Abstract
Although it is clear that the loss of CD4+ T cells is a predisposing factor for the development of Pneumocystis pneumonia, specific T helper mechanisms mediating protection are not well understood. Th1, Th2 and Th17 responses have each been implicated in protective responses during infection. As STAT4 may promote Th1 and Th17 development, yet antagonize Th2 development, we investigated its role in P. murina host defense. STAT4 was required for Th1 and, unexpectedly, Th2 responses in the lungs of C57BL/6 (BL/6) and Balb/c mice 14 days post-challenge, but only Balb/c Stat4-/- mice demonstrated susceptibility to P. murina lung infection. BL/6 Stat4-/-, but not Balb/c Stat4-/-, mice maintained an enhanced alternatively activated (M2) macrophage signature in the lungs, which we have previously reported to be associated with enhanced P. murina clearance. In addition, anti-P. murina class-switched antibodies were increased in BL/6 Stat4-/- mice, but not Balb/c Stat4-/- mice. Supporting our experimental observations, plasma from HIV infected individuals colonized with Pneumocystis jirovecii contained significantly lower levels of the Th2 cytokines IL-4, IL-5 and IL-13 compared to HIV infected individuals who were not colonized. Collectively, our data suggests that robust local and systemic Th2-mediated responses are critical for immunity to Pneumocystis.
Introduction
Pneumocystis jirovecii is an opportunistic fungal pathogen that colonizes the lower airway and alveolar spaces in the lung causing Pneumocystis Pneumonia (PCP). The development of PCP is closely associated with AIDS and it is the leading cause of morbidity and mortality in the HIV-infected patient population (1). Although AIDS patients are highly susceptible to PCP, other individuals with suppressed immune systems are also at risk for Pneumocystis infection. Rheumatoid arthritis (RA) and cancer patients receiving B cell depletion therapies such as rituximab and ofatumumab (2) are susceptible to fatal PCP. Pneumocystis colonization is associated with chronic obstructive pulmonary disease (COPD) severity (3) and is a potential contributor to mortality in infants with sudden unexpected death (SUID) (4). Despite the wide-spread implementation of high active antiretroviral therapy (HAART) and use of antibiotics against Pneumocystis, the mortality rate due to PCP continues to be ≈ 10% (5), and as high as 30% if requiring intensive care (6), indicating that current treatments have reached a limit on the ability to resolve infection.
CD4+ T cells are required for clearance of Pneumocystis (7), yet the mechanism by which they specifically control the infection is not well understood. CD4+ T cell-mediated immunity to Pneumocystis murina is complicated, as mice deficient in the Th1 signature cytokine IFN-γ or the Th2 signature cytokine IL-4 are not more susceptible to infection than wild-type mice (8). One week after P. murina infection there is a 4:1 ratio of Th2:Th1 cell expansion, with a 2:1 ratio during the peak of infection at day 14 (9), suggesting an early role for Th2 responses. Supporting this, within the first 7 days of infection, inflammatory responses and leukocyte recruitment in response to P. murina challenge was defective in Stat6-/- Balb/c mice, suggesting that Th2 responses mediate multiple aspects of anti-P. murina host defense. However, mice deficient in the anti-inflammatory cytokine IL-10 have accelerated lung clearance of P. murina and increased production of IL-12, IL-18, and IFN-γ (10), implicating enhanced Th1-associated responses in augmented protection. Regulatory T cells also play a role in host defense, as depletion of Tregs resulted in enhanced proinflammatory Th1 and Th2 responses during P. murina infection (11). Additionally, antibody-mediated neutralization of IL-17 in CD4-competent mice resulted in a significantly higher fungal burden, suggesting Th17 cells may be involved in immune responses against P. murina (12).
Optimal development of Th1 cells requires the transcription factor T-bet and the activation of STAT4 by IL-12 signaling (13). STAT4 is also downstream of the IL-23 receptor, suggesting that it may play a role in Th17 development (14). Finally, STAT4-mediated CD4 T cell programming antagonizes Th2 development (15). Therefore, to further understand the contribution of STAT4 to CD4+ T cell responses during P. murina infection, we evaluated fungal host defense in C57BL/6 (BL/6) and Balb/c Stat4-/- mice. Unexpectedly, we not only found that Th2 responses mediated protection against Pneumocystis lung infection, but that STAT4 was required for optimal Th2 responses in Balb/c mice.
Materials and Methods
Mice
C57BL/6, Balb/c, and Balb/c Stat4-/- mice were obtained from The Jackson Laboratory (Bangor, ME). Stat4-/- mice on a C57BL/6 background were provided by Dr. Mark Kaplan, Indiana University. All mice used in experiments were 8-12 weeks of age. All animals were housed in a specific pathogen-free, Association for Assessment and Accreditation of Laboratory Animal Care-certified facility and handled according to Public Health Service Office of Laboratory Animal Welfare policies after review by the University of Alabama Institutional Animal Care and Use Committee.
Human subjects
Persons with documented HIV infection who were 18 years of age or older and had at least 1 visit to the University of Pittsburgh Medical Center's HIV/AIDS clinic were recruited between July 1, 2007 and September 30, 2010. Recruitment was performed by using posted advertisements and word of mouth and by contacting patients in a research registry. All participants signed written informed consent forms, and the University of Pittsburgh Institutional Review Board approved the protocol. Participants were excluded if they had new or increasing respiratory symptoms (cough, shortness of breath, and dyspnea) or fevers within the past 4 weeks. All participants also performed an oral wash by gargling with sterile saline for one minute. For determination of Pneumocystis jirovecii colonization, DNA extraction was performed on sputa and oral washes using a DNeasy kit (Qiagen, Valencia, CA). Pneumocystis colonization was determined by nested PCR of the mitochondrial large subunit rRNA as previously described (16). DNA extraction and PCR were carried out in separate rooms, and all PCRs were performed in a UV box. Positive and negative controls were included in each reaction mixture. A subject was considered P. jirovecii-colonized if PCR of either induced sputum or oral wash demonstrated human Pneumocystis by DNA sequencing in duplicate reactions. For determination of T helper cytokine levels, plasma from participants who were colonized with P. jirovecii (n = 50) or were not colonized (n = 53) was analyzed using a human 41-plex cytokine and chemokine kit (Cat. #HCYTMAG-60K-PX41, Millipore) and the Bio-Plex multiplex suspension cytokine array system according to the manufacturer's instructions (Bio-Rad Laboratories). Bio-Plex analysis of plasma samples was conducted at the University of Alabama at Birmingham (UAB) and approved by the UAB Institutional Review Board.
