Abstract
Estrogen status and psychological stress contribute to the expression of several chronic pain conditions including temporomandibular muscle and joint disorders (TMJD). Sensory neurons that supply the temporomandibular joint (TMJ) region terminate in laminae I and V of the spinal trigeminal nucleus (Vc/C1-2 region); however, little is known about lamina specificity and environmental influences on the encoding properties of TMJ brainstem neurons. To test the hypothesis that Vc/C1-2 neurons integrate both interoceptive and exteroceptive signals relevant for TMJ nociception, we recorded TMJ-evoked activity in superficial and deep laminae of ovariectomized rats under high and low estradiol (E2) and stress conditions. Rats received daily injections of low (LE) or high (HE) dose E2 and were subjected to forced swim (FS) or sham swim conditioning for 3 days. The results revealed marked lamina specificity in that HE rats displayed enhanced TMJ-evoked activity in superficial, but not deep, laminae independent of stress conditioning. By contrast, FS conditioned rats displayed increased background firing and TMJ-evoked activity of neurons in deep, but not superficial, laminae independent of E2 status. FS also enhanced TMJ-evoked masseter muscle activity and suggested the importance of deep dorsal horn neurons in mediating evoked jaw muscle activity. In conclusion, E2 status and psychophysical stress play a significant role in modifying the encoding properties of TMJ-responsive medullary dorsal horn neurons with a marked lamina specificity.
Keywords: estradiol, forced swim conditioning, nociception, temporomandibular joint, trigeminal subnucleus caudalis
Introduction
Temporomandibular muscle and joint disorders (TMJD) represent a family of conditions associated with pain in the temporomandibular joint (TMJ) and masticatory muscles. TMJD is often comorbid with other persistent functional pain syndromes such as fibromyalgia, irritable bowel syndrome and chronic pelvic pain [45, 74]. Functional pain syndromes such as TMJD and fibromyalgia share several features, most prominently an association with psychological distress and female gender, environmental factors thought to amplify pain expression [45, 46, 74]. Indeed, the odds of developing TMJD are nearly 3 times greater for females th an for males [64], while the risk of developing major depression or anxiety disorders for women is nearly twice that for men [62] suggesting that stress and female sex hormone status interact to modify pain expression. TMJD often similar to other persistent functional pain syndromes presents with little or no apparent relationship to tissue injury or inflammation, a non-progressive pattern of pain and increased sensitivity to somatic stimuli applied outside the TMJ region [51, 58, 71]. These features suggest that the mechanisms underlying persistent functional pain syndromes central sensitization [74].
The neural basis and possible sites for psychological stress and estrogen status to interact and to modify pain expression are not known. The present study tested three hypothesis concerning estrogen status, behavioral stress and TMJ nociception using neurophysiological recording methods. Do estrogen status and psychophysical stress interact at the level of the Vc/C1-2 region to modify the activity of individual TMJ neurons or, alternatively, do these factors act on separate subpopulations of neurons? Second, do TMJ-responsive neurons in superficial and deep laminae respond similarly to changes in estrogen status and behavioral stress? Third, are changes in jaw muscle EMG activity consistent with those seen by TMJ neurons? Sensory fibers that supply the TMJ and jaw muscles terminate at the spinal trigeminal subnucleus caudalis (Vc) and the upper cervical cord junction (Vc/C 1-2) region [21, 63] and distribute mainly within the superficial and deep laminae (I and V), similar to that seen in spinal dorsal horn for afferents that innervate joints and skeletal muscles of lower body regions [19, 49, 54]. Anatomical and functional differences of nociceptive neurons in superficial and deep laminae have been well documented; however, the significance of these differences remains controversial [9, 19, 24, 56]. Moreover, selected estrogen receptor (ER) subtypes are differentially expressed in superficial and deep laminae of Vc [2, 7]. Despite considerable evidence that estrogen status [8, 27] and behavioral stress [32, 47] are significant determinants of pain expression, little is known about their possible interaction [22, 23, 30]. We used repeated forced swim (FS) conditioning [57] to examine the effects of behavioral stress on TMJ-responsive Vc/C 1-2 neurons in superficial and deep laminae and evoked masseter muscle electromyographic (EMG) activity in female rats under high and low estrogen conditions.
Materials and Methods
The protocols were approved by the Institutional Animal Care and Use Committee of University of Minnesota and conformed to established guidelines set by The National Institutes of Health guide for the care and use of laboratory animals (PHS Law 99-158, revised 2002). All efforts were made to minimize the number of animals used for experiments and their suffering.
Estradiol manipulation and forced swim (FS) conditioning
Ovariectomized (OVX) female rats (250–300 g, Sprague-Dawley, Harlan, Indianapolis, IN) were injected with low (LE, 3 μg/kg) or high (HE, 30 μg/kg) dose 17beta-estradiol-3-benzoate (E2, dissolved in sesame seed oil, Sigma, St. Louis, MO) for three days. Estrogen status was confirmed on the day of the experiment by vaginal smear cytology; LE rats had mainly (> 80%) small nucleated leukocytes, while HE rats had mostly large nucleated epithelial cells. One hour after E2 injection, rats were exposed to repeated forced swim conditioning (FS) by placement in a plastic cylinder (diameter 30 cm, height 50 cm) containing 20 cm water (24-26 C) for 10 min between 09:00 and 11:00 for three days [53]. Sham-conditioned rats served as controls and were placed in an empty swim chamber using the same schedule. During the swim session the “immobility” and “struggling” times were recorded [55]. Immobility was defined as time spent in minimal bodily movement to maintain its head above water, whereas struggling was defined as active diving, jumping, or vigorously moving its limbs to break the surface of the water and attempting to escape the container. Rats were dried in a warm environment (30-33 C) after each swim session and fresh water was used for each rat. On Day 4, rats were anesthetized and prepared for neural or muscle recording. Arterial blood was taken at the end of most experiments and plasma E2 determined by radioimmunoassay (Coat-A-Count, DPC, Los Angeles, CA).
Measurement of grip force
Forelimb grip force was measured each day before the swim session and on the day of recording. Briefly, the rat was held by the tail and passed gently (~10 cm/s) three times over a wire mesh grid attached to a strain gauge [38]. The maximum forelimb grip force (GF) observed during the 3 trials was determined for each of the 4 days and averaged daily for each rat.
Extracellular neural recordings
Rats were anesthetized initially with pentobarbital sodium (70 mg/kg, i.p.) and respired artificially with isoflurane (1.5~ 2.0%) and oxygen-enriched room air after tracheotomy. Catheters were placed in the right femoral artery (blood pressure monitor) and right jugular vein (infusion of gallamine triethiodide). Anesthesia was maintained after surgery with isoflurane (1.2~ 2.0%) and a paralytic agent, gallamine triethiodide (20 mg/kg/h) was infused only after completion of all surgical procedures at the time of neural recording. Adequate depth of anesthesia was confirmed by the absence of corneal and hindlimb withdrawal reflexes prior to gallamine, fully constricted pupils and constant arterial blood pressure and heart rate. Expiratory end-tidal CO2 (3.5-4.5%) and mean arterial pressure (MAP, 90-120 mmHg) were monitored throughout the experiment and body temperature was maintained 38°C with a heating blanket and thermal probe.
Animals were placed in a stereotaxic frame and portions of the C1 and C2 vertebrae were removed to expose the Vc/C1-2 region and the caudal brainstem surface was bathed in warm mineral oil. The Vc/C1-2 region, located approximately 5 to 7 mm caudal to the obex and ipsilateral to the exposed mandibular condyle, was explored for TMJ-responsive units using the entrance of the C2 rootlet as a landmark. Extracellular unit activity was recorded with tungsten microelectrodes (5~9 Mohm, Frederick Haer Inc., Bowdoinham, ME) that penetrated the brainstem tangential (~43° off vertical, 60° off midline for laminae I-II, ~36° off vertical, 45° off midline for lamina V) to the surface. Unit activity was amplified, discriminated, stored and analyzed offline on a computer (Apple G4) using a PowerLab interface board and LabChart software (AD Instruments, Colorado Springs, CO). Spike amplitude and shape were monitored continuously and stored on digital tape to confirm unit isolation in off-line analyses. All units included in this study were identified by a vigorous response to mechanical probing of the exposed dorsal surface of the posterior mandibular condyle [52]. Units were further classified on the basis of cutaneous receptive field (RF) properties as either wide dynamic range (WDR), activated by brush, press and pinch stimulation of facial skin or as nociceptive specific (NS) and activated only by press or pinch of the skin.
