Abstract
Within the immunosuppressive ocular microenvironment, there are constitutively present the immunomodulating neuropeptides alpha-melanocyte stimulating hormone (α-MSH) and Neuropeptide Y (NPY) that promote suppressor functionality in macrophages. In this study, we examined the possibility that α-MSH and NPY modulate phagocytic activity in macrophages. The macrophages treated with α-MSH and NPY were significantly suppressed in their capacity to phagocytize unopsonized E. coli and S. aureus bioparticles, but not antibody-opsonized bioparticles. The neuropeptides significantly suppressed phagolysosome activation, and FcR-associated generation of reactive oxidative species. This suppression corresponds to neuropeptide modulation of macrophage functionality within the ocular microenvironment to suppress the activation of immunogenic inflammation.
Keywords: Macrophages, Bioparticles, Neuroimmunomodulation, Ocular Immune Privilege, Retina
1. INTRODUCTION
The neuropeptides alpha-melanocyte stimulating hormone (α-MSH) and Neuropeptide Y (NPY) are produced by retinal pigment epithelium (RPE) (Kawanaka and Taylor, 2010), the monolayer of pigmented cells at the back of eye upon which the neural retina rests. This intact RPE monolayer induces myeloid suppressor cell-like activities in macrophages and retinal microglial cells that is dependent on the RPE producing both α-MSH and NPY (Kawanaka and Taylor, 2010). These neuropeptides with other immunomodulating factors are constitutively produced within the ocular microenvironment and are part of the mechanisms of ocular immune privilege (Taylor, 2009). Both α-MSH and NPY are considered immunomodulating neuropeptides that can influence macrophages functionality and responses to signals of innate immunity (Dimitrijević et al., 2005; Lam et al., 2006; Li and Taylor, 2008; Taylor, 2005; Zhou et al., 2008). Together they suppress proinflammatory signals and promote an alternative activation of macrophages.
In activated macrophages, α-MSH is a suppressor of Toll-like receptor (TLR) and urate crystal stimulated inflammatory activity (Getting et al., 2006; Li and Taylor, 2008; Sarkar et al., 2003; Taylor, 2005). Treatment of the stimulated macrophages with α-MSH suppresses NF-κB and p38MAPK activation (Brzoska et al., 1999; Ichiyama et al., 1999; Li and Taylor, 2008; Manna and Aggarwal, 1998; Yoon et al., 2003). This suppression of the TLR4 pathways is through α-MSH mediated binding of IRAK-M to IRAK-1, and suppression of both the TLR and urate crystal stimulated activation of p38MAPK is through α-MSH activation cAMP dependent PKA (Li and Taylor, 2008; Yoon et al., 2003). In addition, α-MSH alternatively activates macrophages and dendritic cells and promotes suppressive and tolerogenic functions when the cells act as antigen presenting cells (APC) (Lau and Taylor, 2009; Luger et al., 1999; Taylor, 2005). This has suggested that α-MSH is an immunosuppressive neuropeptide that promotes anti-inflammatory activity and tolerance.
The effects of NPY on macrophages are not as easily characterized and maybe related to age-associated changes in receptor expression (Bedoui et al., 2007; Dimitrijevic and Stanojevic, 2011; Dimitrijević et al., 2005). NPY suppress macrophage migration, but if the macrophages are from aging animals, there is no effect (De la Fuente et al., 2000; Medina et al., 2000; Nave et al., 2004). In addition, NPY suppresses phagocytosis of pathogens, and the production of TNF-α by pathogen-stimulated macrophages (Ahmed et al., 2001; Dimitrijević et al., 2005; Puerto et al., 2005; Stanojevic et al., 2007). However, NPY can stimulate IL-1β production in concanavalin A-stimulated macrophages, and augment phagocytosis of innate substances like opsonized-latex beads, and potentiate reactive oxygen species generation (De la Fuente et al., 2001; Puerto et al., 2005; Stanojevic et al., 2007). While NPY may signal a proinflammatory response in macrophages, and promote APC functionality, NPY suppresses the activation of effector T cells (Prod’homme et al., 2006). Therefore, the effects of NPY on macrophages appear to be more influenced by the microenvironment, the stimulating agents, and aging than the effects of α-MSH.
Soluble factors from healthy RPE monolayer induce peripheral resting macrophages to co-express the arginine substrate enzymes nitric oxide synthase 2 (NOS2) and Arginase 1 (Arg1) (Kawanaka and Taylor, 2010). These treated macrophages express myeloid suppressor cell like characteristics, generate nitric oxide, and promote T cell apoptosis. Neutralization of α-MSH causes the RPE to induce M1 inflammatory activity, while neutralization of NPY causes the RPE to mediate M2 wound/suppressive activity in the resting macrophages (Kawanaka and Taylor, 2010). Individually NPY induces NOS2 expression, while α-MSH promotes expression of Arg1 by resting macrophages (Kawanaka and Taylor, 2010) While individually the neuropeptides promote polarized macrophage functionality, together α-MSH and NPY promote anti-inflammatory and immunosuppressive macrophages. The α-MSH/NPY-induced macrophages suppress inflammation and the activation of effector T cells, which would contribute to the normal immunosuppressive microenvironment of the eye.
Since in the retina α-MSH and NPY mediates an alternative activation of macrophages with suppressor cell functions, it is possible that phagocytic activity is also affected. Changes in phagocytic activity would imply limitations in macrophage/microglial cell clearance of infecting pathogens and apoptotic bodies, and in processing of antigen within the retina. To see if there is an effect, macrophages treated with α-MSH and NPY were assayed for phagocytosis of opsonized and unopsonized bacterial bioparticles, the activation of phagolysosomes, and generation of reactive oxygen species.