Pneumocystis murina isolation and inoculation
P. murina was prepared as previously described (17) (18). In brief, C.B-17 SCID mice previously inoculated with P. murina were injected with a lethal dose of ketamine/xylazine, and the lungs were aseptically removed and frozen at -80°C in 1 ml PBS. Frozen lungs were homogenized through a 70 μm filter and pelleted at 300 × g for 10 min at 4°C. The pellet was resuspended in 1 ml PBS, and a 1:10 dilution was stained with modified Giemsa stain (Diff-Quik). The number of P. murina cysts was quantified microscopically, and the concentration was adjusted to 2 × 106 cysts/ml. For in vivo challenge, mice were anesthetized with isoflurane and administered 2 × 105 cysts in a volume of 0.1 ml via intratracheal inoculation. Some preparations were also adjusted to 2 × 106 cysts/ml, and 50 ml aliquots were placed into tubes containing 200 μl of 90% FBS supplemented with 10% DMSO and stored at -80°C. Using this storage method, stable P. murina viability, as determined by quantitative real-time PCR, can be maintained for >1 year.
CD4+ T cell isolation and culture
Mice were anesthetized with intraperitoneal ketamine/xylazine and sacrificed by exsanguination 14 and 28 days post-inoculation. Both lungs were collected and minced in Iscove's modified Dulbecco's medium (IMDM) (Sigma, St. Louis, MO) supplemented with 1% penicillin-streptomycin-glutamine (Mediatech, Herndon, VA), 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA), and 0.4 mg/ml polymyxin B (Thermo Fisher), followed by incubation for 60 min with 1 mg/ml tissue culture grade type IV collagenase (Sigma, St. Louis, MO) in a 37°C orbital shaker at 100 rpm. The cell suspension was filtered through sterile 70 μm and 40 μm nylon filters, and red blood cells were lysed with red blood cell lysis buffer. Lymph node cells were excised and cells isolated by forcing tissue through a sterile 70 μm nylon filter followed by red blood cell lysis. CD4+ T cells were isolated using mouse CD4 Flowcomp Dynabeads (Cat # 114-61D, Invitrogen, Carlsbad, CA) per the manufactures’ protocol to 90-95% purity. The CD4+ T cells were then plated at 1 × 106 cells/ml in IMDM and stimulated with 2 μg/ml anti-CD3 (clone 145-11) and 1 μg/ml anti-CD28 (Cat # 102112, BioLegend, San Diego, CA) for 48 hours at 37°C in 5% CO2. Controls included cells incubated with medium alone. The supernatants were clarified by centrifugation and the protein levels of 23 cytokines and chemokines were determined using Bio-Plex multiplex suspension cytokine array according to the manufacturer's instructions (Bio-Rad Laboratories). The data were analyzed using Bio-Plex Manager software (Bio-Rad Laboratories).
Real-time PCR analysis of P. murina rRNA in lung tissue
Total RNA was isolated from the right lung of mice inoculated 14 and 28 days prior with P. murina by a single-step method using TRIZOL reagent (Invitrogen Life Technologies) according to the manufacturer's instructions. The RNA was then transcribed to cDNA (iScript cDNA synthesis kit; Bio-Rad), and real-time PCR for P. murina rRNA was performed as described previously (17) (18).
Alternative macrophage activation marker analysis
Total RNA was isolated from the right lung of mice inoculated 14 and 28 days prior with P. murina by a single-step method using TRIZOL reagent (Invitrogen Life Technologies) according to the manufacturer's instructions. The RNA was then transcribed to cDNA (iScript cDNA synthesis kit; Bio-Rad) and real-time PCR for the M2 marker Retnla (Mm00445109_m1; Applied Biosystems) and the M1 marker iNOS (Mm00440485_m1; Applied Biosystems) was performed (iQ Supermix; Bio-Rad). Gene expression was normalized to GAPDH mRNA levels (primers/probe from Applied Biosystems) using the 2-(ΔΔCt) method as previously described (18). The left lung was homogenized in PBS supplemented with Complete Mini protease inhibitor tablets (Roche), clarified by centrifugation and stored at -80°C. CCL17/TARC (Cat # MCC170) levels were determined by ELISA (R&D Systems, Inc., Minneapolis, MN).
Pneumocystis-specific antibody ELISA
Blood was collected weekly for 28 days from the tail vein and sera was frozen at -20°C. A sonicate of P. murina was prepared as previously described (19) (20). Briefly, P. murina inoculum was sonicated and clarified by centrifugation, and then coated onto microtiter plates (Sigma, St. Louis, MO) at 1 μg/well overnight at 4°C. The wells were then blocked with 10% bovine serum albumin (Equitech Bio, Inc., Kerrville, TX) for 1 hour at 37°C. Plates were washed with PBS containing 0.05% Tween-20. Sera were serially diluted and incubated at 37°C for 1 hour. Antibodies were detected by incubating plates with anti-mouse IgM, IgG1, IgG2a, IgG2b, and IgG2c conjugated to HRP (Southern Biotech, Birmingham, AL) for 1 hour at 37°C, followed by incubation with 3,3′,5,5′-Tetramethylbenzidine (TMB) (Sigma, St. Louis, MO). Endpoint data are expressed as the inverse Log2 of the dilution at which the OD450 was 0.1.