Experimental design-neural recording
A single TMJ-responsive neuron was recorded in most animal preparations. Units were recorded in superficial laminae (I-II, see Fig 1A left) or deeper lamina (V, Fig 1A right) and within 1.5 mm rostral to the level of entrance of the C2 rootlets. After confirming the response to posterior condyle stimulation, the face and neck were explored for cutaneous input. The cutaneous RF was tested for responses to “brushing” (camel hair), “press” (arterial clip, ~20 mm2) and “pinch” (stiff arterial clip, ~15 mm2) stimuli applied for 10 s. The high threshold RF area was mapped using a small blunt forceps (~3 mm2) onto a standardized series of rat face drawings. After cutaneous RF testing, a guide cannula (26 gauge) was positioned in the TMJ joint space (~3 mm deep) by a dorsal approach directed at the posterior aspect of the mandibular condyle to allow delivery of chemical stimuli. TMJ units were activated by injection of adenosine triphosphate (ATP) into the joint space [68, 69]. Test solutions were delivered from a microsyringe attached by polyethylene tubing to an inner cannula (33 gauge) that protruded ~ 0.5 mm from the end of the guide cannula. Test solutions were delivered slowly over 30 s (total volume = 20 μl) with an inter-injection interval of 20 min to reduce the likelihood of tachyphylaxis. Previously we found that five consecutive injections of 1 mM ATP at 20 min intervals produced consistent responses [69]. The protocol for chemical injections into the TMJ was: phosphate buffered saline (PBS, pH 7.4) followed by four successive doses of ATP (0.001, 0.01, 0.1, and 1.0 mM, pH 7.4, disodium salt, Sigma, St Louis, MO).
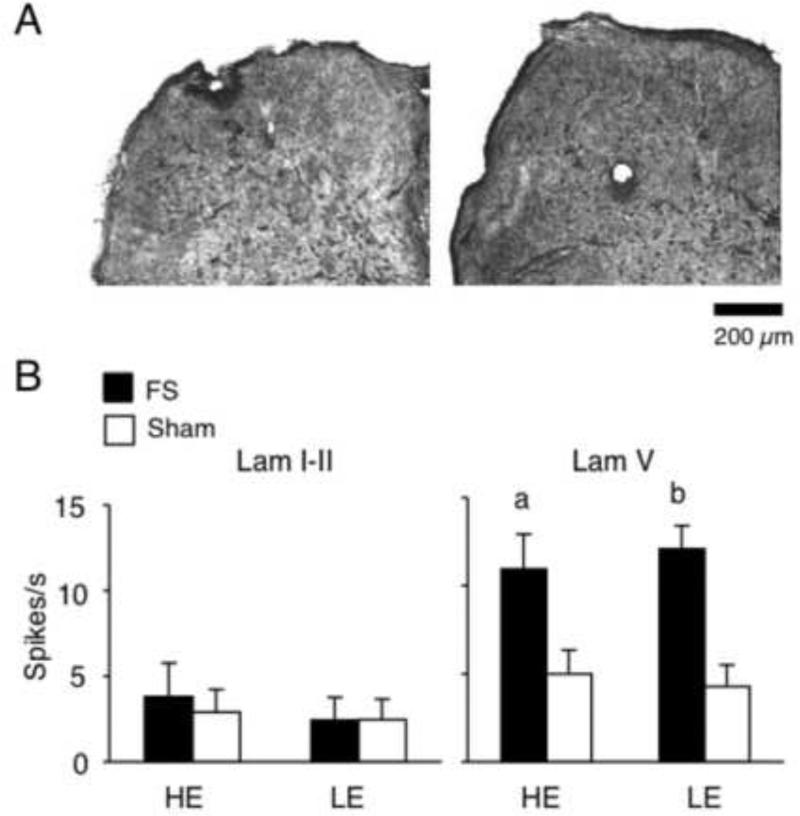
Figure 1.
A. Examples of recording sites in laminae I-II (left) and V (right) at the Vc/C1-2 region.
B. Spontaneous activity of TMJ units in laminae I-II and V. Note that FS increased spontaneous activity of lamina V units only and independent of E2 status. a = P < 0.05, b = P < 0.01 versus sham rats. n = 7-18 units per group.
Data analysis-neural recording
Neural data were acquired and displayed as peristimulus time histograms of spikes per 1 s bins, exported to a spreadsheet and analyzed off-line. Spontaneous activity (spikes/s) was calculated as the average spike count over a 1 min epoch immediately preceding each stimulus. The evoked responses were assessed by calculating the response magnitude (Rmag), determined by subtracting the mean plus 2 times the standard deviation (SD) of background activity from the total spike count for each bin. The total Rmag for each stimulus was defined as the cumulative sum of spikes over contiguous bins in which the spike count minus the background was a positive value. The total Rmag is equivalent to the “area under the curve” for each stimulus period. The response duration was defined as the time interval after stimulus onset until three consecutive bins with a positive spike count occurred above background (initial latency) and until the value of three consecutive bins no longer exceeded the mean + 2 SD above background activity as described previously [67]. Units that failed to show three consecutive bins with positive Rmag values within 100 s after stimulus onset were considered unresponsive to that condition. Units were defined as ATP-responsive if the total Rmag exceeded the response to PBS by > 50%, independent of ATP dose. The threshold dose of ATP was defined as the lowest concentration that produced a total Rmag exceeding that to PBS by > 50%. The total Rmag to mechanical stimulation of the skin overlying the TMJ (e.g., brush, press, pinch) was determined over a 10 s stimulus period. Total Rmag, response duration and latency to chemical and mechanical stimuli were assessed statistically by analysis of variance (ANOVA) corrected for repeated measures and individual comparisons were made by Newman-Keuls after ANOVA. Fisher's Exact Probability test determined if the number of units responding to the lowest concentration of ATP (> 50% versus Rmag response to intra-TMJ injection of PBS) was similar across treatment groups. The cutaneous high threshold RF areas for units were digitized and quantified by a planimetric method using NIH ImageJ software and compared by ANOVA. Values in the text were expressed as mean SEM and P < 0.05 was regarded as significant.
Electromyographic (EMG) recording
Animals were anesthetized with pentobarbital sodium (50 mg/kg i.p.) and a catheter was positioned in the right femoral artery for monitoring MAP. After tracheotomy, animals were respired artificially with isoflurane (1.0-2.0%) and oxygen-enriched room air. End-tidal CO2, MAP and body temperature were monitored throughout the experiment and kept within normal range. Animals were placed in a stereotaxic frame and the TMJ region was exposed for guide cannula implantation in the joint space as described above. A small skin incision was made to expose the surface of the left masseter muscle and paired wire electrodes (0.12 mm diameter, 5 mm interpolar distance) were implanted ~1 mm into the central portion of the muscle. EMG activity was sampled at 1000 Hz, amplified (x10k), filtered (bandwidth 300-3000 Hz), displayed and stored for off-line analyses.
Data analysis-EMG activity
EMG activity was sampled continuously for 6 min, beginning 3 min before and after each TMJ stimulus. Activity was rectified and stored as 1 s bins for off-line analyses. Baseline activity was quantified as the area under the curve (AUC) for the 3 min epoch (μV per 3 min) sampled immediately prior to stimulation. TMJ-evoked EMG activity was calculated as AUC post-ATP injection minus baseline AUC. The response latency (onset) was defined as the time that AUC first exceeded the average baseline (activity/s). Results were assessed statistically by ANOVA, corrected for repeated measures and individual comparisons were made by Newman-Keuls after ANOVA. The threshold dose of ATP was defined as the lowest concentration that produced an AUC exceeding that to PBS by > 50%. Fisher's Exact Probability test determined if the number of rats responding to the lowest concentration of ATP (> 50% versus AUC to intra-TMJ injection of PBS) was different across treatment groups.
