephalogram (EEG)/electromyogram (EMG)] wires, fiber optics and infusion lines). Telemetric devices avoid the tethers, but they still restrict the animal’s movement because they are very heavy for a mouse (i.e., smallest telemetry transponder weighs ~4 g or ~20% of its body weight). It is expected that restraining mouse movement would negatively influence experimental results because waking activity changes various physiological parameters in the mouse. In fact, evidence suggests that sleep is induced through metabolic substances including extracellular adenosine triphosphate and adenosine (Zielinski and Krueger, 2011; Brown et al., 2012). Thus, it is likely that animals that have restricted movement have impaired metabolic-related behavioral responses confounding sleep analysis results.
Non-rapid eye movement sleep (NREMS) EEG delta power (~0.5–4 Hz range) [also called slow-wave activity (SWA)] is generated in thalamo-cortical loops (Brown et al., 2012). SWA is highly positively correlated with enhanced NREMS duration after sleep deprivation (Davis et al., 2012), although the mechanisms that regulate it are not fully understood. Consequently, SWA was used in the development of the two-process model of sleep that involves a homeostatic process (Process S) and a circadian process (Process C) (Borbely, 1982). SWA is altered by pro-inflammatory sleep regulatory substances and substances that induce vasodilation, such as neuronal nitric oxide synthase, that are enhanced from waking activity (Zielinski et al., 2011; Gerashchenko et al., 2011). Thus, natural movement is imperative to accurate biological and behavioral sleep analysis.
A hallmark of accurate sleep analysis is the ability to have clear signals for sleep stage analysis and frequency analysis of the EEG signals. In fact, a big limitation in EEG power analysis occurs from movement artifacts that alter the amplitude of these signals that are typically analyzed by Fast Fourier transformation of the signal. The majority of these biopotential signal artifacts occur during wakefulness. However, movement artifacts also occur during NREMS and rapid eye movement sleep (REMS), and these mostly occur during transition points between sleep and wakefulness when animals jostle cables tethered to the animal or within the animal (such as often occurs in telemetry systems). Signal artifacts lead to more laborious sleep scoring and analysis and limit the number of epochs sampled providing less reliable results and potentially missing valuable data.
In the present study, we developed and tested a new system for biopotential analysis that has the capabilities of allowing multiple sets of different biopotential leads (e.g., multi-channel EEG electrodes) to be connected to the mouse simultaneously and high levels of mobility for the mouse compared to telemetry systems (Fig 1 and 2). Our new system assembly is composed of a counterbalanced lightweight tethered housing that encapsulates a wireless transponder that receives biopotential information such as EEG and EMG signals for sleep analysis.
Fig 1.
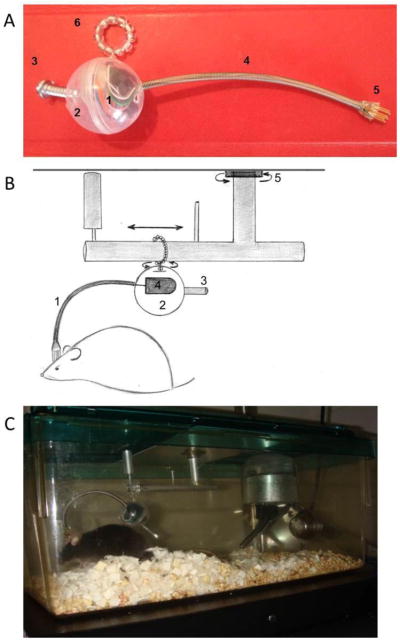
Photograph and schematic of the components of the new system and a photograph of a mouse using the new system to measure biopotentials. (A) (1) Telemetry transponder. (2) Container encapsulating the telemetry transponder. (3). Weighted counterbalance. (4) Flexible protective sheath containing the wires from the telemetry transponder. (5) Pins connecting the wires from the transponder to the EEG/EMG electrodes on the head of mouse. (6) O-ring holding the aforementioned components that rotates 360 degrees and slides on a metallic bar. Thus, this apparatus minimizes the weighted pressure of the cable on the animal and does not restrict mobility. (B) The mouse head cap containing EEG/EMG electrodes is connected to a telemetry transponder (4) encapsulated in a protective covering (2) by short cables contained in a lightweight protective sheath (1). This system is counterbalanced (3), rotates on an O-ring that swivels 360 degrees and slides horizontally between two contained ends, and possesses an additional swivel allowing the maximal range of movement within the mouse housing (5). (C) Photograph of a mouse using the new system.
Fig 2.
(A and B) Videos of mouse using the new system for measuring biopotentials.