Statistical analysis
Data were analyzed using GraphPad Prism statistical software (GraphPad Software, San Diego, CA). Comparisons between groups were made with paired or unpaired two-tailed Student t-test. For comparisons of plasma cytokines between P. jirovecii-colonized and non-colonized participants, a non-parametric Mann-Whitney test was employed. Significance was accepted at p≤0.05.
Results
Differential susceptibility to P. murina lung infection between Stat4-/- mice on BL/6 vs. Balb/c backgrounds
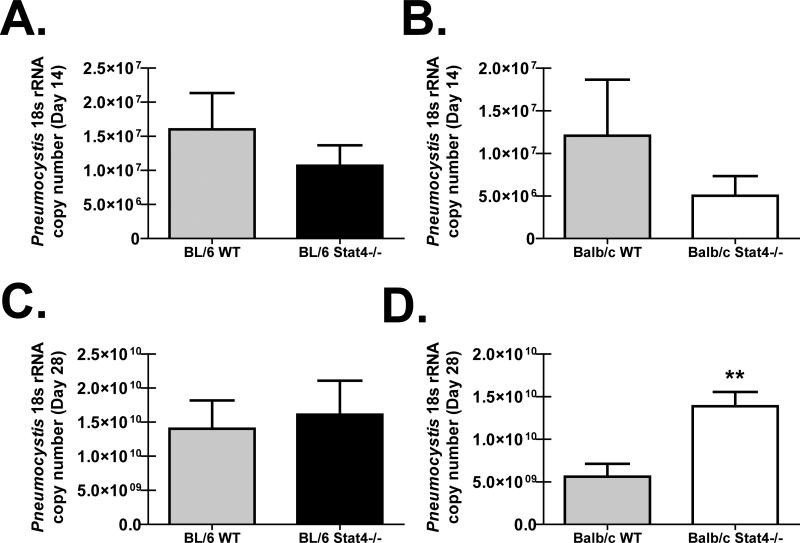
Mice deficient in the IFN-αR (21) (22), IL-12p35 (23), or IL-23p19 (12), all of which signal through STAT4 (13) (14) (24), have delayed clearance of P. murina, suggesting that STAT4 contributes to anti-P. murina responses in the lung. As Stat4-/- mice are available on the “Th1 skewed” BL/6 background and the “Th2 skewed” Balb/c background, BL/6 and Balb/c WT and Stat4-/- mice were challenged with P. murina and fungal burden was determined 14 and 28 days thereafter. There was no difference in lung burden between BL/6 WT and BL/6 Stat4-/- mice (Figure 1A) or Balb/c WT and Balb/c Stat4-/- mice (Figure 1B) 14 days post-challenge. In contrast, whereas BL/6 Stat4-/- mice had similar P. murina lung burden as BL/6 WT mice (Figure 1C), Balb/c Stat4-/- mice had a significantly higher burden in the lungs compared to Balb/c WT mice (Figure 1D). Thus, despite both strains having a deficiency in STAT4, susceptibility to P. murina lung infection was uniquely observed in mice on the Balb/c background.
Figure 1. Differential susceptibility to P. murina lung infection between Stat4-/- mice on BL/6 vs. Balb/c backgrounds.
C57BL/6 WT and Stat4-/- mice and Balb/c WT and Stat4-/- mice were administered 2 × 105 Pneumocystis cysts via intratracheal inoculation. (A/B) Fourteen and (C/D) twenty-eight days post-inoculation, lungs were collected and Pneumocystis burden was determined by real-time PCR for Pneumocystis rRNA copy number. The Figure illustrates representative data from one of two independent studies with an n = 5 mice per group. Data is expressed as mean Pneumocystis rRNA copy number. Data are expressed as mean + SEM. ** represents a P value of < 0.01 (Unpaired two-tailed Student's t test).
BL/6 and Balb/c Stat4-/- mice demonstrate impaired CD4+ Th2 responses in the lung
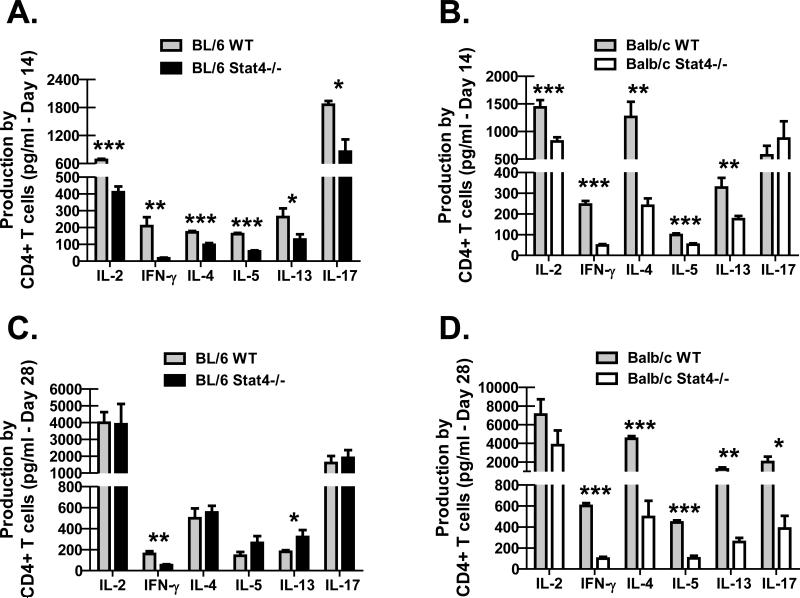
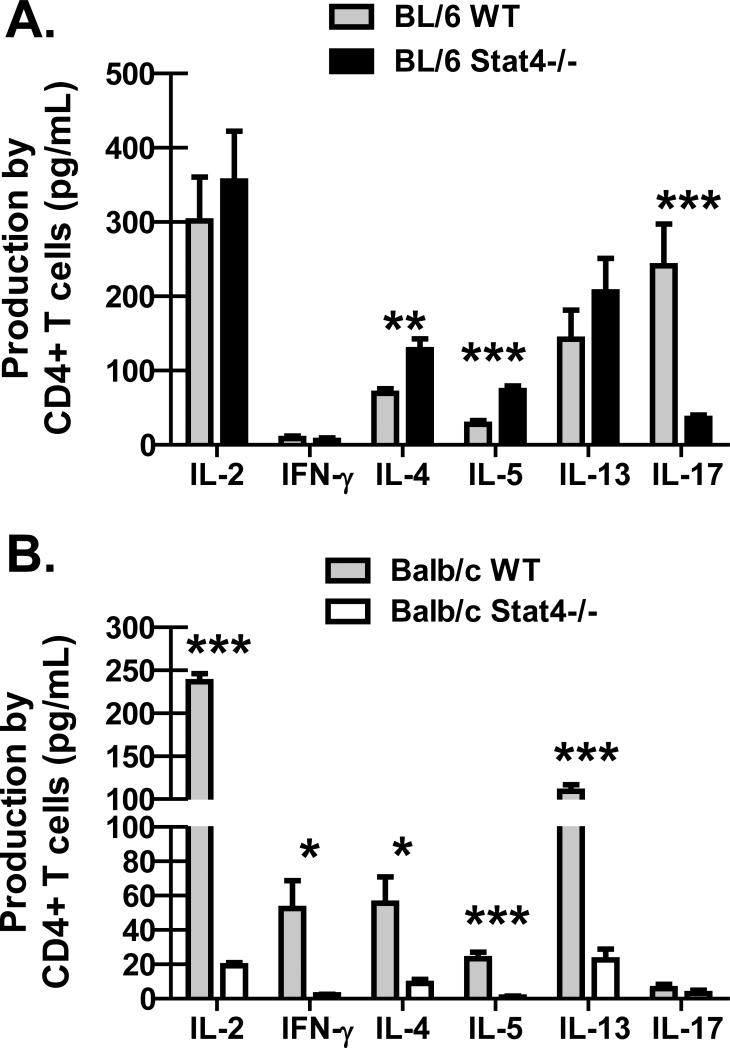