Histology
At the end of each neural recording experiment, rats were given a bolus dose of pentobarbital (100 mg/kg, i.p.) and perfused through the heart with 10% formalin. The recording site was marked electrolytically (5 μA, 20 s). Recording and injections sites were confirmed from transverse sections (40 μm) and drawn onto a standard series of rat brainstem outlines [67].
Results
General and behavioral effects of stress
A total of 128 rats were used for neural (n = 95) and EMG (n = 33) recording experiments. Prior to stress conditioning the body weights were similar for FS (HE, n = 36, 294 ± 3 g; LE, n = 30, 297 ± 3 g) and sham (HE, n = 35, 294 ± 3 g; LE: n = 27, 298 ± 5 g). Three days of FS caused a significant reduction in body weight in HE (-4.4 ± 0.4 %, F3, 105 = 77, P < 0.01) and LE (-4.1 ± 0.4 %, F3, 90 = 51.1, P < 0.01) animals, while sham conditioning also caused a small but significant reduction (HE, -2.6 ± 0.4 %, F3, 105 = 21.6, P < 0.01; LE, -2.3 ± 0.4 %, F3, 78 = 22.5, P < 0.01). The reductions in body weight were similar when assessed across all treatment groups (F3, 124 = 0.8, P > 0.1).
Plasma samples were collected on day 4 at the end of the experiment and assayed for estradiol (E2). High E2-FS treated animals averaged 85.3 12.8 pg/ml E2 (n = 20) and 78.3 10.3 pg/ml for HE-sham rats (n = 21). Low E2-FS treated animals averaged 11.2 3.2 pg/ml E2 (n = 22) and 10.7 3.9 pg/ml for LE-sham rats (n = 20). Plasma E2 levels for HE groups were significantly greater compared to LE rats (F3, 79 = 23.4, P < 0.01); however, FS did not affect E2 levels in HE (F1, 39 = 0.2, P > 0.05) or LE groups (F1, 40 = 0.1, P > 0.05) compared to sham controls.
Immobility time (IT), as a percentage of the 10 min swim session, increased significantly after 3 days of FS compared to the initial session in HE (96.3 ± 1.4% versus 84.8 ± 1.2%) and LE (95.6 ± 0.6% versus 85.7 ± 1.2%) rats (F2, 128 = 17.4, P < 0.01) similarly, suggesting that FS-evoked depression-like behavior was not affected by E2 status (F1, 64 = 0.32, P > 0.1). There were no group differences in maximum grip force (GF) prior to the conditioning sessions (HE-FS: 1042 ± 17 g, HE-sham = 1063 ± 14 g), LE-FS: 1058 ± 18 g, LE-sham = 1044 ± 21 g). After 3 days of FS, GF was reduced similarly for HE-FS (-12.1 ± 2.5 %, F 3, 105 = 7.9, P < 0.01) and LE-FS rats (-12.3 ± 2.3 %, F3, 87 = 12.8, P < 0.001), whereas sham conditioning had no effect (HE-sham -4.2 ± 1.7% and LE-sham -1.3 ± 4.0%, P > 0.1). These results indicated that the FS protocol reliably reduced body weight, increased depression-like behavior and increased forelimb muscle weakness and/or hyperalgesia independent of E2 status in agreement with our previous report [23].
TMJ-responsive neurons: general properties
A total of 104 TMJ-responsive units were recorded in superficial (n = 63) and deep laminae (n = 41) and all sites were located within 1.5 mm rostral to the level of entrance of the C2 rootlets (see examples in Fig 1A). The depth of recording was similar across treatment groups for neurons recorded in superficial (range = 10-287 μm) and deep laminae (range = 675-1710 μm). However, since an acute angle of penetration was used, the exact vertical distance from the dorsal brainstem surface was not determined.
All units displayed spontaneous activity prior to stimulation. The mean discharge rate for neurons in superficial laminae was similar for all treatment groups (Fig 1B, left panel; F 3, 59 = 1.3, P > 0.1). The spontaneous activity of neurons in deep laminae from HE-FS and LE-FS rats was significantly elevated compared to their sham controls (Fig 1B, right panel, F3, 49 = 6.8, P < 0.01). These findings indicated that spontaneous activity of TMJ-responsive neurons in deep, but not superficial, laminae were markedly increased by FS conditioning.
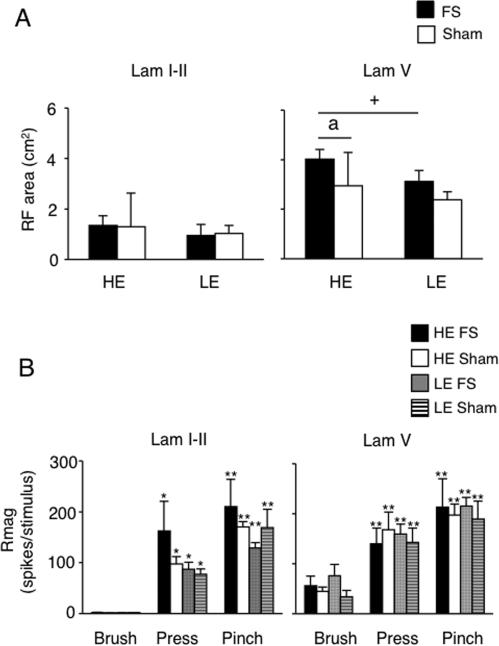
All TMJ units received convergent input from facial skin. Based on the responses to mechanical stimulation of the skin, units from superficial laminae were further classified as NS, while those from deep laminae were classified as WDR units. In all cases the cutaneous RF was positioned over the TMJ and extended anterior and ventral within the maxillary and mandibular territories of the trigeminal nerve. The average cutaneous RF area of neurons in deep laminae was significantly larger than for units in superficial laminae for all groups (Fig 2A, F 7, 96 = 19.4, P < 0.001). Further, units in deep laminae of HE-FS treated rats had significantly larger RF areas than those from HE-sham rats (P < 0.05, NK test) or LE-FS treated rats (P < 0.05, NK test). TMJ units in superficial laminae (NS units) did not respond to brush, but did display vigorous responses to both press and pinch (Fig 2B, left panel, F2,26 = 595, P < 0.001). TMJ units in deep laminae (WDR units) had vigorous responses to brush, press and pinch (Fig 2B, right panel, F2, 44 = 51, P < 0.001). The evoked response to mechanical stimulation of facial skin of superficial and deep laminae units was not altered by FS or E2 status (superficial, F3, 13 = 1.0, P > 0.1; deep, F3, 22 = 0.2, P > 0.1). These results indicated that units in deep laminae from HE-FS rats had larger RF areas than deep units from other treatment groups; however, the magnitude of the response to mechanical stimulation of the skin was not affected by E2 status or stress conditioning.
Figure 2.
A. High threshold convergent cutaneous receptive field (RF) areas of TMJ units in laminae I-II and V. Sample sizes: laminae I-II units: HE-FS, n = 17; HE-sham, n = 18; LE-FS, n = 14; LE-sham, n = 14; lamina V units: HE-FS, n = 11; HE-sham, n = 13; LE-FS, n = 10; LE-sham, n = 7. a = P < 0.05 versus sham; + = P < 0.05 HE versus LE.
B. Response magnitude of TMJ units in laminae I-II and V to mechanical stimulation of facial skin overlying the TMJ. Sample sizes: laminae I-II units: HE-FS, n = 5; HE-sham, n = 4; LE-FS, n = 4; LE-sham, n = 4; laminae V units: HE-FS, n = 5; HE-sham, n = 11; LE-FS, n = 6; LE-sham, n = 4. *P < 0.05, **P < 0.01 versus response to brush stimulation.