This system fits into most standardized animal caging, has a rotary and sliding joint, and a counterbalance that allows the animal to move freely throughout the housing chamber (Fig 1 and 2). Our aim was to develop and validate a novel system that is less surgically invasive than wireless systems and does not restrict movement for polysomnography analysis of sleep state and EEG spectral power analysis during spontaneous sleep, sleep deprivation, and sleep rebound responses after sleep deprivation.
2. Methods
2.1 Animals
Twelve 8–11 week old male C57Bl/6J mice (Jackson Laboratories, Bar Harbor, ME, USA) were used for the following experiments. All mice were individually housed and maintained on a 12:12 h light/dark cycle [light onset, Zeitgeber time (ZT) = 0 h] under ambient temperatures (22 ± 3 C°) and humidity (40–50%). Mice were provided food and water ad libitum. All experimental protocols were approved by Harvard University and Veteran Affairs Boston Healthcare system Animal Care and Use Committee and were in compliance with the National Institutes of Health guidelines.
2.2 New System for measuring biopotentials in rodents
Diagrams, pictures, and video of the new system are presented in Fig 1–2. We used a round lightweight protective plastic housing enclosure (weight: 2 grams; diameter 27 mm) that contained a wireless transponder [F20-EET transponders (Data Sciences International, St. Paul, MN, USA)] (total weight: 4.5 grams) (Fig 1A and 1B). The transponder contained wire leads that were designed for EEG/EMG signals that were implanted into the animal. The wires were housed in a flexible protective sheath. We used these wires to connect the transponder to a pedestal (Plastics One, Roanoke, VA) that attached the EEG/EMG electrodes (the animal surgeries are described below in section 2.3). The transponder enclosure contained an insert that was comprised of an O-ring that slides bidirectionally on an L-shaped bar that rotated 360 degrees. This insert was placed into standardized commercially available mouse caging (Fig 1C). However, this insert can easily be altered or manufactured to insert into most animal caging. Further, the transponder enclosure and connecting biopotential leads were counterbalanced with a length adjustable screw thereby dismissing weighted pressure to the head of the mouse. Thus, the movement of the tethered animal was not restricted (Fig 2). Additionally, a chew guard, water bottle (5 fluid oz. capacity), and water bottle holder (Petco, San Diego, CA, U.S.A.) were placed in the corner of a standard mouse housing cage to provide water ad libitum and were used to allow the swivel to not be impeded upon rotation (Fig 1C).
2.3 Surgery
Mice were randomly assigned to either a group that had telemetry transponders (Data Sciences International, St. Paul, MN, USA) internally implanted (i.e., wireless system) or a group that used our new biopotential recording system (i.e., new system). All mice that underwent surgeries for polysomnographic analysis using the wireless system and new system were used in these experiments and were included in these data. Surgical procedures for polysomnography analysis were performed under isoflurane gas anesthesia for all mice. Six mice were internally implanted into the peritoneal cavity with F20-EET transponders (Data Sciences International, St. Paul, MN, USA) as described by the manufacturer. Briefly, mice using the wireless system were implanted with an EEG electrode over the left parietal cortex (−1 mm from bregma and 1 mm left from central) and a reference electrode over the cerebellum (−0.5 from lambda placed centrally) that were fixed to the skull with dental cement (Paxinos et al., 2004). Additionally, two EMG electrodes were sutured into the nuchal muscles for muscle activity. A separate group of six mice were implanted with an EEG electrode over the left parietal cortex, a reference electrode over the cerebellum, and two EMG electrodes in the nuchal muscles. These electrodes were mounted to the mice heads with dental cement and the mice were tethered to the transponders via a pedestal as described below (Fig 1C and 2).