Due to the unexpected susceptibility difference between BL/6 Stat4-/- mice and Balb/c Stat4-/- mice, we questioned whether this could be explained by differences in lung CD4+ T cell responses. On day 14 after P. murina inoculation, CD4+ T cells were purified from enzymatic lung digest cell suspensions and stimulated for 48 hours with anti-CD3 and anti-CD28. As assessed by Bio-Plex, production of the Th1 signature cytokines IL-2 and IFN-γ by CD4+ T cells from the lungs of BL/6 Stat4-/- mice (Figure 2A) and Balb/c Stat4-/- mice (Figure 2B) were significantly reduced compared to their WT counterparts as expected. However unexpectedly, IL-4, IL-5 and IL-13 production by lung CD4+ T cells were also significantly reduced in BL/6 Stat4-/- mice (Figure 2A) and Balb/c Stat4-/- mice (Figure 2B). Thus, Stat4-/- mice on both backgrounds exhibited global defects in Th1-type and Th2-type cytokine production during P. murina infection. As a role for STAT4 in IL-17A production by CD4+ T cells has been reported (14), we questioned its production by lung CD4+ T cells during P. murina infection. IL-17A production by CD4+ T cells isolated from the lungs of Balb/c Stat4-/- mice, which were susceptible to P. murina infection, was similar to CD4+ T cells isolated from the lungs of Balb/c WT mice (Figure 2B). In contrast, lung CD4+ T cell-mediated production of IL-17A was significantly less in BL/6 Stat4-/- mice (Figure 2A), which were protected from P. murina infection (compared to Balb/c Stat4-/- mice). By 28 days post-challenge, IL-4, IL-5 and IL-13 production by lung CD4+ T cells returned in BL/6 Stat4-/- mice, as did IL-17A, although IFN-γ remaining impaired (Figure 2C). However, lung CD4+ T cells in Balb/c Stat4-/- mice continued to display significantly impaired production of IFN-γ, IL-4, IL-5 and IL-13 (Figure 2D). Furthermore, IL-17A production by lung CD4+ T cells was now also was impaired at 28 days post-challenge. Thus, resistance to P. murina lung infection in BL/6 Stat4-/- mice was associated with increased lung CD4+ Th2 responses whereas susceptibility to P. murina lung infection in Balb/c Stat4-/- mice correlated with significantly attenuated lung CD4+ Th1, Th2 and Th17 responses.
Figure 2. BL/6 and Balb/c Stat4-/- mice demonstrate impaired CD4+ Th2 responses in the lung.
C57BL/6 WT and Stat4-/- mice and Balb/c WT and Stat4-/- mice were administered 2 × 105 Pneumocystis cysts via intratracheal inoculation. (A/B) Fourteen and (C/D) twenty-eight days post-inoculation, the lungs were collected, enzymatically digested and CD4+ T cells were isolated via Dynabeads followed by stimulation with 2 μg/mL anti-CD3 and 1 μg/mL anti-CD28 for 48 h. T helper cytokine levels were quantified in clarified co-culture supernatants by Bio-Plex. Cumulative data are shown from two to three independent experiments with cells cultured in duplicate or triplicate. Data is expressed as mean pg/ml + SEM. For both graphs, *, ** and *** represent P values of < 0.05, < 0.01 and < 0.001, respectively (Unpaired two-tailed Student's t test).