2. MATERIALS AND METHODS
Cell Lines and Materials
Murine monocytic leukemic cell line RAW 264.7 cells (ATCC, Manassas, VA) were maintained in DMEM supplemented with 0.01 M HEPES, 1x nonessential amino acids, 1x glutamine, and 1 mM sodium pyruvate (Lonza Walkersville, Walkersville, MD), 1mM gentamycin (Sigma-Aldrich, St Louis, MO) and 5% Fetal Bovine Serum (Life Technologies-Gibco, Grand Island, NY). Cells were incubated at 37°C, 5% CO2. The Alexa Fluor 488 (AF488)-conjugated and pHrodo-red-conjugated E. coli and S. aureus bioparticles with their respective opsonizing reagents were purchased from Life Technologies-Molecular Probes (Grand Island, NY). The neuropeptides α-MSH and NPY (Bachem, Torrance, CA) were reconstituted in sterile 1x PBS, aliquotted and stored at −80°C before use. The scavenger receptor antibodies were FITC-conjugated anti-MARCO antibody, and AF488-conjugated anti-CD206 antibody from AbD Serotec (Raleigh, NC). Highly reactive oxygen species were detected using hydroxyphenyl fluorescein (HPF) from Cell Technologies (Mountain View, CA).
Phagocytosis assay
The macrophages were plated at 5 × 105 cells into the wells of a 24 well plate under serum free conditions, similar to the our previous studies with organotypic RPE cultures (Kawanaka and Taylor, 2010; Lau and Taylor, 2009; Zamiri et al., 2006). The serum-free media consists of the growth media described above with the serum substituted with 1/500 dilution of ITS+ supplement (Sigma, St Louis, MO). The macrophages were treated with α-MSH and NPY at 1 ng/ml each this is the concentration of the neuropeptides in the RPE conditioned media produced by in vitro culturing of healthy RPE monolayers for 24 hours(Kawanaka and Taylor, 2010). The cells were incubated for 30 min then added to the cultures 40 μg AF488-conjugates of either S. aureus or E. Coli bioparticles. These bioparticles were either unopsonized, or opsonized with their respective S. aureus or E. Coli antibodies. The cells were incubated for 24 hrs. at 37°C, 5% CO2, collected, washed and assayed by flow cytometry. The flow results were analyzed by FlowJo software to measure both the number of cells and the intensity of the material taken up by the treated macrophages.
Detection of Phagolysosome Activation
The macrophages were cultured and treated in the same manner as described except they were plated at 1 × 105 cells/well of a 96 well flat bottom plate. Added to the wells of α-MSH and NPY treated macrophages were S. aureus or E. coli pHrodo-red bioparticles at 0.1 mg/well. The bioparticles were either unopsonized or opsonized with their respective antibodies. The pHrodo-red beads increase in intensity under acidic conditions and have a maximum fluorescence at pH=4, and a minimal fluorescence at pH=7. After the cultures were incubated for 24 hours, the medium in each well was replaced with PBS (pH=7.0) to minimize the fluorescence of the non-phagocytized particles, and the plate was spun down at 2000 RPM for 5 minutes. An additional well with the pHrodo-red bioparticle suspend in 0.01M PBS (pH=7.0) was used as background control. The phagocytized particles in an active phagolysosome (pH = 4.0) were detected by imaging the plate using a Bio-Rad Versadoc with green LED excitation. The fluorescent intensity was measured for each well, background was subtracted (bioparticles at pH=7), and the relative intensity to the untreated group was calculated for each condition and experiment.
Fluorescent microscopy for bioparticle uptake and reactive oxygen species generation
To image the cells by microscopy the macrophages were cultured and plated as described but this time 1 × 105 cells were cultured in 60mm petri dishes. The macrophages were treated with α-MSH and NPY, and given a mixture of opsonized pHrodo-red (25 μg) and AF488-conjugated (10 μg) S. aureus or E. coli bioparticles. This concentration of bioparticles allowed for complete uptake of the bioparticles within 24 hours to observe intracellular bioparticles. After incubation for 24 hours, the cultures were washed once with 0.01M PBS (pH = 7.0), and PBS was added to the culture dishes. The macrophage cultures were digitally imaged with the FSX100 digital fluorescent microscope (Olympus, Center Valley, PA) using a 40x objective and a 1/13 second exposure time. Both green (AF488-conjugated bioparticles) and red (pHrodo bioparticles) fluorescence were imaged. The images were corrected for background and overlaid to make the presented images using the FSX100 software.
For detection of reactive oxygen species generation, the macrophages were treated with α-MSH and NPY as before. The treated macrophages were given opsonized pHrodo-red bioparticles, because these bioparticles have a fluorescent excitation at 560nm, since HPF after reacting with hydroxyl radicals and peroxinitrite is fluorescently excited at 488nm. The cultures were incubated for 24 hours and in the last hour HPF was added to the cultures. The cells were examine by FSX100 fluorescent microscopy and florescent intensity of 30 – 40 cells per treatment of two independent experiments were measured using the FSX100 analysis software. The relative intensity over background was calculated.
Flow cytometry analysis for MARCO and CD206 expression
The macrophages were cultured in a 24 well plates and treatment with α-MSH and NPY as described before. In addition, added to the cultures was E. coli O155 lipopolysaccharide (Sigma) at 1 μg/ml. The cultures were incubated for 24 hours, and 5 × 106 cells were collected, washed, and resuspended in 0.01M PBS. The Fc-receptors were blocked with mouse IgG, and the cells were fixed with 4% paraformaldehyde. The fixed cells were stained with conjugated anti-CD206 antibody, or anti-MARCO antibody for 30 min. Cells were washed, assayed by flow cytometry, and fluorescent intensity analyzed by FlowJo software.