2.4 Biopotential analysis
Mice that used either the wireless system or new system groups were placed on receiver plates (PhysioTel receiver RPC-1; Data Sciences International, St. Paul, MN, USA) that detected an FM signal specific to each individual mouse transponder in a wireless system or new system. The transponder frequencies and EEG, EMG, and movement signals were detected by two receiving antennae that are positioned at right angles that lessen signal loss from movements around the receiver plate. The receiver plate was connected to a data exchange matrix (Data Sciences International, St. Paul, MN, USA) that automatically forwarded the biopotential data to the Dataquest A.R.T. system (Data Sciences International, St. Paul, MN, USA). EEG, EMG, and movement signals were amplified and recorded (500 Hz sampling rate) using the Dataquest A.R.T. system (Data Sciences International, St. Paul, MN, USA). EEG signals were converted to European data files (.EDF files) and then filtered below 0.1 Hz and above 40 Hz and EMG signals were analyzed in 10 second epochs using SleepSign Software (Kissei Comtec Co., Ltd., Japan). Vigilance states of NREMS, REMS, and waking were determined manually off-line as previously described (Zielinski et al., 2012b). NREMS was identified by high-amplitude EEG signals and low EMG activity. Regular low-amplitude EEG and minimal EMG activity characterized REMS. Wake periods were recognized by low amplitude fast EEG and high EMG activity. Vigilance state durations were calculated in 1 h time blocks. Vigilance state episode durations and frequencies were calculated in 12 h light and dark time blocks. Fast Fourier transformations of EEG signals (μV2) within each epoch were utilized for NREMS EEG SWA (0.5–4 Hz) power analysis and EEG power spectra in 0.5 Hz bins in the frequency range of 0.5–20 Hz for 24 h periods. EEG SWA was determined as a percentage of total EEG SWA across a 24 h period in 2 h time bins for each individual mouse. Power spectra analysis was normalized as a percentage of power spectra within the 0.5–20 Hz range. Power spectra analysis was determined during light and dark periods for NREMS, REMS, and waking. Further, power spectra analysis of NREMS EEG was determined in the first two hours after sleep deprivation (i.e. ZT 5–6 h) compared to spontaneous time-of-day measures—a time when NREMS EEG power is enhanced in mice post-sleep deprivation. Additionally, NREMS EEG power responses during the negative rebound in NREMS EEG delta power (i.e., 8–20 h post-sleep deprivation; ZT 12-0 h) compared to spontaneous sleep during identical time-of day measures were analyzed.
2.5 Spontaneous sleep and sleep responses after sleep deprivation
Mice were given at least seven days of recovery from the surgical procedure and a two day acclimation period. Spontaneous sleep was recorded continuously for 24 h. Mice were sleep deprived by the gentle handling method as previously described (Zielinski et al., 2012b). Sleep deprivation occurred continuously for 4 h at the beginning of light onset (ZT 0–4 h)—a time when sleep pressure is high. Recovery sleep was recorded for 24 h immediately after sleep deprivation.
2.6 Statistics
Light and dark period diurnal differences in spontaneous NREMS and REMS durations, NREMS and REMS episode frequency and episode durations, and NREMS EEG SWA between the two recording systems were analyzed using 2-way analysis of variance (ANOVA). Spontaneous NREMS, REMS, and waking EEG power spectrum were analyzed with 3-way ANOVA [independent factors: wireless system vs. new system and frequency (0–20 Hz); repeated factor: light period vs. dark period]. NREMS and REMS duration and NREMS EEG SWA during or after SD were analyzed by 3-way ANOVA (independent factor: wireless system vs. new system; repeated factor: spontaneous sleep vs. sleep deprivation or recovery sleep, time) in 2 h time blocks. NREMS and REMS episode durations and episode frequencies during or after sleep deprivation were analyzed using 3-way ANOVA (repeated factors: wireless system vs. new system, light period vs. dark period; repeated factor: spontaneous sleep vs. sleep deprivation or recovery sleep). NREMS EEG power spectrum responses after sleep deprivation were analyzed by 3-way ANOVA [independent factor: wireless system vs. new system, frequency (0–20 Hz); repeated factor: spontaneous sleep vs. recovery sleep]. Motion activity counts were determined by 2-way ANOVA [light period vs. dark period or baseline vs. sleep deprivation or baseline vs. sleep recovery]. Post-hoc comparisons were made with independent or paired t-tests when appropriate. Significance was set at p < 0.05.