TMJ-responsive neurons and ATP-evoked responses
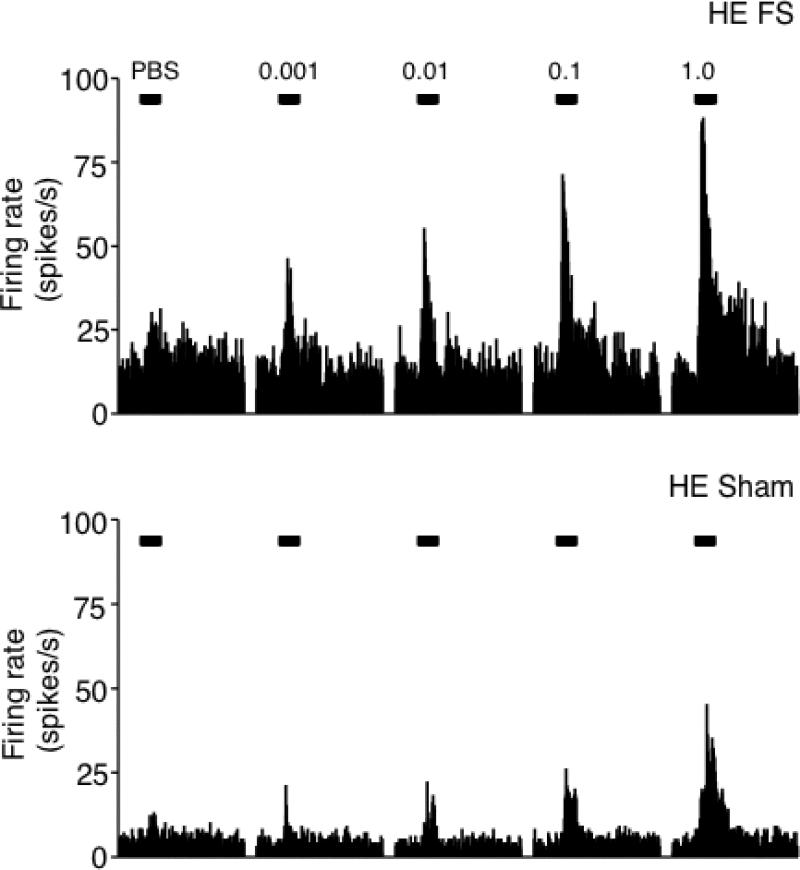
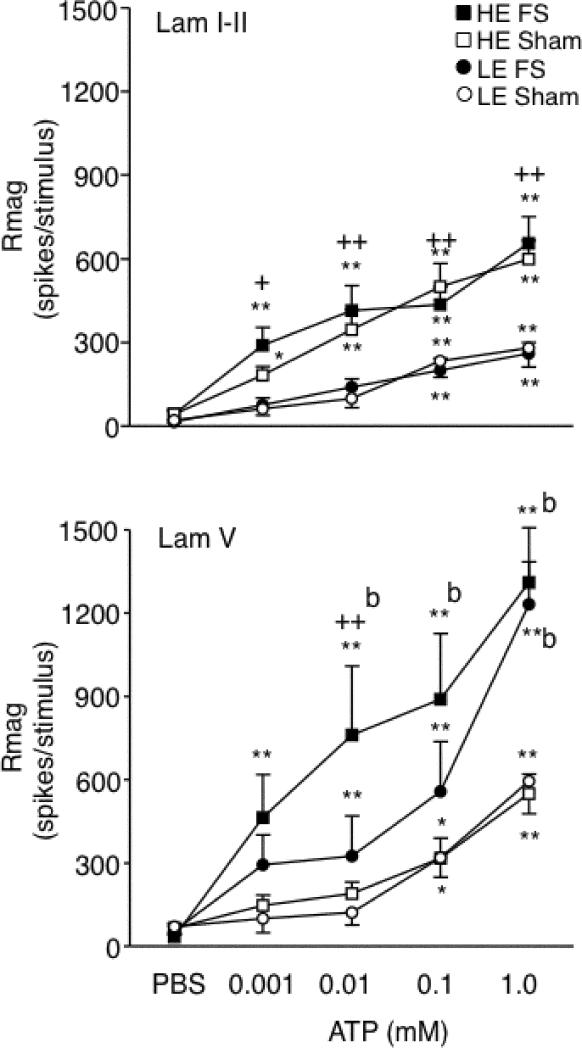
Figure 3 presents examples of neural activity from units in deep laminae evoked by intra-TMJ injections of ATP in HE-FS and HE-sham rats. The pattern of ATP-evoked activity was similar for units in superficial and deep laminae; however, E2 status and FS influenced the responses in a laminae-specific manner. In superficial laminae, high E2 status markedly enhanced the magnitude of the ATP-evoked neural response under sham conditions (F3, 59 = 7.8, P < 0.001), while FS had no effect (Fig 4, upper panel). Response duration also increased significantly with higher doses of ATP (F4, 370 = 112, P < 0.001); however, this increase was similar across treatment groups (duration after 1 mM ATP: HE-FS = 53 ± 6s; HE-sham = 52 ± 5s; LE-FS = 37 ± 5s; LE-sham = 47 ± 3s). Response latency was reduced with higher doses of ATP for all treatment groups (F4, 239 = 38, P < 0.001). The lowest dose of ATP (0.001 mM) evoked a significant neural response (>50% above PBS) in 29 of 35 units from HE-treated rats (FS + sham) versus 13 of 28 units from LE-treated (FS + sham) rats (P = 0.002, Fisher's exact probability). These results indicated that E2 status, but not FS, significantly affected both the response magnitude and the threshold dose of ATP necessary to activate TMJ units in superficial laminae.
Figure 3.
Examples of peristimulus time histograms of the responses to intra-TMJ injections of ATP of units recorded in lamina V at the Vc/C1-2 region from HE-FS (upper panel) and HE-sham (lower panel) rats. Calibration bars (30 s) indicate the time of intra-TMJ injections of PBS, 0.001, 0.01, 0.1 1.0 mM ATP (20 μl). Twenty minutes elapsed between each injection.
Figure 4.
Total response magnitude (Rmag) to intra-TMJ injections of ATP on TMJ units recorded in superficial (upper panel) and deep laminae (lower panel) defined as ATP responsiveness from FS and sham rats under HE and LE conditions. Sample sizes: laminae I-II, HE-FS = 17, HE-sham = 18, LE-FS = 14, LE-sham = 14; lamina V, HE-FS = 11, HE-sham = 13, LE-FS = 10, LE-sham = 7 units. *P < 0.05, **P < 0.01 versus response to PBS; b = P < 0.01 versus sham group; + = P < 0.05, ++ = P < 0.01 HE- versus corresponding LE-treated group.
In deep laminae, FS enhanced the magnitude of the ATP-evoked neural response (F3, 37 = 4.3, P < 0.01), while E2 status had only minor effects (Fig 4, lower panel). Response duration increased significantly and across all treatment groups with higher doses of ATP (F4, 147 = 58, P < 0.001; duration after 1 mM ATP: HE-FS = 82 ± 6s; HE-sham = 54 ± 8s; LE-FS = 72 ± 6s; LE-sham = 59 ± 7s). Increasing doses of ATP caused a significant and similar decrease in response latency across all treatment groups (F4, 147 = 22, P < 0.001). The lowest dose of ATP (0.001 mM) evoked a significant neural response (>50% above PBS) in 18 of 21 units from FS-treated (HE+LE) rats versus 11 of 20 units from sham-treated (HE+LE) rats (P = 0.05, Fisher's exact probability). These results indicated that FS conditioning, but not E2 status, significantly affected both the response magnitude and threshold dose of ATP necessary to activate TMJ units in deep laminae.