Data and statistical analysis
The histograms presented are representative of 3 experiments with similar results. The bar grafts of geometric mean fluorescent intensity (MFI), average fluorescent intensity, and average percentage of cells are calculated from 3 experiments. For the HPF staining the bar grafts are the mean ± SEM of the relative intensities of 30 – 40 cells for each condition. Statistically significant differences, as indicated on the figures, of at least P ≤ 0.05 were calculated by one-way ANOVA and post-test Tukey comparison analysis using Prism software (GraphPad Software Inc, La Jolla, CA).
3. RESULTS
Phagocytosis of S. aureus bioparticles
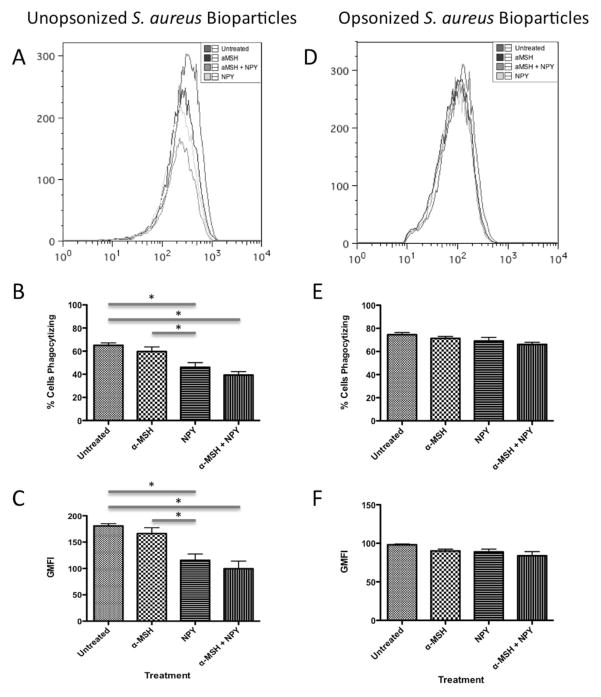
The macrophages were treated with α-MSH and NPY at a concentration of 1 ng/ml each, which is the concentration of these neuropeptides produced by organotypic cultures of healthy RPE monolayers (Kawanaka and Taylor, 2010). The α-MSH/NPY co-treatment make the macrophages express characteristics and functions similar to myeloid suppressor cells (Kawanaka and Taylor, 2010). The treated macrophage were given unopsonized AF488-conjugated S. aureus bioparticles to phagocytize (Fig. 1A–1C). When the macrophages treated with NPY or with α-MSH/NPY co-treatment were analyzed by flow cytometry there was a significant decrease in the percentage of macrophages positive for S. aureus bioparticles (Fig. 1B). There was a similar significant decrease in fluorescent intensity of macrophages with S. aureus bioparticles (Fig. 1C) indicating a decrease in the amount taken up by the macrophages. These results demonstrate that there is a decrease in the uptake of unopsonized S. aureus bioparticles by macrophages treated with NPY suggesting suppression of phagocytosis. In contrast, if the bioparticles are opsonized then there was no change in the percent of macrophages positive for the bioparticles or in the intensity of S. aureus bioparticles taken up by the macrophages (Fig. 1D–1F). This suggests that the neuropeptides had no effect on FcR-mediated phagocytosis of gram-positive bioparticles.
Figure 1. The effects of α-MSH and NPY on the phagocytosis of unopsonized and opsonized S. aureus bioparticles.
The macrophages were treated with α-MSH, NPY, or both and given unopsonized (A, B, C) or opsonized (D, E, F) AF488-conjugated S. aureus bioparticles. After 24 hours incubation the cells were washed, and analyzed by flow cytometry. A, D) The histograms show the flow cytometry results of analyzing the macrophages for AF488. The histogram is representative of 3 independent experiments. B, E) Presented are the percentage ± SD of AF488 positive cells as percent of cells phagocytizing the bioparticles. C, F) Presented are the geometric mean fluorescent intensity ± SD of AF488 expression by the cells. *Significant differences (P ≤ .05) were measured between NPY and co-treated macrophages and untreated macrophages. Also, there was a significant difference in the percent and intensity of AF488 positive cells was between NPY and α-MSH treated macrophages. There were no significant differences between neuropeptide treated and untreated macrophages in AF488 expression with opsonized bioparticles. B, C, E, F) Data are from 3 independent experiments.
Phagocytosis of E. coli bioparticles
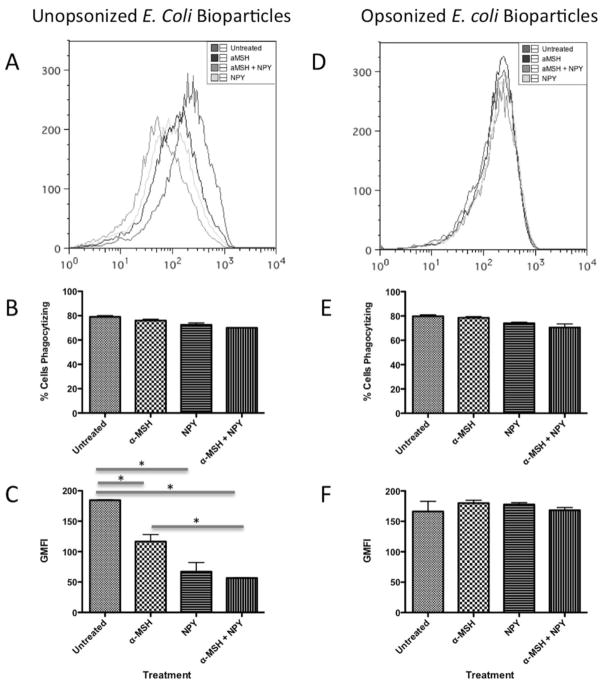
To see if there was an effect of the neuropeptides on gram-negative bioparticle uptake, the macrophages were treated with α-MSH and NPY and were given AF488-conjugated E. coli bioparticles to phagocytize. When the bioparticles were unopsonized there was no significant change in percent of macrophages positive for E. coli bioparticles (Fig. 2A, 2B); however, the fluorescent intensity of macrophages with E. coli bioparticles was significantly suppressed by macrophages treated with α-MSH and NPY individually and together (Fig. 2C). The suppression of bioparticle uptake by NPY and the co-treated macrophages was significantly greater than the suppression mediated by α-MSH treatment (Fig. 2C). This suggests that while the number of macrophages phagocytizing the gram-negative bioparticles did not change, it was the amount of phagocytized bioparticles that was suppressed by the neuropeptides. Therefore, the neuropeptides suppress the macrophage response to gram-negative bioparticles reducing phagocytic activity. Just as with the opsonized S. aureus bioparticles, there was no significant effect of treating the macrophages with neuropeptides on FcR-mediated phagocytosis of opsonized E. coli bioparticles (Fig. 2D–E). This further demonstrates that FcR-mediated phagocytosis is not affected by neuropeptide treatment.