3. Results
3.1 Spontaneous Sleep
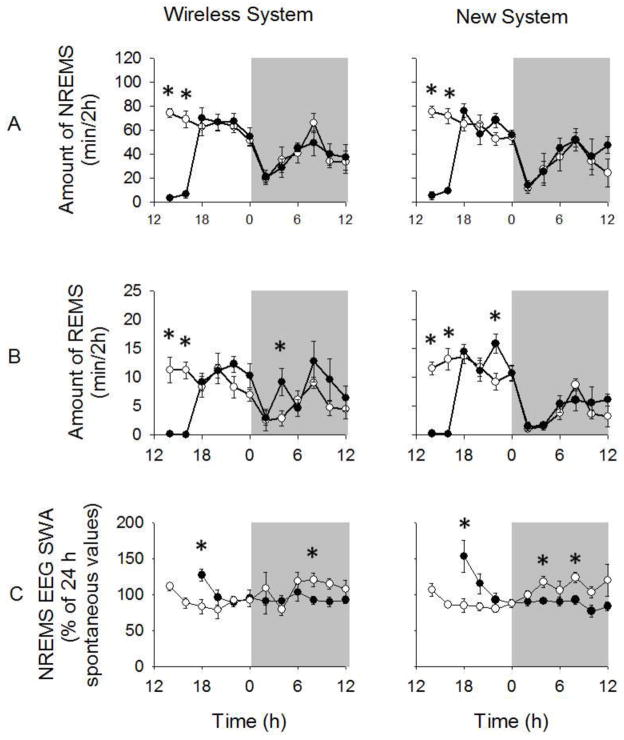
NREMS, REMS, and waking EEG and EMG signals in the new system were similar to the wireless system and representative signals from both recording system are shown in Fig 3. A diurnal rhythm in NREMS duration was found with greater NREMS durations occurring during the light period compared to the dark period for both mice that underwent surgery for implanted wireless telemetry transponder system and those that were used for the new system [F (1, 10) = 73.038, p < 0.001](Fig 4A). The diurnal variation in NREMS duration occurred, in part, from enhanced NREMS episode frequencies during the light period [(wireless system: 165.17 ± 26.36; new system: 189.83 ± 23.62) compared to dark period (wireless system: 99.67 ± 19.30; new system: 98.33 ± 19.36; F (1, 10) = 31.351, p < 0.001]. Nevertheless, no significant diurnal variations in NREMS episode duration were found for either recording system during the light (wireless system: 2.62 ± 0.38 min; new system: 2.27 ± 0.45 min) or dark periods (wireless system: 2.57 ± 0.31 min; new system: 2.11 ± 0.34 min). No significant differences in spontaneous NREMS duration were found between the mice in the two recording systems during the light or dark periods.
Fig 3.
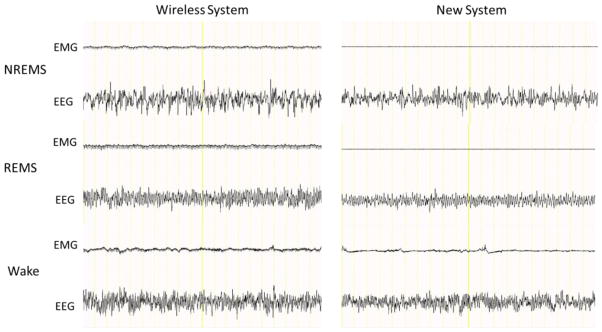
Representative EEG and EMG signals (20 seconds total) from mice using the wireless system and the new system. Mice in both systems exhibited high-amplitude EEG signals and low EMG activity during NREMS, regular low-amplitude EEG and little EMG activity during REMS, and low amplitude fast EEG and high EMG activity during wake periods.
Fig 4.
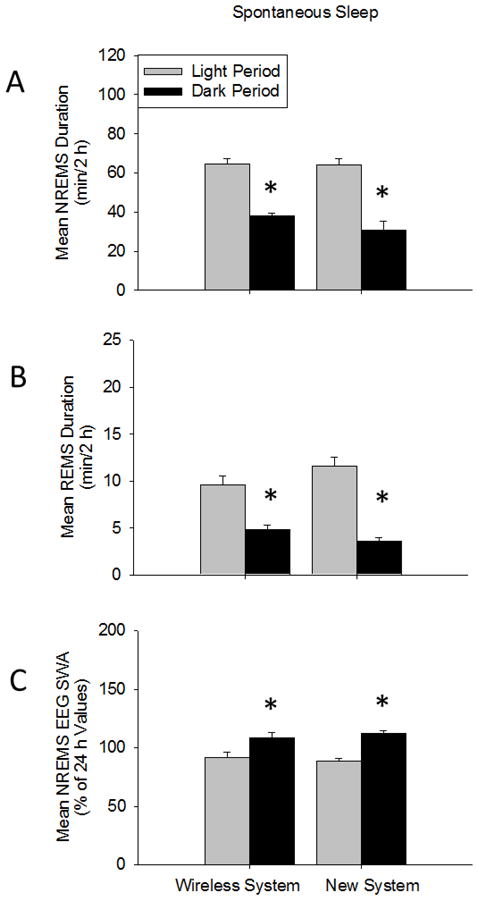
NREMS and REMS duration and NREMS EEG SWA during light and dark periods during spontaneous sleep using both recording systems. (A and B) Diurnal rhythms of enhanced NREMS and REMS duration during the light period compared to the dark period were found for mice in both systems. (C) Further, mice in both systems exhibited the typical diurnal rhythm of enhanced NREMS EEG SWA occurring during the dark period vs. light period. NREMS and REMS duration and NREMS EEG SWA were similar between mice in the two systems. (*) = significant difference between light and dark periods. Significance was set at p < 0.05.