BL/6 Stat4-/- mice demonstrate enhanced lung M2 macrophage polarization
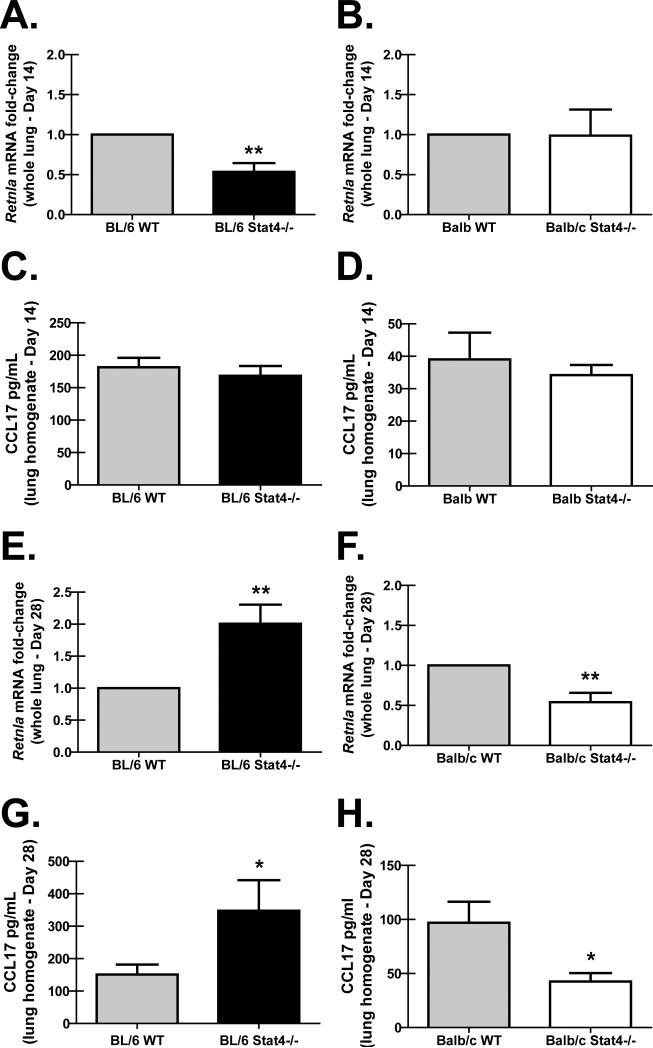
We have recently reported that an increase in alternatively activated (M2) alveolar macrophages correlated with an enhanced ability to clear P. murina (18). To determine whether M2 responses were different between BL/6 Stat4-/- mice and Balb/c Stat4-/- mice, we assessed markers of M2 macrophage populations in the lung. Fourteen days post-P. murina challenge, there were lower lung mRNA levels of the M2 macrophage marker Retnla (RELM-alpha/FIZZ-1) (Figure 3A) in BL/6 Stat4-/- mice, but not Balb/c Stat4-/- mice (Figure 3B), despite both of these strains demonstrating lower Th2 responses by lung CD4+ T cells (Figure 2A, 2B). Lung levels of CCL17, a chemokine produced by M2 macrophages, was also not different between WT BL/6 mice and BL/6 Stat4-/- mice (Figure 3C) and WT Balb/c mice and Balb/c Stat4-/- mice (Figure 3D) 14 days post-challenge. In contrast, by twenty-eight days post-P. murina challenge, there was significantly higher lung Retnla (RELM-alpha/FIZZ-1) mRNA levels (Figure 3E) and CCL17 protein levels (Figure 3G) in the lungs of BL/6 Stat4-/- mice, indicating that along with increased lung CD4+ Th2 responses (Figure 2C), BL/6 Stat4-/- mice had increased M2 macrophage activation. In contrast, Balb/c Stat4-/- mice had significantly lower lung mRNA levels of Retnla (RELM-alpha/FIZZ-1) compared to Balb/c WT mice (Figure 3F) as well as a significant reduction in the concentration of CCL17 in the lungs of Balb/c Stat4-/- mice (Figure 3H), which correlated with attenuated lung CD4+ Th2 responses (Figure 2D). Of note, naïve BL/6 Stat4-/- mice did not demonstrate evidence of increased CCL17 or Retnla mRNA levels (data not shown). There was no difference in the mRNA levels of the M1 macrophage marker Nos2 between BL/6 Stat4-/- mice and Balb/c Stat4-/- mice compared to their respective WT controls (data not shown). We further did not observe any differences in the pro-M2 cytokine IL-33 in either strain of WT or Stat4-/- mice (data not shown). Thus, Balb/c Stat4-/- mice, which are susceptible to P. murina infection (relative to BL/6 Stat4-/- mice), had diminished M2 macrophage activation, suggesting that intact/enhanced M2 macrophage activation in BL/6 Stat4-/- mice is a possible protective mechanism.
Figure 3. BL/6 Stat4-/- mice demonstrate enhanced lung M2 macrophage polarization.
C57BL/6 WT and Stat4-/- mice and Balb/c WT and Stat4-/- mice were administered 2 × 105 Pneumocystis cysts via intratracheal inoculation. (A/B/C/D) Fourteen and (E/F/G/H) twenty-eight days post-inoculation, the right lung was collected and total RNA isolated, transcribed to cDNA and quantitative real-time PCR was performed for Retnla (A/B/E/F). Gene expression was normalized to Gapdh and fold changes between WT (set at 1) and Stat4-/- mice were determined using the 2-ΔΔCt method. Of note, there were no differences in the delta Ct values (Ct value of Retnla minus Ct value of Gapdh) when comparing C57BL/6 WT mice and Balb/c WT mice at 14 and 28 days post-challenge. For CCL17 analysis (C/D/G/H), mice were infected as described and twenty-eight days post-inoculation, the left lung was collected and homogenized in PBS supplemented with Complete Mini protease inhibitor tablets and supernatants clarified by centrifugation. CCL17 levels were determined in lung homogenate supernatants by ELISA. Cumulative data are shown from three independent studies with n = 4-6 mice/group per study. (A/B/E/F) Data is expressed as mean fold-change. ** represents a P value of < 0.01 (Paired two-tailed Student's t test). (C/D/G/H) Data is expressed as mean pg/ml + SEM. * represents a P value of < 0.05 (Unpaired two-tailed Student's t test).