Figure 2. The effects of α-MSH and NPY on the phagocytosis of unopsonized and opsonized E. coli bioparticles.
The macrophages were treated with α-MSH, NPY, or both and given unopsonized (A, B, C) or opsonized (D, E, F) AF488-conjugated E. coli bioparticles. After 24 hours incubation the cells were washed, and analyzed by flow cytometry. A, D) The histograms show the flow cytometry results of analyzing the macrophages for AF488. The histogram is representative of 3 independent experiments. B, E) Presented are the percentage ± SD of AF488 positive cells as percent of cells phagocytizing the bioparticles. C, F) Presented are the geometric mean fluorescent intensity ± SD of AF488 expression by the cells. There was no significant difference in percent of AF488 expressing cells between the untreated and any of the neuropeptide treated macrophages *Significant differences (P ≤ .05) were measured between untreated macrophages and the neuropeptide treated macrophages. There were no significant differences between neuropeptide treated and untreated macrophages in AF488 expression with opsonized bioparticles. B, C, E, F) Data are from 3 independent experiments.
Scavenger receptor CD206 and MARCO expression
To see if the neuropeptide suppression of the uptake of unopsonized bioparticles was due to suppression of scavenger receptors CD206 and MARCO expression on the treated macrophages, the neuropeptide treated macrophages were assayed by flow cytometry for CD206 and MARCO expression. The macrophages were activated with endotoxin and treated with α-MSH, NPY or both. Flow cytometry data showed that there was no change in either CD206 or MARCO expression on the neuropeptide treated macrophages (Fig. 3). Such data suggests that the suppressive effects of the neuropeptides on phagocytic activity are not by down regulating the expressions of two dominate scavenger receptors CD206 or MARCO in activated macrophages, but through suppressing the activation of the phagocytic pathways.
Figure 3. Expression of CD206 and MARCO on activated macrophages treated with α-MSH and NPY.
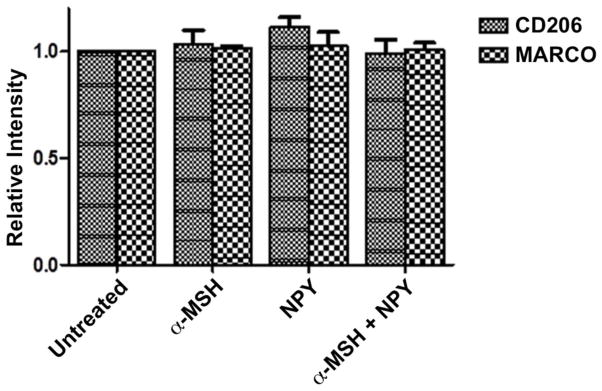
The macrophages were treated with a-MSH, NPY or both and stimulated with endotoxin. After 24 hours incubation the cells were stained with fluorescent-conjugated antibodies against CD206 or MARCO, and analyzed by flow cytometry. Presented are the relative intensity ± SD of CD206 and MARCO expression on the treated macrophages in relationship to their expression on LPS-stimulated macrophages that were not treated with the neuropeptides (untreated). No significant differences were measured between the untreated and neuropeptide treated macrophage expression of CD206 and MARCO. The data are from 3 independent experiments.
Imaging of bioparticle uptake
To visualize the internalization of opsonized S. aureus and E. coli bioparticles the treated macrophages were given a mixture of FITC-conjugated and pHrodo-conjugated bioparticles to phagocytize. Combining the beads allowed for visualization of the binding/uptake of the particles (Green) with the activation of the phagolysosome (Red). Using digital microscopy single pictures were taken for both opsonized S. aureus (Fig. 4A–D) and E. coli bioparticles (Fig. 4E–H) after 24 hours incubation. The untreated macrophages took up and moved the particles into active phagolysosomes, which is seen as intense red bioparticles with some unprocessed opsonized green bioparticles remaining in the cells (yellow color, Fig. 4A, 4E). Almost no red bioparticles, S. aureus or E. coli, were seen in the macrophages treated with α-MSH, NPY or α-MSH/NPY co-treated (Fig. 4B–D and 4F–H). The figure shows that most of the particles were taken up by the neuropeptide-treated macrophages, but were not moved into active phagolysosomes. This suggests that the neuropeptides are suppressing opsonized bioparticle processing.
Figure 4. Visualization of bioparticle uptake and phagolysosome activation.