Spontaneous REMS duration exhibited a diurnal rhythm with greater amounts of REMS duration occurring during the light vs. dark period for mice in both recording systems [F (1, 10) = 80.840, p < 0.001](Fig 4B). This effect was evident with enhanced numbers of REMS episode frequencies occurring during the light period (wireless system: 57.83 ± 5.17; new system: 78.33 ± 12.10) compared to the dark period [wireless system: 30.50 ± 4.36; new system: 25.17 ± 2.99; F (1, 10) = 38.419, p < 0.001]. No significant differences in spontaneous REMS episode durations were found between systems during light (wireless system: 0.98 ± 0.03 min; new system: 0.93 ± 0.09 min) or dark periods (wireless system: 0.99 ± 0.08 min; new system: 0.90 ± 0.12 min). Spontaneous REMS durations were similar between mice in both recording systems during light and dark periods.
Spontaneous NREMS EEG SWA (0.5–4 Hz range) exhibited a diurnal rhythm with greater NREMS EEG SWA occurring during the dark vs. light period [F (1, 10) = 220.679, p < 0.001](Fig 4C). No significant differences in NREMS EEG SWA were found between the treatment groups. NREMS EEG power (0.5–20 Hz range) was greater during the dark period compared to light period for mice in both systems [F (1, 400) = 74.633, p < 0.001](Supplementary Fig 1A), although the enhanced NREMS EEG power in the dark vs. light period occurred mostly in the delta power range [F (39, 400) = 6.299, p < 0.001]. NREMS EEG power was similar between treatment groups. A main REMS EEG power (0.5–20 Hz) effect was found during the dark period compared to the light period for both systems [F (1, 400) = 8.222, p = 0.004](Supplementary Fig 1B). Waking EEG power values were similar between the light period and dark periods for mice in both systems (Supplementary Fig 1C).
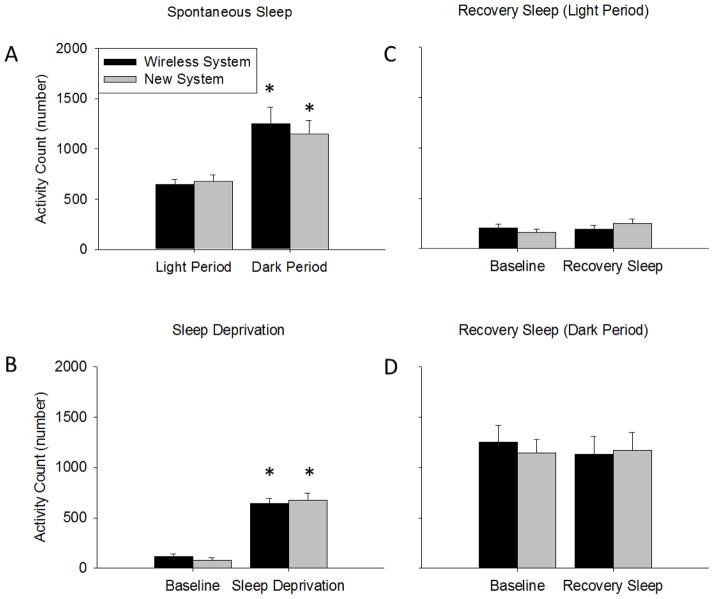
The number of motion detected activity bouts occurring during spontaneous sleep recordings exhibited a diurnal rhythm with greater activity occurring during the dark period compared to the light period [F (1, 10) = 26.490, p < 0.001] (Fig 5A). No significant differences in activity bouts occurring during spontaneous sleep recordings were found between mice in the two recording systems.
Fig 5.
Motion detected activity counts. (A) Mice using both recording systems both had enhanced activity counts during the dark period compared to the light period. (B) During the sleep deprivation procedure mice in both systems had enhanced activity counts compared to time-of-day matched baseline spontaneous sleep times. (C and D) Activity counts during recovery sleep after sleep deprivation were similar to baseline values. Activity counts were similar between mice in the two recording systems. (*) = significant difference between light and dark periods or baseline and sleep deprivation or recovery sleep. Significance was set at p < 0.05.