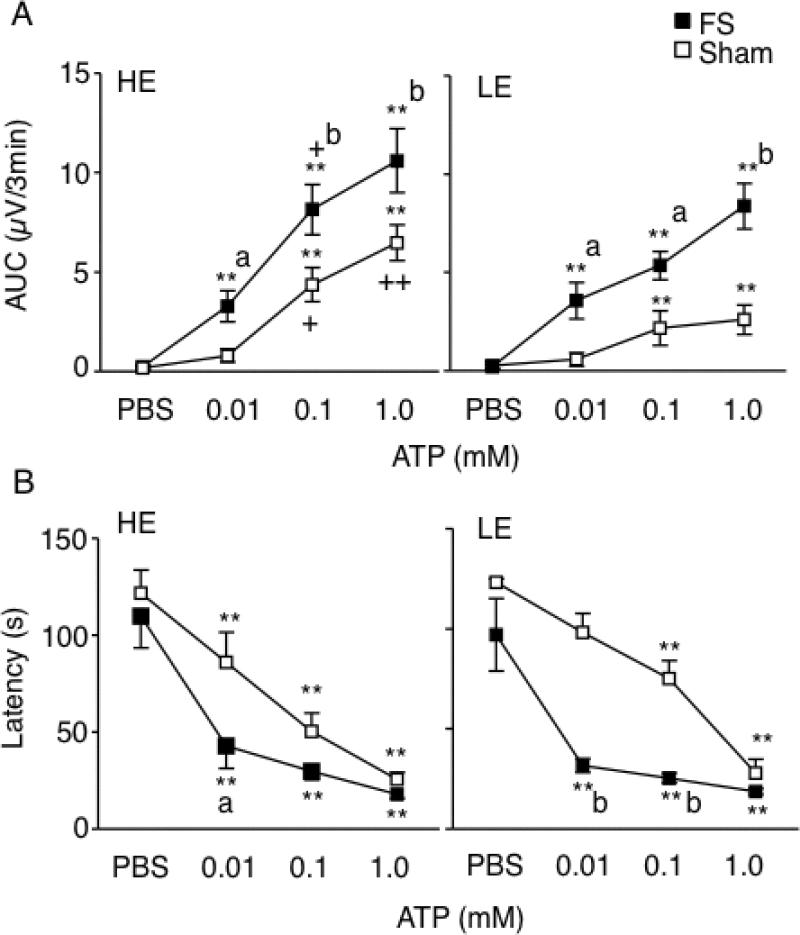
Masseter muscle electromyographic (EMG) activity
Resting masseter muscle EMG activity was sampled over 3 min prior to the initial intra-TMJ injection of PBS and found to be similar across all treatment groups (F3, 29 = 0.7, P > 0.1). The increase in EMG after intra-TMJ injections of ATP was dose-dependent across all treatment groups (F3, 87 = 63.6, P < 0.001, Fig 5A). FS markedly enhanced the ATP-evoked EMG activity compared to sham conditioning for both HE and LE-treated rats. Estrogen status had a small but significant effect on evoked EMG activity as seen by the enhanced response to 0.1 mM ATP in HE-treated rats compared to LE-treated rats after both FS and sham conditioning (F3,107 = 8.9, P < 0.01). The number of rats displaying a significant (>50% above PBS) increase in EMG activity after intra-TMJ injection of the lowest dose of ATP (0.01 mM) was not different across treatment groups (HE-FS, 8 of 9; LE-FS, 8 of 8; HE-sham, 5 of 8; LE-sham, 6 of 8 rats) and suggested that neither E2 status or FS altered the threshold dose of ATP necessary to evoke EMG activity. However, we cannot exclude that lower doses of ATP may have revealed group differences in threshold. Intra-TMJ injections of ATP also caused a dose-dependent decrease in EMG response latency for all groups (F3, 87 = 67.3, P < 0.01, Fig 5B). Group comparisons revealed that FS significantly decreased the latency compared to sham rats (F3, 29 = 7.0, P < 0.005) independent of E2 status. These findings indicated that stress conditioning was a significant modulator of TMJ-evoked masseter muscle EMG activity; however, E2 status also contributed to evoked jaw muscle activity in females.
Figure 5.
Effect of E2 status and psychophysical stress on masseter muscle EMG activity evoked by intra-TMJ injections of ATP. A. TMJ-evoked masseter muscle EMG activity increased significantly in a dose-dependent manner after FS and sham conditioning and under HE and LE conditions. B. Response latency decreased significantly in all treatment groups. Sample sizes: HE-FS = 9, HE-sham = 8, LE-FS = 8, LE-sham = 8 rats. **P < 0.01 versus PBS; a = P < 0.05, b = P < 0.01 versus sham; + = P < 0.05, ++ = P < 0.01 HE- versus corresponding LE-treated group.
Discussion
Anatomical and physiological differences between superficial and deep laminae of spinal dorsal horn have been well documented, and although their roles remain controversial, most studies have focused on effects due to injury and not environmental factors [19, 24, 55]. Our present results indicated that although neurons in superficial and deep laminae encoded TMJ stimulus intensity, there were marked differential effects of estrogen status and behavioral stress on neurons in each lamina. High E2 conditions significantly enhanced the TMJ-evoked responses of neurons in superficial laminae independent of stress conditioning, whereas neurons in deep laminae were not affected. By contrast, FS conditioning increased the TMJ-evoked responses of neurons in deep laminae independent of estrogen status, whereas neurons in superficial laminae were not affected. TMJ-evoked EMG activity was markedly enhanced after FS; however, high E2 also caused smaller yet significant increases suggesting that neurons in superficial and deep dorsal horn contribute to masseter muscle EMG activity induced by TMJ stimulation. These findings suggest that internal (estrogen status) and external influences (stress) contribute to TMJ nociception through changes in the response properties at the Vc/C 1-2 region in a laminar-specific manner.
The basis for the lamina specificity in this study is not certain; however, three sites of action may be considered. Estrogen status and behavioral stress may act peripherally to selectively influence subpopulations of primary afferent neurons. Second, they may act locally to engage select subpopulations of dorsal horn neurons or third, they may selectively modify the recruitment and/or outflow of supraspinal endogenous pain control pathways. Sensory fibers that innervate the knee joint [20] and hindlimb muscle [49, 54] terminate in the superficial and deep laminae of the spinal dorsal horn. A similar laminar pattern is seen for TMJ and jaw muscles afferents at the Vc/C 1-2 region [5, 21, 63]. The TMJ and jaw muscles are supplied mainly by small diameter sensory fibers [40, 68] that express TRPV1 [18, 35] or isolectin B4 (IB4) [3], while the expression of TRPV1 [6] and IB4 [41, 65] at the Vc/C1-2 region is restricted to the superficial laminae. Thus, a peripheral basis for estrogen status and stress induced lamina specificity in medullary dorsal horn could derive from preferential actions on unmyelinated C fibers. Most estrogen receptor (ER)-positive trigeminal ganglion (TG) neurons are small to medium-sized cells [7, 44]; however, it is not known what percentage also supply the TMJ or jaw muscles or what percentage are associated with unmyelinated fibers. In isolated TMJ-labeled TG neurons E2 treatment increased neuronal excitability overall; however, E2 did not affect neurons activated by capsaicin or labeled with IB4 suggesting no preferential effect on C fibers [28]. Glutamate-evoked TMJ afferent activity was greatest for the slowest conducting fibers; however, estrogen status did not affect this activity [12]. Behavioral stress increased mechanical-evoked excitability of hindlimb muscle afferents in male rats; however, no preferential effect on unmyelinated and myelinated fibers was noted [15]. Also, peripheral effects of stress on non-neural tissue cannot be excluded [16]. In this study FS increased the spontaneous activity and reduced the threshold dose of ATP required to activate TMJ units in deep, but not superficial laminae, consistent with a peripheral effect; however, the responses to mechanical stimulation of facial skin were not altered. Thus, although our data suggested that estrogen status and behavioral stress influence some aspects of TMJ neural activity through peripheral mechanisms, these actions alone were not sufficient to account for the lamina specificity.