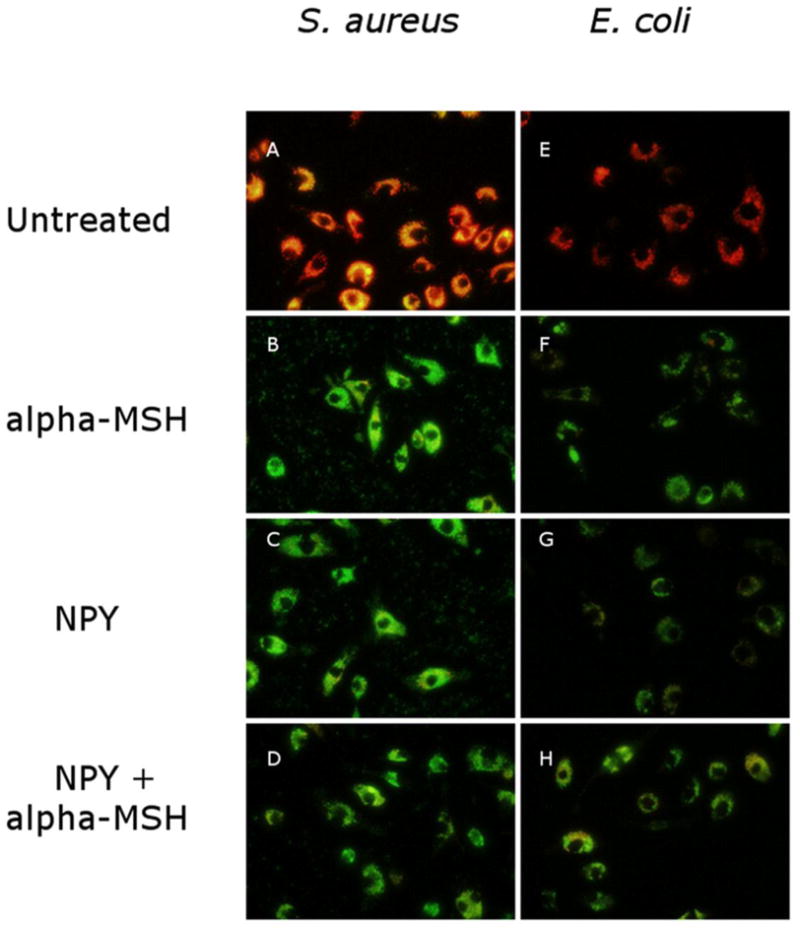
The macrophages were treated with α-MSH, NPY or both and given equal portions of opsonized pHrodo-red bioparticles and opsonized AF488-conjugated bioparticles to phagocytize. After 24 hours incubation, the cells were washed, and digitally photographed though a fluorescent microscope. Presented are the fluorescent overlays of the green (AF488 expression) and red (pHrodo-red expression) images of the macrophages given opsonized (AD) S. aureus or (E–H) E. coli bioparticles. The untreated macrophages showed that within 24 hours most of the cells were expressing pHrodo-red with little AF488 expression indicating that the particles were phagocytized and moved into active phagolysosomes. The neuropeptide treated macrophages were highly expressing AF488 with little to no pHrodo-red expression. There was no difference seen between treating the macrophages with α-MSH, NPY or both. This indicates that the neuropeptide treated macrophages have phagocytic activity (green particles) but are suppressed in phagolysosome activity. The figures are representative of 3 independent experiments.
Phagolysosome activity
To measure the effects of the neuropeptides on the activation of the phagolysosome the macrophages were treated with the neuropeptides and given S. aureus or E. coli pHrodo bioparticles. After incubation the macrophages were assayed for fluorescent intensity of the pHrodo bioparticles associated with the bioparticles in an acidic vesicle. The relative intensity of the treated macrophages to the untreated macrophages was compared for opsonized bioparticle uptake (Fig. 5). The fluorescent intensity of the bioparticles was significantly suppressed in all macrophages treated with the neuropeptides (Fig. 5). There was a significant suppression in the fluorescent intensity of the phagolysosomes of macrophages taking up opsonized pHrodo-red bioparticles (Fig. 5A and 5B). The same effect was seen with both the Gram-positive and Gram-negative bioparticles. Similar suppression of detectible fluorescence was seen in macrophages treated with the neuropeptides and given unopsonized pHrodo bioparticles to phagocytize (data not shown). These results demonstrate that as there is no suppression by the neuropeptides in the phagocytosis of opsonized bioparticles, there is suppression in phagolysosome activation. In addition, the α-MSH and NPY co-treatment is additive to the effects of the individual neuropeptides on phagolysosome activation with opsonized bioparticles (Fig. 5A and 5B).
Figure 5. The effects of the neuropeptides on the expression of phagocytized opsonized pHrodo-red bioparticles in macrophages.
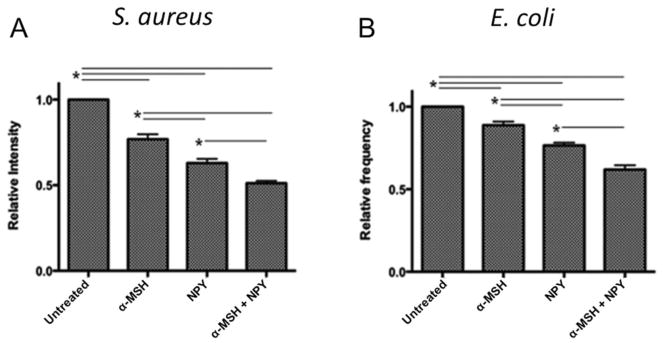
The macrophages were treated with α-MSH, NPY, or both and given opsonized A) S. aureus or B) E. coli pHrodo-red-conjugated bioparticles. After 24 hours incubation the cells were washed, and analyzed by flow cytometry. Presented are the relative fluorescent intensity ± SD of pHrodo-red expression in the macrophages related to the pHrodo-red expression in the untreated macrophages. *These groups of treated macrophages were significantly (P ≤ 0.05) different from each other. The neuropeptide treated macrophages were significantly suppressed in pHrodo-red expression compared to untreated macrophages with both S aureus and E. coil bioparticles, suggesting suppressed phagolysosome activation. The data are from 3 independent experiments.