3.2 Sleep Deprivation
Sleep deprivation using the gentle handling method inhibited NREMS duration during the sleep deprivation procedures compared to spontaneous values [F (1, 10) = 319.182, p < 0.001; wireless (−133.53 ± 10.58); new system (−132.36 ± 10.46)](Fig 6A). However, only a few bouts of NREMS occurred during the four hour sleep deprivation protocol in both the wireless system and tethered systems (15.50 ± 4.57 and 32.50 ± 4.25, respectively). These bouts were very short in episode duration for both the wireless and new system (0.53 ± 0.09 min and 0.41 ± 0.09 min, respectively). The amounts of NREMS during sleep deprivation did not differ between animals in the different recording systems.
Fig 6.
NREMS and REMS duration and NREMS EEG SWA responses during spontaneous sleep and after sleep deprivation. (A and B) NREMS and REMS duration were reduced during the 4 h sleep deprivation procedures compared to spontaneous sleep responses for mice in both systems (p < 0.001). Mice in both systems exhibited NREMS and REMS durations after sleep deprivation that were largely similar to spontaneous values. (C) Enhanced NREMS EEG SWA during the first 2 h post-sleep deprivation and a negative rebound in NREMS EEG SWA were found compared to spontaneous sleep values for mice in both systems (p = 0.005 and p = 0.002, respectively). White background = light period; gray background = dark period. White circles = spontaneous sleep; black circles = responses during and after sleep deprivation. (*) = significant difference between spontaneous sleep and sleep responses after sleep deprivation. Significance was set at p < 0.05.
Sleep deprivation also inhibited REMS duration during sleep deprivation procedures compared to spontaneous values [F (1, 10) = 150.221, p < 0.001; wireless system: −22.31 ± 2.55 min; new system: −24.31 ± 2.82 min](Fig 6B). Only one mouse in each sleep recording system entered REMS during the sleep deprivation period (wireless system: 0.17 ± 0.17 min; new system: 0.67 ± 0.67 min) and the bouts were extremely short (wireless system: 0.11 ± 0.11 min; new system: 0.05 ± 0.05 min). No significant differences in REMS duration were found between mice in the two recording systems during sleep deprivation procedures.
Mice undergoing both recording conditions exhibited greater motion detected activity bouts during sleep deprivation procedures compared to time matched spontaneous sleep values [F (1, 10) = 157.467, p < 0.001; change in activity bouts—wireless system: + 528.5 ± 49.3; new system: + 594.5 ± 74.7](Fig 5B). However, the number of activity bouts during the sleep deprivation protocol was similar in mice in both recording systems.
3.3 Sleep deprivation recovery
No significant differences in NREMS duration were found during recovery sleep after sleep deprivation for mice for either recording system compared to spontaneous sleeping values (Fig 6A). Neither were there any significant differences in REMS duration during the recovery sleep period compared to spontaneous values (Fig 6B).
NREMS EEG SWA was enhanced in the first 2 h of sleep recovery after sleep deprivation compared to spontaneous values in both mice that used the wireless and new systems [F (1, 10) = 12.828, p = 0.005](Fig 6C). No significant differences in NREMS EEG SWA were found between mice using the new system compared to the wireless system during this time. A negative rebound in NREMS EEG SWA was found 8–20 h post-sleep deprivation (i.e., ZT 12-0 h) for mice in both treatment groups [F (1, 10) = 17.639, p = 0.002]. This negative rebound effect did not significantly differ between mice in the two recording systems.
A main effect of sleep deprivation enhancing NREMS EEG power during the first 2 h post-sleep deprivation (i.e., ZT 5–6 h) compared to spontaneous sleep values was found for mice in both recording systems [F (1, 400) = 61.285, p < 0.001](Supplementary Fig 2A). This enhancement in NREMS EEG power 2 h post-sleep deprivation occurred mostly in the delta power range [F (39, 400) = 4.379, p < 0.001]. Mice in both systems had attenuated NREMS EEG power 8–20 h post-sleep deprivation (i.e., ZT 12-0 h) compared to spontaneous sleep values [F (1, 400) = 131.138, p < 0.001](Supplementary Fig 2B). This negative rebound in NREMS EEG power during recovery sleep occurred mostly in the delta power range. Positive and negative rebound NREMS EEG power responses to sleep deprivation were similar for mice in both systems.
No significant differences in motion detected activity bouts were found during recovery sleep light or dark periods compared to the time-of-day matched period during spontaneous sleep for mice in either recording system (Fig 5C and 5D). Further, activity bouts during recovery sleep light and dark periods were similar between mice in both systems.