Lamina specificity in TMJ nociception may have resulted from local effects of estrogen status and behavioral stress on subpopulations of dorsal horn neurons. The superficial laminae at Vc/C1-2 region contain many more ER-positive neurons than deeper laminae [3, 7] consistent with the selective effects of E2 on TMJ neurons seen in this study and our previous study [70]. A high percentage of ER-positive neurons in superficial, but not deep laminae at Vc/C1-2 region also express preproenkephalin mRNA or Opioid receptor-like receptor [1, 29]. Enkephalin-producing neurons and opioid receptors in dorsal horn are key components of pain modulatory pathways and are regulated by estrogens [1, 29], suggesting that lamina specificity could derive from trophic influences of E2 treatment on subpopulations of local circuit neurons. Previously, we reported that morphine-induced inhibition of TMJ units in superficial, but not deep, laminae depended on estrogen status in female rats [69]. Stimulus modality also may have contributed to the selective effects on neurons in superficial laminae since ATP-evoked responses were enhanced by high E2 while responses to cutaneous input was not affected. Evidence that lamina specific effects from behavioral stress were due to actions on select subpopulations of dorsal horn neurons has not been consistent. Repeated FS increased the phosphorylation of potassium channels mainly in superficial spinal dorsal horn [36]; however, expression of phospho-CREB positive neurons in medullary dorsal horn was increased in both superficial and deep laminae and independent of estrogen status [23]. Thus, local actions of E2 at the dorsal horn could account for the lamina-specific effects on TMJ units, while the site(s) of behavioral stress effects on TMJ processing are less certain.
Pain modulatory pathways from the periaqueductal gray-rostral ventromedial medulla (PAG-RVM) have long been associated with stress-induced hyperalgesia [31, 47]. Behavioral stress activated neurons in the RVM [33], while local inhibition of RVM prevented hyperalgesia induced by repeated FS [34] and other chronic stress models [60, 61]. RVM efferent fibers project to superficial and deep laminae of the spinal dorsal horn [4, 26]; however, both anatomical [37, 42] and physiological [14, 48] evidence exist to support differential control of nociceptive neurons in superficial versus deep laminae. Notably, Lumb and colleagues [31] have reported that stimulation of the PAG-RVM system preferentially modified C fiber input to spinal dorsal horn neurons, although results from superficial and deep laminae were not compared. Selective ablation of NK1-positive neurons in superficial laminae of spinal dorsal horn reduced nociceptive behavior [50] and sensitization of deep dorsal horn neurons [66]; however, when neurons from superficial and deep laminae were included no preferential effects were seen [39]. Since C fiber input to deep dorsal horn is sparse [9], one possible pathway to account for lamina-specificity and FS on TMJ units involves dorsal horn interneurons in superficial laminae that receive C fiber input and also project to deeper laminae [25, 43, 59]. Stimulus modality also may have contributed to these results since FS had no effect on the magnitude of facial skin-evoked responses of TMJ units in the present study. Earlier studies also reported that descending controls had greater effects on deep tissue input compared to cutaneous input to dorsal horn neurons [17, 72]. Thus, these studies suggested that the lamina-specific effect of FS on TMJ units in deep dorsal horn could be mediated by differential recruitment and/or outflow from descending controls in the brainstem.
TMJ-evoked masseter muscle EMG activity was markedly enhanced after FS; however, high E2 also caused a significant increase. This suggested that neurons in both superficial and deep laminae at the Vc/C 1-2 region play a role in modifying masseter muscle EMG activity. Previously we reported that local blockade of the Vc/C 1-2 region prevented TMJ-evoked increases in EMG activity in male rats [53]. Earlier studies in female rats revealed that TMJ-evoked increase in jaw muscle activity was enhanced under high E2 conditions [13], in agreement with the present results. Jaw muscle EMG activity has used been extensively as a behavioral measure for TMJ nociception [10, 11, 12, 73].
Estrogen status and behavioral stress, risk factors thought to play critical roles in persistent jaw pain, produced marked lamina-specific effects on the encoding properties of TMJ-responsive neurons at the Vc/C1-2 region. These results support the hypothesis that TMJ-responsive neurons in superficial and deep laminae at the Vc/C1-2 region contribute to different aspects of TMJ nociception in females that may contribute to altered TMJ-evoked jaw muscle activity during persistent psychophysical stress.
Acknowledgements
The authors thank Dr. Zheng Chang for excellent technical assistance. This study was supported by a grant from the National Institute of Dental and Craniofacial Research: DE12758 (DAB) and the Office of Research on Women's Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial or other relationships to report that might lead to a conflict interest.
References
- 1.Amandusson A, Hallbeck M, Hallbeck AL, Hermanson O, Blomqvist A. Estrogen-induced alterations of spinal cord enkephalin gene expression. Pain. 1999;83:243–248. doi: 10.1016/s0304-3959(99)00109-8. [DOI] [PubMed] [Google Scholar]
- 2.Amandusson A, Hermanson O, Blomqvist A. Estrogen receptor-like immunoreactivity in the medullary and spinal dorsal horn of the female rat. Neurosci Lett. 1995;196:25–28. doi: 10.1016/0304-3940(95)11828-k. [DOI] [PubMed] [Google Scholar]
- 3.Ambalavanar R, Moritani M, Dessem D. Trigeminal P2X(3) receptor expression differs from dorsal root ganglion and is modulated by deep tissue inflammation. Pain. 2005;117:280–291. doi: 10.1016/j.pain.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 4.Antal M, Petko M, Polgar E, Heizmann CW, Storm-Mathisen J. Direct evidence of an extensive GABAergic innervation of the spinal dorsal horn by fibres descending from the rostral ventromedial medulla. Neuroscience. 1996;73:509–518. doi: 10.1016/0306-4522(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 5.Arvidsson J, Raappana P. An HRP study of the central projections from primary sensory neurons innervating the rat masseter muscle. Brain Res. 1989;480:111–118. doi: 10.1016/0006-8993(89)91573-4. [DOI] [PubMed] [Google Scholar]
- 6.Bae YC, Oh JM, Hwang SJ, Shigenaga Y, Valtschanoff JG. Expression of vanilloid receptor TRPV1 in the rat trigeminal sensory nuclei. J Comp Neurol. 2004;478:62–71. doi: 10.1002/cne.20272. [DOI] [PubMed] [Google Scholar]
- 7.Bereiter DA, Cioffi JL, Bereiter DF. Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Arch Oral Biol. 2005;50:971–979. doi: 10.1016/j.archoralbio.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Bereiter DA, Okamoto K. Neurobiology of estrogen status in deep craniofacial pain. Int Rev Neurobiol. 2011;97:251–284. doi: 10.1016/B978-0-12-385198-7.00010-2. [DOI] [PubMed] [Google Scholar]
- 9.Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Broton JG, Sessle BJ. Reflex excitation of masticatory muscles induced by algesic chemicals applied to the temporomandibular joint of the cat. Arch Oral Biol. 1988;33:741–747. doi: 10.1016/0003-9969(88)90008-8. [DOI] [PubMed] [Google Scholar]