Effects of the neuropeptides on FcR-mediated activation of reactive oxygen species generation
The suppression of phagolysosome acidification could be a result of the neuropeptides suppressing the FcR-mediated activation of the macrophages, or by potentiating the generation of highly reactive oxidative reactants that would bind the protons generated in the phagolysosome and neutralize the pH (Haas, 2007; Jankowski and Grinstein, 2002). Since NPY is known to possibility potentiate the generation of reactive oxygen species, and that α-MSH/NPY co-treatment induces peroxinitrite production in macrophages (Kawanaka and Taylor, 2010; Stanojevic et al., 2007), the macrophages were treated with the neuropeptides and opsonized pHrodo-red bioparticles. The macrophages were incubated, loaded with HPF, and assayed for fluorescent expression of HPF reacting with hydroxyl radicals and peroxinitrites (Fig. 6). The neuropeptide treatment significantly suppressed the detection of HPF fluorescence in the macrophages that opsonized S aureus or E. coli bioparticles (Fig. 6A and 6B). Therefore, the effect of the neuropeptides on FcR-mediated phagocytosis is in suppressing the FcR-response and activation of the phagolysosome in the macrophages.
Figure 6. The effects of the neuropeptides on FcR-stimulated generation of reactive oxygen species.
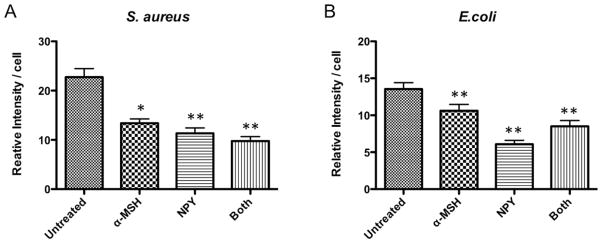
The macrophages were treated with α-MSH, NPY, or both and given opsonized A) S. aureus or B) E. coli pHrodo-red-conjugated bioparticles. After 23 hours incubation the cells were loaded with HPF and incubated for one more hour. The cells were washed and imaged under a fluorescent microscope, and the intensity of fluorescent HPF was measured from digital images of each cells. The relative intensity per cell ± SD for each macrophage group (30 – 40 cells from two independent experiments) was calculated. Significant differences (*P≤0.01, **P≤0.001) were seen in HPF fluorescence between the neuropeptide treated macrophages and the untreated macrophages.
4. DISCUSSION
These results demonstrate that the neuropeptides α-MSH and NPY, two important regulators of macrophage activity in the retina, influence the process of phagocytosis. One of the newest findings is that together the neuropeptides suppressed the activation of phagolysosomes, and FcR-mediated generation of reactive oxygen species. These results suggest that within the immune privileged retina, macrophages/microglial cells are limited in uptake of bacterial particles, and more importantly suppressed in phagolysosome activation. This has an implication on how the healthy ocular microenvironment can clear pathogens, and process antigens.
Previously, it has been demonstrated that macrophages co-treated with α-MSH and NPY express characteristics of myeloid suppressor cells (Kawanaka and Taylor, 2010). In the current study, such treated cells are also suppressed in phagocytosis of unopsonized bacterial particles, but not when phagocytizing antibody coated bioparticles. In addition, the macrophages were suppressed in phagolysosome activation and FcR-mediated generation of reactive oxygen species. While our results demonstrated a similarity in the individual effects of α-MSH and NPY on FcR-mediated phagocytosis, activation of phagolysosomes, and reactive oxygen species generation, the neuropeptides had different individual effects on the uptake of unopsonized bioparticles. This was related to whether the particles were Gram positive or negative. The suppression of unopsonized E. coli bioparticles was in the amount of bioparticles phagocytized, and not in the number of cells capable of phagocytic activity. This suggests that the neuropeptides are suppressing the phagocytic response to TLR4 stimulation. Where as the phagocytosis of unopsonized S. aureus bioparticles was suppressed by only NPY treatment, and it was in suppressing the number of cells that have phagocytic activity. This suggests that NPY suppresses the activation of the macrophages through TLR2, which then appears as suppression of phagocytosis. The suppression of TLR4-stimulated activity by α-MSH has been clearly documented (Li and Taylor, 2008; Lipton and Catania, 1997; Taylor, 2005), and there is a hint that α-MSH may have no affect on TLR2 dominated signaling pathways in macrophages (Taylor, 2005), which is further supported by our results. While the literature presents contradictory information on the effects of NPY on macrophage phagocytic activity (Bedoui et al., 2007; Dimitrijevic and Stanojevic, 2011; Dimitrijević et al.,2005), our results clearly demonstrate that NPY is a suppressor of phagocytosis of unopsonized Gram-positive and Gram-negative bacterial bioparticles. In addition, the results show NPY and α-MSH suppressors of phagolysosome activation and reactive oxygen species generation. Therefore, the neuropeptides suppress processing of material taken up by the phagocytes.
A consequence of phagocytic activity by macrophages in clearing pathogens and dangerous material is to generate antigens for presentation. The phagosome fusion with the lysosome is part of the processing of proteins into peptide antigens for presentation. However, our results suggest that within the ocular microenvironment the constitutive presence of α-MSH, and NPY means that resident retinal macrophages/microglial cells are not processing phagocytized material in an expected manner. The finding that macrophages treated with both α-MSH and NPY induces myeloid suppressor cell-like activity (Kawanaka and Taylor, 2010) means that the macrophages are receiving multiple signals that are anti-inflammatory, suppressive in phagolysosome processing, and inducing immunosuppressive functions. It is known that APC influenced by the ocular microenvironment present antigen in a manner that suppresses effector T cell activation and stimulates Treg cell activation (Niederkorn, 2007). This suggests that the uptake, processing, or presentation of antigen is altered in a way that restricts or limits the range of T cells that can be activated within the healthy eye. This is ideal for the maintenance of ocular immune privilege, and the prevention of accidental processing and presentation of autoantigens.