P. murina-specific antibody levels are elevated in serum of BL/6 Stat4-/- mice, but not Balb/c Stat4-/-, mice
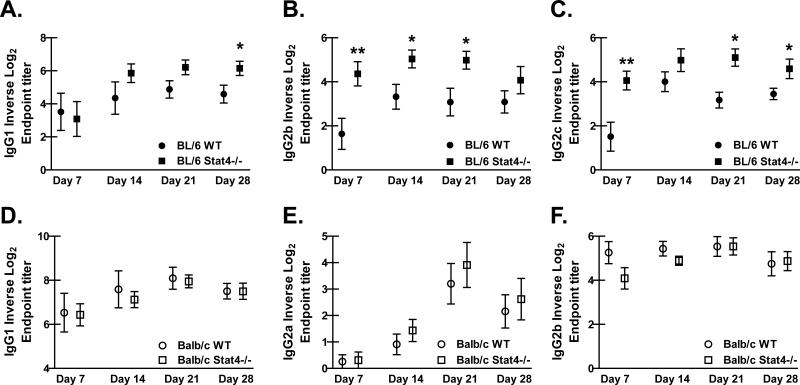
To gain further insight into potential mechanisms of resistance and susceptibility between BL/6 Stat4-/- mice and Balb/c Stat4-/- mice, P. murina-specific serum antibody levels were assessed. BL/6 and Balb/c WT and Stat4-/- mice were challenged with P. murina and sera were collected weekly for 28 days and analyzed by ELISA. The level of anti-P. murina IgM was similar between BL/6 and Balb/c Stat4-/- mice and their respective controls, suggesting that STAT4 does not play a role in the production of preexisting P. murina-specific natural IgM or in IgM produced during the immune response against P. murina (data not shown). However, BL/6 Stat4-/- mice had enhanced anti-P. murina class-switched antibody production compared to BL/6 WT mice. Whereas P. murina-specific IgG1 production by B cells in BL/6 Stat4-/- mice was significantly enhanced 28 days post-challenge (Figure 4A), IgG2b was enhanced earlier at days 7, 14 and 21 (Figure 4B). The production of P. murina-specific IgG2c was also significantly increased throughout the immune response in BL/6 Stat4-/- mice compared to BL/6 WT mice (Figure 4C). In contrast, there was no difference in the levels of anti-P. murina antibodies of any isotype at any time point examined in Balb/c Stat4-/- mice compared to Balb/c WT mice (Figures 4D, E and F). Thus, enhanced anti-fungal antibody production in addition to increased M2 macrophage activation in BL/6 Stat4-/- mice is sufficient for protection during P. murina infection in the absence of robust CD4+ T cell responses in the lung (14 days post-challenge, Figure 2A). Moreover, while Balb/c Stat4-/- mice also exhibited defective CD4+ T cell responses in the lung (14 and 28 days post-challenge, Figures 2B and 2D), these mice had decreased M2 macrophage activation and no difference in anti-P. murina antibody production, rendering these mice susceptible to P. murina infection.
Figure 4. P. murina-specific antibody levels are elevated in serum of BL/6 Stat4-/-, but not Balb/c Stat4-/-, mice.
(A/B/C) C57BL/6 WT and Stat4-/- mice and (D/E/F) Balb/c WT and Stat4-/- mice were administered 2 × 105 Pneumocystis cysts via intratracheal inoculation followed by bleeding mice weekly for 28 days. P. murina-specific (A/D) IgG1, (B/E) IgG2b, (C) IgG2c (C57BL/6) and (F) IgG2a (Balb/c) and were determined by ELISA. Cumulative data are shown from three independent studies with n = 3-4 mice/group per study. Data is expressed as the mean per group of the natural log of the dilution at which the OD450 is 0.1 + SEM. * and ** represent P values of < 0.05 and < 0.01, respectively (Unpaired two-tailed Student's t test).
CD4+ Th2 responses in the draining lymph nodes are elevated in BL/6 Stat4-/- mice but significantly impaired in Balb/c Stat4-/- mice
The observation of higher P. murina-specific IgG levels in sera from BL/6 Stat4-/- prompted us to determine whether a difference in systemic CD4+ T cell responses between BL/6 and Balb/c WT and Stat4-/- mice existed. CD4+ T cells from the mediastinal lymph nodes (MLN) of BL/6 and Balb/c WT and Stat4-/- mice were isolated 14 days after challenge with P. murina and stimulated ex vivo with anti-CD3 and anti-CD28 for 48 hours. Similar to CD4+ T cells from the lungs, the production of IL-4, IL-5, IL-13, IL-2 and IFN-γ by CD4+ T cells from the MLN of Balb/c Stat4-/- mice were significantly diminished compared to CD4+ T cells from the MLN of Balb/c WT mice (Figure 5A). In contrast, CD4+ T cells from the MLN of BL/6 Stat4-/- mice produced significantly more IL-4, IL-5 and IL-13 compared to CD4+ T cells from the MLN of BL/6 WT mice (Figure 5B). IL-17A production was significantly reduced in MLN CD4+ T cells from BL/6 Stat4-/- mice yet there was no difference in IFN-γ production (Figure 5B). Thus, the enhanced production of anti-P. murina class-switched antibodies observed in sera from BL/6 Stat4-/- mice correlated with lack of susceptibility of these mice in the presence of CD4+ T cell defects. In contrast, no changes in anti-P. murina class-switched antibodies in sera from Balb/c Stat4-/- mice, also in the presence of CD4+ T cell defects, correlated with higher lung burden.
Figure 5. CD4+ Th2 responses in the draining lymph nodes are elevated in BL/6 Stat4-/- mice but significantly impaired in Balb/c Stat4-/- mice.