4. Discussion
Herein, we developed a novel, simple, easy to use system to measure biopotentials in freely moving mice. Using our new system, mice exhibited the diurnal rhythms in sleep states and NREMS EEG SWA that are typically found in mice (Olivadoti and Opp, 2008; Zielinski et al., 2012b). Further, enhanced NREMS EEG SWA after sleep deprivation was found in mice using the new system as expected. Overall, this new system for analyzing biopotentials in mice was similar to the wireless system. The new system exhibited fewer EEG artifacts allowing for enhanced data sampling and reduced EEG power variability. Moreover, our new system was far less surgically invasive and allowed for free range in movements as assessed by similar activity counts to the wireless system. These findings are consistent with the idea that more “normal” activity behaviors can lead to more accurate experimental biopotential results.
Spontaneous NREMS and REMS durations were similar during the light and dark periods using both systems and were within ranges reported in other studies involving C57BL/6 mice (Olivadoti and Opp, 2008; Zielinski et al., 2012b). We found similar NREMS and REMS episode duration and frequencies between mice in both recording systems suggesting that the new system is indeed adequate in assessing these measures. Sleep episode frequencies and episode durations can indicate valuable information about fragmented sleep and insights into sleep responses to various experimental treatments or disease models. Nevertheless, a major limitation of episode frequency and duration analysis is that one sleep state scored epoch can divide particular sleep state episode duration in half skewing the results. The majority of EEG signal artifacts occur during state transition leading to a higher propensity of invalid sleep state scored epochs at these times. Thus, the validity of this variable can be influenced by the quality of the signal and makes the validity of this variable questionable. Further, mice using the new system demonstrated typical EEG power and NREMS EEG SWA patterns as previously reported in mice (Olivadoti and Opp, 2008; Zielinski et al., 2012b). Collectively, these findings suggest that our new system is a viable method for sleep state analysis in mice.
Enhanced waking activity in the form of sleep deprivation in animals is a tool of sleep research to investigate both mechanisms of sleep and behavioral responses of prolonged wakefulness. In rodents, many techniques have been used to deprive sleep including mechanical discs and wheels (Bergmann et al., 1989; Zielinski et al., 2012a; Kim et al., 2012), rotating bars (Naylor et al., 2012), motorized treadmills (Xu et al., 2010), or the introduction of novel objects (Nelson et al., 2010). However, the most widely used and validated method for acutely deprive sleep is the gentle handling method. In mice, the gentle handling method has been previously reported to almost completely inhibit sleep (Hasan et al., 2012), which is consistent with our current findings using both the wireless telemetry and new system. Moreover, the sleep deprivation-induced enhancements in NREMS EEG SWA responses followed by a negative rebound that were exhibited in mice using the new system are consistent with previous studies indicating that using this new system mice exhibit typical sleep measure responses to sleep deprivation (Zielinski et al., 2012b).
Current biopotential systems often utilize rotary electrical switches (also known as commutators) that are very expensive and often do not rotate when the animal twists and moves due to the heavy weight of long cable relative to the mouse size. That the new system is counterbalanced, uses very short cables, and has multiple rotational/sliding joints thereby reduces strain on the animal and allows the animal to move freely. Mice in our new system generated similar activity counts relative to the wireless system suggesting that this apparatus allows relatively free movement and activity patterns. To our knowledge the smallest EEG/EMG telemetry transponders currently available are the F20-EET produced by DSI international, which is very large compared to the mouse, occupies much of the peritoneum, and uses signal wires connecting the implanted transponder to the brain and muscle potentially altering behavior. Additionally, the surgical procedure for EEG/EMG telemetry systems is more invasive and takes longer to complete than the procedure using our new system.
Our presented system has several advantages over tethered and wireless telemetry systems (Table 1). For instance, it can be inserted into most standardized mouse housing cages and easily modified to fit into non-traditional caging. This system was designed specifically for mice to eliminate added weighted pressure and impairing natural mobility induced on mice. This new system allows for the fusion of drugs or sampling of microdialysates without restricting movement, if a miniature pump is added to the protective plastic housing enclosure. Our system can also be applied for laser light delivery via fiber optic rotary joints. Our system reduces experimental artifacts, especially in the active wake periods thereby providing for more reliable sampling that cannot be achieve by current methodologies. Moreover, this system can be applied to other species including but not limited to rats, rabbits, guinea pigs, hamsters and pups. In conclusion, we have developed a biopotential system for recording sleep in mice that can be applied to most sleep and neuroscience applications.
Table 1.