While the effects of α-MSH and NPY on macrophages may be beneficial for the maintenance of immune privilege, it could be associated with a weakened anti-microbial defense. The mechanical barrier of the intact cornea is the most important defense against ocular infections. This is normally quite effective in that the incidence of exogenous ocular infection (endophthalmitis) is very low (5 cases per 10,000 patients). However, when there is an infection often introduced by surgery, it is quite severe and requires aggressive antibiotic therapy (Connell et al., 2011; Ness et al., 2007; Taban et al., 2005). This suggests that the anti-microbial actions of resident ocular phagocytes are not normally optimal. This could be related to the immunosuppressive activity of the neuropeptides to modulate resident macrophage functionality. In addition, α-MSH and NPY affect the migratory activity of macrophages (Catania et al., 1996; Manna et al., 2006; Medina et al., 2000; Nave et al., 2004) further weakening the response to microbial infection within the ocular microenvironment.
We report a unique action of the neuropeptides α-MSH and NPY in the suppression of phagocytosis, the activation of phagolysosomes, and FcR-mediated generation of reactive oxygen species. Our results demonstrate an important contribution of these two neuropeptides in the maintenance of the immunosuppressive microenvironment of the eye.
Highlights.
The neuropeptides α-MSH and NPY suppress phagocytosis of unopsonized bioparticles.
The neuropeptides α-MSH and NPY do not suppress FcR-mediated phagocytosis
α-MSH and NPY suppress phagolysosome activation of FcR-mediated phagosomes
α-MSH and NPY suppress FcR-mediated generation of reactive oxygen species
Acknowledgments
This work is supported in part by the U.S. National Institutes of Health grant EY01752 and the Massachusetts Lions Eye Research Foundation to A.W.T. Thanks are given to David Yee, and Hari N. Mylvaganam for their technical support, and to the BU flow cytometry core facility.
Footnotes
AUTHORSHIP
T.A.P. and A.W.T. performed the research, interpreted the data, and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed AA, Wahbi AH, Nordlin K. Neuropeptides modulate a murine monocyte/macrophage cell line capacity for phagocytosis and killing of Leishmania major parasites. Immunopharmacology and immunotoxicology. 2001;23:397–409. doi: 10.1081/iph-100107339. [DOI] [PubMed] [Google Scholar]
- Bedoui S, von Horsten S, Gebhardt T. A role for neuropeptide Y (NPY) in phagocytosis: implications for innate and adaptive immunity. Peptides. 2007;28:373–376. doi: 10.1016/j.peptides.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Brzoska T, Kalden DH, Scholzen T, Luger TA. Molecular basis of the alpha-MSH/IL-1 antagonism. Ann N Y Acad Sci. 1999;885:230–238. doi: 10.1111/j.1749-6632.1999.tb08680.x. [DOI] [PubMed] [Google Scholar]
- Catania A, Rajora N, Capsoni F, Minonzio F, Star RA, Lipton JM. The neuropeptide alpha-MSH has specific receptors on neutrophils and reduces chemotaxis in vitro. Peptides. 1996;17:675–679. doi: 10.1016/0196-9781(96)00037-x. [DOI] [PubMed] [Google Scholar]
- Connell PP, O’Neill EC, Fabinyi D, Islam FM, Buttery R, McCombe M, Essex RW, Roufail E, Clark B, Chiu D, Campbell W, Allen P. Endogenous endophthalmitis: 10-year experience at a tertiary referral centre. Eye. 2011;25:66–72. doi: 10.1038/eye.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Fuente M, Del Rio M, Medina S. Changes with aging in the modulation by neuropeptide Y of murine peritoneal macrophage functions. Journal of neuroimmunology. 2001;116:156–167. doi: 10.1016/s0165-5728(01)00297-1. [DOI] [PubMed] [Google Scholar]
- De la Fuente M, Medina S, Del Rio M, Ferrandez MD, Hernanz A. Effect of aging on the modulation of macrophage functions by neuropeptides. Life sciences. 2000;67:2125–2135. doi: 10.1016/s0024-3205(00)00799-2. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic M, Stanojevic S. The intriguing mission of neuropeptide Y in the immune system. Amino acids. 2011 doi: 10.1007/s00726-011-1185-7. [DOI] [PubMed] [Google Scholar]
- Dimitrijević M, Stanojević S, Vujić V, Beck-Sickinger A, von Hörsten S. Neuropeptide Y and its receptor subtypes specifically modulate rat peritoneal macrophage functions in vitro: counter regulation through Y1 and Y2/5 receptors. Regulatory peptides. 2005;124:163–172. doi: 10.1016/j.regpep.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Getting SJ, Lam CW, Chen AS, Grieco P, Perretti M. Melanocortin 3 receptors control crystal-induced inflammation. Faseb J. 2006;20:2234–2241. doi: 10.1096/fj.06-6339com. [DOI] [PubMed] [Google Scholar]
- Haas A. The phagosome: compartment with a license to kill. Traffic. 2007;8:311–330. doi: 10.1111/j.1600-0854.2006.00531.x. [DOI] [PubMed] [Google Scholar]
- Ichiyama T, Zhao H, Catania A, Furukawa S, Lipton JM. alpha-melanocyte-stimulating hormone inhibits NF-kappaB activation and IkappaBalpha degradation in human glioma cells and in experimental brain inflammation. Exp Neurol. 1999;157:359–365. doi: 10.1006/exnr.1999.7064. [DOI] [PubMed] [Google Scholar]
- Jankowski A, Grinstein S. Modulation of the cytosolic and phagosomal pH by the NADPH oxidase. Antioxidants & redox signaling. 2002;4:61–68. doi: 10.1089/152308602753625861. [DOI] [PubMed] [Google Scholar]