(A) C57BL/6 WT and Stat4-/- mice and (B) Balb/c WT and Stat4-/- mice were administered 2 × 105 Pneumocystis cysts via intratracheal inoculation. Fourteen days post-inoculation, the mediastinal lymph nodes were collected, manually digested and CD4+ T cells were isolated via Dynabeads followed by stimulation with 2 μg/mL anti-CD3 and 1 μg/mL anti-CD28 for 48 h. T helper cytokine levels were quantified in clarified co-culture supernatants by Bio-Plex. Cumulative data are shown from two to three independent experiments with cells cultured in duplicate or triplicate. Data is expressed as mean pg/ml + SEM. For both graphs, *, ** and *** represent P values of < 0.05, < 0.01 and < 0.001, respectively (Unpaired two-tailed Student's t test).
Lower Th2 cytokine levels in plasma correlate with Pneumocystis jirovecii colonization in HIV-infected individuals
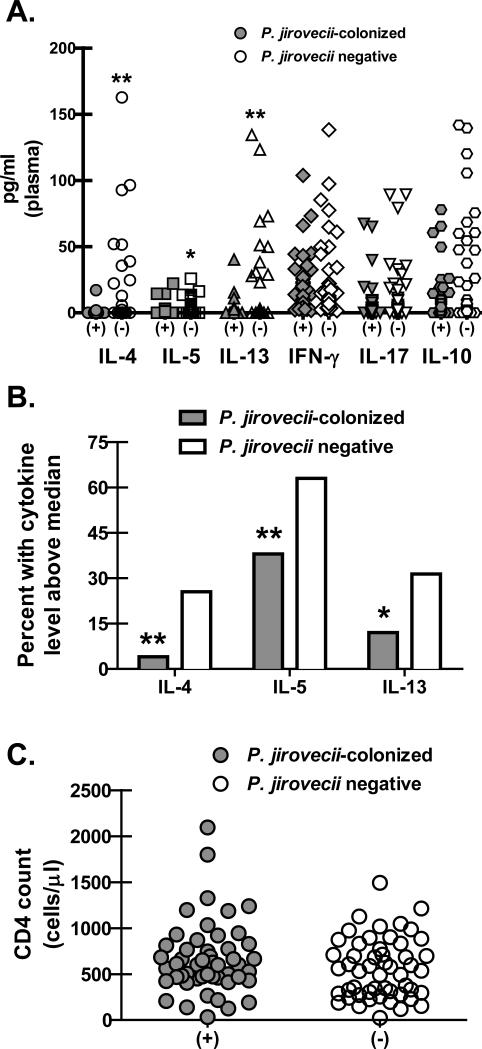
Observations thus far suggest that Th2 immunity mediates protection from P. murina infection. Therefore, to determine the cytokine response in the periphery in humans during P. jirovecii colonization, we examined the levels of T helper cytokines in plasma from a cohort of HIV-infected individuals who were documented to be colonized with P. jirovecii using nested PCR compared to HIV-infected individuals who were not colonized. Assessment of Th1, Th2, Th17 and Treg-associated cytokine levels in plasma from this cohort revealed no differences in the Th1 cytokine IFN-γ, the Th17 cytokine IL-17A and the Treg cytokine IL-10 (Figure 6A). In contrast, HIV-infected individuals who were colonized with P. jiroveci had significantly lower concentrations of the Th2 cytokines IL-4, IL-5 and IL-13 (Figure 6A) compared to HIV-infected individuals that who were not colonized. Dichotomizing Th2 cytokine levels as above and below the median for the cohort demonstrated that HIV-infected individuals who were colonized with P. jirovecii were significantly more likely to have levels of the Th2 cytokines IL-4, IL-5 and IL-13 that were below the cohort median compared to HIV-infected individuals who were not colonized (Figure 6B). Of note, CD4 cell numbers were not different between colonized vs. non-colonized individuals (Figure 6C). Thus, these data suggest that colonization with P. jirovecii is less likely if Th2 responses are induced.
Figure 6. Lower Th2 cytokine levels in plasma correlate with Pneumocystis jirovecii colonization in HIV-infected individuals.
(A) Plasma was collected from a cohort of HIV-infected individuals who were subsequently confirmed to be colonized with P. jirovecii via nested PCR (n = 50). Controls included HIV-infected individuals who were negative for P. jirovecii by nested PCR (n = 53). T helper cytokine levels were quantified in clarified co-culture supernatants by Bio-Plex. Data is expressed as pg/ml (each symbol represents a single individual. * and ** represent P values of < 0.05 and < 0.01, respectively (Non-parametric two-tailed Mann-Whitney test). (B) Percentage of P. jirovecii colonized vs. non-colonized individuals with detectable Th2 cytokines above the median for each. * and ** represent P values of < 0.05 and < 0.01, respectively (Chi square test). (C) CD4 cell numbers in peripheral blood of P. jirovecii colonized vs. non-colonized HIV-infected individuals.
Discussion
Although CD4+ T cells are the central effector cell mediating host defense against Pneumocystis, the type of T helper response that is required for clearance is not clear. As stated previously, mice deficient in the IFN-αR (21) (22), IL-12p35 (23), or IL-23p19 (12) display delayed (significant differences in P. murina lung burden at 14 to 21 days post-challenge), yet ultimately intact, (no significant differences in P. murina lung burden at 28 days post-challenge) organism clearance, suggesting that some aspects of Th1 responses may provide protection against Pneumocystis. However, by comparing the immune response against P. murina in Stat4-/- on the BL/6 and Balb/c backgrounds, we demonstrate here that STAT4 paradoxically contributes to Th2-mediated responses, which significantly contribute to multiple aspects of P. murina host defense.