Comparison of sleep recording systems in mice.
| Tethered System | Wireless System | New System | |
|---|---|---|---|
| Commutator | Needed | Not needed | Not needed |
| Housing cages | Specialized | Standard | Standard |
| Weighted pressure on the mouse | Present (caused by tether) | Present (caused by transponder) | Minimal |
| Surgical invasiveness | Minimal | Large | Minimal |
| Typical surgery duration | Less than 30 min | More than 30 min | Less than 30 min |
| Duration of EEG/EMG recordings | Unlimited | Limited (batteries allow continuous use of transponder for ~2 weeks) | Unlimited (transponder batteries can be changed during experiments) |
| EEG/EMG telemetry transponders | Not used | Mouse transponder (~4g) | Either mouse or a heavier rat transponder can be used |
| Core body temperature analysis | No | Yes | No |
| Motion detection | No | Direct | Indirect |
Disadvantageous features of the systems are shown in bold.
Supplementary Material
Highlights.
We developed a new system for measuring biopotentials in freely moving animals.
This new system is easy to use, inexpensive, and non-restrictive.
Surgical invasiveness is reduced with our new system compared to telemetry systems.
Seep and activity responses using the new system are similar to wireless systems.
Acknowledgments
This work was funded by the National Institutes of Health (NINDS, R01NS064193) awarded to Dr. Dmitry Gerashchenko.
Footnotes
Conflict of interest
Dr. Mark R. Zielinski and Dr. Svetlana A. Karpova have no conflicts of interest to disclose. Dr. Ludmila Gerashchenko, CEO of Neurotargeting Systems, Inc., filed a provisional patent application for the new system. Dr. Dmitry Gerashchenko has part-time employment at Neurotargeting Systems, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergmann BM, Kushida CA, Everson CA, Gilliland MA, Obermeyer W, Rechtschaffen A. Sleep deprivation in the rat: II. Methodology Sleep. 1989;12:5–12. doi: 10.1093/sleep/12.1.5. [DOI] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1528–36. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Davis CJ, Clinton JM, Jewett KA, Zielinski MR, Krueger JM. Delta wave power: an independent sleep phenotype or epiphenomenon? J Clin Sleep Med. 2011;7:S16–8. doi: 10.5664/JCSM.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraschchenko D, Wisor JP, Kilduff TS. Sleep-active cells in the cerebral cortex and their role in slow-wave activity. Sleep Biol Rhythms. 2011;9:71–7. doi: 10.1111/j.1479-8425.2010.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S, Dauvilliers Y, Mongrain V, Franken F, Tafti M. Age-related changes in sleep in inbred mice are genotype dependent. Neurobiology of Aging. 2012;33:13–26. doi: 10.1016/j.neurobiolaging.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bolortuya Y, Chen L, Basheer R, McCarley RW, Strecker RE. Decoupling of sleepiness from sleep time and intensity during chronic sleep restriction: evidence for a role of the adenosine system. Sleep. 2012;35:861–9. doi: 10.5665/sleep.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor E, Aillon DV, Barrett BS, Wilson GS, Johnson DA, Johnson DA, Harmon HP, Gabbert S, Petillo PA. Lactate as a biomarker for sleep. Sleep. 2012;35:1209–22. doi: 10.5665/sleep.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Faraguna U, Tononi G, Cirelli C. Effects of anesthesia on the response to sleep deprivation. Sleep. 2010;33:1659–67. doi: 10.1093/sleep/33.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivadoti MD, Opp MR. Effects of I.C.V. administration of interleukin-1 on sleep and body temperature of interleukin-6-deficient mice. Neuroscience. 2008;153:338–48. doi: 10.1016/j.neuroscience.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. In: Franklin KBJ, editor. Gulf Professional Publishing. University of New South Wales; Sydney, Australia: 2004. [Google Scholar]
- Xu A, Sakurai E, Kuramasu A, Zhang J, Li J, Okamura N, Zhang D, Yoshikawa T, Watanabe T, Yanai K. Roles of hypothalamic subgroup histamine and orexin neurons on behavioral responses to sleep deprivation induced by the treadmill method in adolescent rats. J Pharmacol Sci. 2010;114:444–53. doi: 10.1254/jphs.10177fp. [DOI] [PubMed] [Google Scholar]
- Zielinski MR, Davis JM, Fadel JR, Youngstedt SD. Influence of chronic moderate sleep restriction and exercise on inflammation and carcinogenesis in mice. Brain Behav Immun. 2012a;26:672–9. doi: 10.1016/j.bbi.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski MR, Krueger JM. Sleep and innate immunity. Front Biosci (Schol Ed) 2011;3:632–42. doi: 10.2741/s176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski MR, Taishi P, Clinton JM, Krueger JM. 5′-Ectonucleotidase-knockout mice lack non-REM sleep responses to sleep deprivation. Eur J Neurosci. 2012b;35:1789–98. doi: 10.1111/j.1460-9568.2012.08112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.