- Kawanaka N, Taylor AW. Localized retinal neuropeptide regulation of macrophage and microglial cell functionality. J Neuroimmunol. 2010 doi: 10.1016/j.jneuroim.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CW, Perretti M, Getting SJ. Melanocortin receptor signaling in RAW264.7 macrophage cell line. Peptides. 2006;27:404–412. doi: 10.1016/j.peptides.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Lau CH, Taylor AW. The immune privileged retina mediates an alternative activation of J774A.1 cells. Ocul Immunol Inflamm. 2009;17:380–389. doi: 10.3109/09273940903118642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Taylor AW. Diminishment of alpha-MSH anti-inflammatory activity in MC1r siRNA-transfected RAW264.7 macrophages. J Leukoc Biol. 2008;84:191–198. doi: 10.1189/jlb.0707463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton JM, Catania A. Anti-inflammatory actions of the neuroimmunomodulator alpha-MSH. Immunology Today. 1997;18:140–145. doi: 10.1016/s0167-5699(97)01009-8. [DOI] [PubMed] [Google Scholar]
- Luger TA, Kalden D, Scholzen TE, Brzoska T. alpha-melanocyte-stimulating hormone as a mediator of tolerance induction. Pathobiology. 1999;67:318–321. doi: 10.1159/000028089. [DOI] [PubMed] [Google Scholar]
- Manna SK, Aggarwal BB. Alpha-melanocyte-stimulating hormone inhibits the nuclear transcription factor NF-kappa B activation induced by various inflammatory agents. J Immunol. 1998;161:2873–2880. [PubMed] [Google Scholar]
- Manna SK, Sarkar A, Sreenivasan Y. Alpha-melanocyte-stimulating hormone down-regulates CXC receptors through activation of neutrophil elastase. Eur J Immunol. 2006;36:754–769. doi: 10.1002/eji.200535209. [DOI] [PubMed] [Google Scholar]
- Medina S, Del Rio M, Hernanz A, De la Fuente M. The NPY effects on murine leukocyte adherence and chemotaxis change with age. Adherent cell implication. Regulatory peptides. 2000;95:35–45. doi: 10.1016/s0167-0115(00)00134-8. [DOI] [PubMed] [Google Scholar]
- Nave H, Bedoui S, Moenter F, Steffens J, Felies M, Gebhardt T, Straub RH, Pabst R, Dimitrijevic M, Stanojevic S, von Horsten S. Reduced tissue immigration of monocytes by neuropeptide Y during endotoxemia is associated with Y2 receptor activation. Journal of neuroimmunology. 2004;155:1–12. doi: 10.1016/j.jneuroim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Ness T, Pelz K, Hansen LL. Endogenous endophthalmitis: microorganisms, disposition and prognosis. Acta ophthalmologica Scandinavica. 2007;85:852–856. doi: 10.1111/j.1600-0420.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY. The induction of anterior chamber-associated immune deviation. Chem Immunol Allergy. 2007;92:27–35. doi: 10.1159/000099251. [DOI] [PubMed] [Google Scholar]
- Prod’homme T, Weber MS, Steinman L, Zamvil SS. A neuropeptide in immune-mediated inflammation, Y? Trends in Immunology. 2006;27:164–167. doi: 10.1016/j.it.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Puerto M, Guayerbas N, Alvarez P, De la Fuente M. Modulation of neuropeptide Y and norepinephrine on several leucocyte functions in adult, old and very old mice. Journal of neuroimmunology. 2005;165:33–40. doi: 10.1016/j.jneuroim.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Sreenivasan Y, Manna SK. alpha-Melanocyte-stimulating hormone inhibits lipopolysaccharide-induced biological responses by downregulating CD14 from macrophages. FEBS Lett. 2003;553:286–294. doi: 10.1016/s0014-5793(03)01029-9. [DOI] [PubMed] [Google Scholar]
- Stanojevic S, Mitic K, Vujic V, Kovacevic-Jovanovic V, Dimitrijevic M. Exposure to acute physical and psychological stress alters the response of rat macrophages to corticosterone, neuropeptide Y and beta-endorphin. Stress. 2007;10:65–73. doi: 10.1080/10253890601181289. [DOI] [PubMed] [Google Scholar]
- Taban M, Behrens A, Newcomb RL, Nobe MY, Saedi G, Sweet PM, McDonnell PJ. Acute endophthalmitis following cataract surgery: a systematic review of the literature. Arch Ophthalmol. 2005;123:613–620. doi: 10.1001/archopht.123.5.613. [DOI] [PubMed] [Google Scholar]
- Taylor AW. The immunomodulating neuropeptide alpha-melanocyte-stimulating hormone (alpha-MSH) suppresses LPS-stimulated TLR4 with IRAK-M in macrophages. J Neuroimmunol. 2005;162:43–50. doi: 10.1016/j.jneuroim.2005.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AW. Ocular immune privilege. Eye (Lond) 2009;23:1885–1889. doi: 10.1038/eye.2008.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SW, Goh SH, Chun JS, Cho EW, Lee MK, Kim KL, Kim JJ, Kim CJ, Poo H. alpha-Melanocyte-stimulating hormone inhibits lipopolysaccharide-induced tumor necrosis factor-alpha production in leukocytes by modulating protein kinase A, p38 kinase, and nuclear factor kappa B signaling pathways. J Biol Chem. 2003;278:32914–32920. doi: 10.1074/jbc.M302444200. [DOI] [PubMed] [Google Scholar]
- Zamiri P, Masli S, Streilein JW, Taylor AW. Pigment epithelial growth factor suppresses inflammation by modulating macrophage activation. Invest Ophthalmol Vis Sci. 2006;47:3912–3918. doi: 10.1167/iovs.05-1267. [DOI] [PubMed] [Google Scholar]
- Zhou JR, Xu Z, Jiang CL. Neuropeptide Y promotes TGF-beta1 production in RAW264.7 cells by activating PI3K pathway via Y1 receptor. Neurosci Bull. 2008;24:155–159. doi: 10.1007/s12264-008-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]