- 11.Cairns BE, Sessle BJ, Hu JW. Evidence that excitatory amino acid receptors within the temporomandibular joint region are involved in the reflex activation of the jaw muscles. J Neurosci. 1998;18:8056–8064. doi: 10.1523/JNEUROSCI.18-19-08056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cairns BE, Sessle BJ, Hu JW. Characteristics of glutamate-evoked temporomandibular joint afferent activity in the rat. J Neurophysiol. 2001;85:2446–2454. doi: 10.1152/jn.2001.85.6.2446. [DOI] [PubMed] [Google Scholar]
- 13.Cairns BE, Sim Y, Bereiter DA, Sessle BJ, Hu JW. Influence of sex on reflex jaw muscle activity evoked from the rat temporomandibular joint. Brain Res. 2002;957:338–344. doi: 10.1016/s0006-8993(02)03671-5. [DOI] [PubMed] [Google Scholar]
- 14.Cervero F, Schaible H-G, Schmidt RF. Tonic descending inhibition of spinal cord neurones driven by joint afferents in normal cats and in cats with an inflamed knee joint. Exp Brain Res. 1991;83:675–678. doi: 10.1007/BF00229846. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Green PG, Levine JD. Stress enhances muscle nociceptor activity in the rat. Neuroscience. 2011a;185:166–173. doi: 10.1016/j.neuroscience.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YJ, Huang F, Zhang M, Shang HY. Psychological stress alters ultrastructure and energy metabolism of masticatory muscle in rats. J Biomed Biotechnol. 2011b;2010:302693. doi: 10.1155/2010/302693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang CY, Hu JW, Sessle BJ. Parabrachial area and nucleus raphe magnus-induced modulation of nociceptive and nonnociceptive trigeminal subnucleus caudalis neurons activated by cutaneous or deep inputs. J Neurophysiol. 1994;71:2430–2445. doi: 10.1152/jn.1994.71.6.2430. [DOI] [PubMed] [Google Scholar]
- 18.Connor M, Naves LA, McCleskey EW. Contrasti ng phenotypes of putative proprioceptive and nociceptive trigeminal neurons innervating jaw muscle in rat. Mol Pain. 2005;1:31. doi: 10.1186/1744-8069-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- 20.Craig AD, Hepplemann B, Schaible H-G. The projection of the medial and posterior articular nerves of the cat's knee to the spinal cord. J Comp Neurol. 1988;276:279–288. doi: 10.1002/cne.902760210. [DOI] [PubMed] [Google Scholar]
- 21.Dessem D, Moritani M, Ambalavanar R. Nociceptive craniofacial muscle primary afferent neurons synapse in both the rostral and caudal brain stem. J Neurophysiol. 2007;98:214–223. doi: 10.1152/jn.00990.2006. [DOI] [PubMed] [Google Scholar]
- 22.Devall AJ, Liu ZW, Lovick TA. Hyperalgesia in the setting of anxiety: sex differences and effects of the oestrous cycle in Wistar rats. Psychoneuroendocrinology. 2009;34:587–596. doi: 10.1016/j.psyneuen.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Duenes SL, Thompson R, Chang Z, Okamoto K, Bereiter DA. Psychophysical stress increases the expression of phospho-CREB, Fos protein and neurokinin-1 receptors in superficial laminae of trigeminal subnucleus caudalis in female rats. Neurosci Lett. 2010;486:207–210. doi: 10.1016/j.neulet.2010.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckert WA, 3rd, Julius D, Basbaum AI. Differential contribution of TRPV1 to thermal responses and tissue injury-induced sensitization of dorsal horn neurons in laminae I and V in the mouse. Pain. 2006;126:184–197. doi: 10.1016/j.pain.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 25.Eckert WA, McNaughton KK, Light AR. Morphology and axonal arborization of rat inner lamina II neurons hyperpolarized by m-opioid-selective agonists. J Comp Neurol. 2003;458:240–256. doi: 10.1002/cne.10587. [DOI] [PubMed] [Google Scholar]
- 26.Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol. 1995;74:1742–1759. doi: 10.1152/jn.1995.74.4.1742. [DOI] [PubMed] [Google Scholar]
- 27.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flake NM, Bonebreak DB, Gold MS. Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons. J Neurophysiol. 2005;93:1585–1597. doi: 10.1152/jn.00269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flores CA, Shughrue P, Petersen SL, Mokha SS. Sex-related differences in the distribution of opioid receptor-like 1 receptor mRNA and colocalization with estrogen receptor mRNA in neurons of the spinal trigeminal nucleus caudalis in the rat. Neuroscience. 2003;118:769–778. doi: 10.1016/s0306-4522(02)01000-x. [DOI] [PubMed] [Google Scholar]
- 30.Green PG, Levine JD. Sexual dimorphism in the effect of nonhabituating stress on neurogenic plasma extravasation. Eur J Neurosci. 2005;21:486–492. doi: 10.1111/j.1460-9568.2005.03872.x. [DOI] [PubMed] [Google Scholar]
- 31.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imbe H, Iwai-Liao Y, Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Front Biosci. 2006;11:2179–2192. doi: 10.2741/1960. [DOI] [PubMed] [Google Scholar]
- 33.Imbe H, Murakami S, Okamoto K, Iwai-Liao Y, Senba E. The effects of acute and chronic restraint stress on activation of ERK in the rostral ventromedial medulla and locus coeruleus. Pain. 2004;112(3):361–371. doi: 10.1016/j.pain.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 34.Imbe H, Okamoto K, Donishi T, Senba E, Kimura A. Involvement of descending facilitation from the rostral ventromedial medulla in the enhancement of formalin-evoked nocifensive behavior following repeated forced swim stress. Brain Res. 2010;1329:103–112. doi: 10.1016/j.brainres.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Ioi H, Kido MA, Zhang JQ, Yamaza T, Nakata S, Nakasima A, Tanaka T. Capsaicin receptor expression in the rat temporomandibular joint. Cell Tissue Res. 2006;325:47–54. doi: 10.1007/s00441-006-0183-7. [DOI] [PubMed] [Google Scholar]
- 36.Ippolito DL, Xu M, Bruchas MR, Wickman K, Chavkin C. Tyrosine phosphorylation of K(ir)3.1 in spinal cord is induced by acute inflammation, chronic neuropathic pain, and behavioral stress. J Biol Chem. 2005;280(50):41683–41693. doi: 10.1074/jbc.M507069200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones SL, Light AR. Termination patterns of serotoninergic medullary raphespinal fibers in the rat lumbar spinal cord: an anterograde immunohistochemical study. J Comp Neurol. 1990;297:267–282. doi: 10.1002/cne.902970209. [DOI] [PubMed] [Google Scholar]
- 38.Kehl LJ, Trempe TM, Hargreaves KM. A new animal model for assessing mechanisms and management of muscle hyperalgesia. Pain. 2000;85:333–343. doi: 10.1016/S0304-3959(99)00282-1. [DOI] [PubMed] [Google Scholar]
- 39.Khasabov SG, Rogers SD, Ghilardi JR, Peters CM, Mantyh PW, Simone DA. Spinal neurons that possess the substance P receptor are required for the development of central sensitization. J Neurosci. 2002;22:9086–9098. doi: 10.1523/JNEUROSCI.22-20-09086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kido MA, Kiyoshima T, Ibuki T, Shimizu S, Kondo T, Terada Y, Tanaka T. A topographical and ultrastructural study of sensory trigeminal nerve endings in the rat temporomandibular joint as demonstrated by anterograde transport of wheat germ agglutinin-horseradish peroxidase (WGA-HRP). J Dent Res. 1995;74:1353–1359. doi: 10.1177/00220345950740070601. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi Y, Matsumura G. Central projections of primary afferent fibers from the rat trigeminal nerve labeled with isolectin B4-HRP. Neurosci Lett. 1996;217:89–92. [PubMed] [Google Scholar]
- 42.Li J-L, Kaneko T, Nomura S, Li Y-Q, Mizuno N. Association of serotonin-like immunoreactive axons with nociceptive projection neuons in the caudal spinal trigeminal nucleus of the rat. J Comp Neurol. 1997;384:127–141. doi: 10.1002/(sici)1096-9861(19970721)384:1<127::aid-cne8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 43.Light AR, Kavookjian AM. Morphology and ultrastructure of physiologically identified substantia gelatinosa (Lamina II) neurons with axons that terminate in deeper dorsal horn laminae (III-V). J Comp Neurol. 1988;267:172–189. doi: 10.1002/cne.902670203. [DOI] [PubMed] [Google Scholar]
- 44.Liverman CS, Brown JW, Sandhir R, McCarson KE, Berman NE. Role of the oestrogen receptors GPR30 and ERalpha in peripheral sensitization: relevance to trigeminal pain disorders in women. Cephalalgia. 2009;29:729–741. doi: 10.1111/j.1468-2982.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maixner W. Temporomandibular joint disorders. In: Mayer E, Bushnell M, editors. Functional pain syndromes: presentation and pathology. IASP Press; Seattle: 2009. pp. 55–69. [Google Scholar]
- 46.Maixner W, Diatchenko L, Dubner R, Fillingim RB, Greenspan JD, Knott C, Ohrbach R, Weir B, Slade GD. Orofacial Pain Prospective Evaluation and Risk Assessment study--the OPPERA study. J Pain. 2011;12(11 Suppl):T4–11. e11–12. doi: 10.1016/j.jpain.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martenson ME, Cetas JS, Heinricher MM. A possible neural basis for stress-induced hyperalgesia. Pain. 2009;142:236–244. doi: 10.1016/j.pain.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMahon SB, Wall PD. Descending excitation and inhibition of spinal cord lamina I projection neurons. J Neurophysiol. 1988;59:1204–1219. doi: 10.1152/jn.1988.59.4.1204. [DOI] [PubMed] [Google Scholar]
- 49.Mense S, Craig AD. Spinal and supraspinal terminations of primary afferent fibers from the gastrocnemius-soleus muscle in the cat. Neuroscience. 1988;26:1023–1035. doi: 10.1016/0306-4522(88)90117-0. [DOI] [PubMed] [Google Scholar]
- 50.Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- 51.Ohrbach R, Dworkin SF. Five-year outcomes in TMD: relationship of changes in pain to changes in physical and psychological variables. Pain. 1998;74:315–326. doi: 10.1016/s0304-3959(97)00194-2. [DOI] [PubMed] [Google Scholar]
- 52.Okamoto K, Hirata H, Takeshita S, Bereiter DA. Response properties of TMJ neurons in superficial laminae at the spinomedullary junction of female rats vary over the estrous cycle. J Neurophysiol. 2003;89:1467–1477. doi: 10.1152/jn.00795.2002. [DOI] [PubMed] [Google Scholar]
- 53.Okamoto K, Tashiro A, Chang Z, Thompson R, Bereiter DA. Temporomandibular joint-evoked responses by spinomedullary neurons and masseter muscle are enhanced after repeated psychophysical stress. Eur J Neurosci. 2012;36:2025–2034. doi: 10.1111/j.1460-9568.2012.08100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panneton WM, Gan Q, Juric R. The central termination of sensory fibers from nerves to the gastrocnemius muscle of the rat. Neuroscience. 2005;134:175–187. doi: 10.1016/j.neuroscience.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 55.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 56.Price DD, Greenspan JD, Dubner R. Neurons involved in the exteroceptive function of pain. Pain. 2003;106:215–219. doi: 10.1016/j.pain.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 57.Quintero L, Moreno M, Avila C, Arcaya J, Maixner W, Suarez-Roca H. Long-lasting delayed hyperalgesia after subchronic swim stress. Pharmacol Biochem Behav. 2000;67(3):449–458. doi: 10.1016/s0091-3057(00)00374-9. [DOI] [PubMed] [Google Scholar]
- 58.Rammelsberg P, LeResche L, Dworkin S, Mancl L. Longitudinal outcome of temporomandibular disorders: a 5-year epidemiologic study of muscle disorders defined by research diagnostic criteria for temporomandibular disorders. J Orofac Pain. 2003;17:9–20. [PubMed] [Google Scholar]
- 59.Renehan WE, Jacquin MF, Mooney RD, Rhoades RW. Structure-function relationships in rat medullary and cervical dorsal horns. II. Medullary dorsal horn cells. J Neurophysiol. 1986;55:1187–1201. doi: 10.1152/jn.1986.55.6.1187. [DOI] [PubMed] [Google Scholar]
- 60.Reynolds J, Bilsky EJ, Meng ID. Selective ablation of mu-opioid receptor expressing neurons in the rostral ventromedial medulla attenuates stress-induced mechanical hypersensitivity. Life Sci. 2011;89:313–319. doi: 10.1016/j.lfs.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 61.Rivat C, Becker C, Blugeot A, Zeau B, Mauborgne A, Pohl M, Benoliel JJ. Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain. 2010;150:358–368. doi: 10.1016/j.pain.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 62.Seedat S, Scott KM, Angermeyer MC, Berglund P, Bromet EJ, Brugha TS, Demyttenaere K, de Girolamo G, Haro JM, Jin R, Karam EG, Kovess-Masfety V, Levinson D, Medina Mora ME, Ono Y, Ormel J, Pennell BE, Posada-Villa J, Sampson NA, Williams D, Kessler RC. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry. 2009;66:785–795. doi: 10.1001/archgenpsychiatry.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shigenaga Y, Sera M, Nishimori T, Suemune S, Nishimura M, Yoshida A, Tsuru K. The central projection of masticatory afferent fibers to the trigeminal sensory nuclear complex and upper cervical spinal cord. J Comp Neurol. 1988;268:489–507. doi: 10.1002/cne.902680403. [DOI] [PubMed] [Google Scholar]
- 64.Slade GD, Bair E, By K, Mulkey F, Baraian C, Rothwell R, Reynolds M, Miller V, Gonzalez Y, Gordon S, Ribeiro-Dasilva M, Lim PF, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Dampier D, Knott C, Ohrbach R. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. J Pain. 2011;12(11 Suppl):T12–26. doi: 10.1016/j.jpain.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sugimoto T, Fujiyoshi Y, He Y-F, Xiao C, Ichikawa H. Trigeminal primary projection to the rat brain stem sensory trigeminal nuclear complex and surrounding structures revealed by anterograde transport of cholera toxin B subunit-conjugated and Bandeiraea simplicifolia isolectin B4-conjugated horseradish peroxidase. Neurosci Res. 1997;28:361–371. doi: 10.1016/s0168-0102(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- 67.Takeshita S, Hirata H, Bereiter DA. Intensity coding by TMJ-responsive neurons in superficial laminae of caudal medullary dorsal horn of the rat. J Neurophysiol. 2001;86:2393–2404. doi: 10.1152/jn.2001.86.5.2393. [DOI] [PubMed] [Google Scholar]
- 68.Takeuchi Y, Toda K. Subtypes of nociceptive units in the rat temporomandibular joint. Brain Res Bull. 2003;61:603–608. doi: 10.1016/s0361-9230(03)00219-3. [DOI] [PubMed] [Google Scholar]
- 69.Tashiro A, Okamoto K, Bereiter DA. Morphine modulation of temporomandibular joint-responsive units in superficial laminae at the spinomedullary junction in female rats depends on estrogen status. Eur J Neurosci. 2008;28:2065–2074. doi: 10.1111/j.1460-9568.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 70.Tashiro A, Okamoto K, Milam SB, Bereiter DA. Differential effects of estradiol on encoding properties of TMJ units in laminae I and V at the spinomedullary junction in female rats. J Neurophysiol. 2007;98:3242–3253. doi: 10.1152/jn.00677.2007. [DOI] [PubMed] [Google Scholar]
- 71.Turp JC, Kowalski CJ, O'Leary N, Stohler CS. Pain maps from facial pain patients indicate a broad pain geography. J Dental Res. 1998;77:1465–1472. doi: 10.1177/00220345980770061101. [DOI] [PubMed] [Google Scholar]
- 72.Yu X-M, Mense S. Response properties and descending control of rat dorsal horn neurons with deep receptive fields. Neuroscience. 1990;39:823–831. doi: 10.1016/0306-4522(90)90265-6. [DOI] [PubMed] [Google Scholar]
- 73.Yu X-M, Sessle BJ, Vernon H, Hu JW. Effects of inflammatory irritant application to the rat temporomandibular joint on jaw and neck muscle activity. Pain. 1995;60:143–149. doi: 10.1016/0304-3959(94)00104-M. [DOI] [PubMed] [Google Scholar]
- 74.Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36:339–356. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]