The production of signature cytokines from the Th1 and Th2 lineages of CD4+ T cells in the lungs were all affected by Stat4-deficiency in both strains of mice during P. murina infection. STAT4 phosphorylation is critical in IL-12-induced IFN-γ production (13), and in the absence of STAT4, IFN-γ production by CD4+ T cells from the lungs was significantly decreased during P. murina infection. IL-2 production by CD4+ T cells was also reduced three-fold on both genetic backgrounds, indicating the expected impaired Th1 development during P. murina infection in the lungs of Stat4-/- mice. However, a striking finding was that lung CD4+ T cell-mediated production of Th2-type cytokines was also significantly reduced in BL/6 and Balb/c Stat4-/- mice 14 days post-challenge. This was unexpected, because in the absence of STAT4, CD4+ T cells are known to be biased towards the development of Th2 cells in other infection models (15). Th2 cytokine production did return 28 days post-infection in BL/6 Stat4-/- mice, but not Balb/c Stat4-/- mice, suggesting that STAT4 is required at some level for Th2 responses in Balb/c mice more than BL/6. It was of interest to note that P. murina lung burden in Balb/c WT mice at 28 days post-challenge was three-fold lower compared to that in BL/6 WT mice, lending additional support for Th2 responses being important in P. murina host defense. P. murina host defense was not dependent on T-bet, the master regulator of the Th1 cell lineage, as Balb/c Tbx21-/- mice had no defect in P. murina clearance 28 days post-challenge (unpublished data). As the intrinsic parameters for cytokine signaling in determining T-helper cell fate have been well characterized, the mechanism for the apparent STAT4-dependent, T-bet-independent effect on Th2 immunity in the lung during P. murina infection may be due to extrinsic factors that have yet to be examined.
In addition to Th1 and Th2 cytokines, IL-17A production by CD4+ T cells from the lungs was also negatively affected by the absence of STAT4, albeit with different kinetics: 14 days after infection in BL/6 Stat4-/- mice (but not Balb/c Stat4-/- mice) and 28 days after infection in Balb/c Stat4-/- mice (but not BL/6 Stat4-/- mice). STAT4 is thought to be partially involved in Th17 differentiation by mediating IL-23R signaling (14) and IL-17A has been shown to be STAT4 dependent on both the BL/6 (14) and Balb/c backgrounds (25). A role for IL-17A has been implicated in the clearance of P. murina (12). Neutralization of IL-17A with a mAb resulted in a higher fungal burden, and mice deficient in IL-23p19 (12), which plays a role in expanding and maintaining the Th17 fate (26), have delayed clearance of P. murina. IL-23p19 is an activator STAT4, so it was unclear in this study whether the delayed clearance of P. murina was due to impaired Th17 responses or compromised STAT4-mediate host defense. The current study suggests IL-17A production by CD4+ T cells likely did not play a critical role in P. murina host defense in BL/6 Stat4-/- mice, however, we cannot exclude a role for IL-17A in protective responses in Balb/c Stat4-/- mice.
Despite a global defect in CD4+ T cell-mediated cytokine production in the lungs of BL/6 and Balb/c Stat4-/- mice, only Balb/c Stat4-/- mice had significantly higher fugal burdens 28 days post-challenge. As this suggested that Balb/c Stat4-/- mice were susceptible to infection while BL/6 Stat4-/- mice were protected, this led us to hypothesize that other mechanisms of host defense were likely enhanced in BL/6, but not Balb/c Stat4-/- mice. Phagocytosis by alveolar macrophages is the predominant mechanism for clearance of P. murina from the lungs (27). Previous work from our lab has shown that increased M2 macrophage polarization correlated with enhanced clearance of P. murina (18). Indeed, BL/6 Stat4-/- mice had increased M2 macrophage activation late during infection, whereas M2 macrophage activation in Balb/c Stat4-/- mice was significantly impaired, suggesting that enhanced M2 macrophage activation contributed to protection from P. murina infection in BL/6 Stat4-/- mice. CD4+ T cell-mediated production of IL-4 and IL-13 in the lungs are normally critical for M2 macrophage activation, but they were decreased in BL/6 Stat4-/- mice 14 days after infection. However, Th2 responses in the lungs returned by 28 days post-challenge and only in BL/6 Stat4-/- mice. There may also be alternative cellular sources of IL-4 or IL-13, such as basophils or type-2 innate helper cells (28), in the lungs of BL/6 mice 14 or 28 days post-challenge mice that may serve to initiate M2 macrophage polarization and these populations are currently being investigated. Additionally, immune complexes binding to Fc receptors on macrophages also induce alternative activation (29); consequently, the increase in anti-P. murina antibody production in BL/6 28 days post-challenge mice may have also contributed to the enhanced M2 activation.
In addition to increased M2 macrophage activation, BL/6 Stat4-/- mice, but not Balb/c Stat4-/- mice, had increased production of anti-P. murina antibodies. While it is clear that CD4+ T cells are required for P. murina host defense, B cells and antibodies are also important contributors to host defense (30) (31), (32) (33). P. murina-specific IgG2b was significantly increased in BL/6 Stat4-/- mice early in the immune response, whereas P. murina-specific IgG1 was significantly increased late. IgG1 is associated with Th2-type immunity (34) and the enhanced production was consistent with increased Th2 cytokine production in the MLN. Isotype-switching to IgG2b has been associated with either Th1-type (35) or Th2-type cytokines (36) in various experimental models. Although increased IgG2b production was observed in BL/6 Stat4-/- mice during P. murina infection, it is not clear if this could be a result of increased Th2 cytokine production in the MLN. Curiously, P. murina-specific IgG2c was also enhanced in BL/6 Stat4-/- mice. IgG2c is associated with Th1 immunity (37), so it was unexpected that the production of this isotype was also elevated over BL/6 WT mice.
Despite the importance of PCP as an opportunistic infection associated with HIV, there is little evidence to suggest which is the dominate CD4+ T cell response against P. jiroveci in humans. PBMC stimulated with the major surface glycoprotein (MSG) of P. jiroveci from HIV-positive individuals with a previous history of PCP produced significantly higher concentrations of IL-4 compared with HIV-positive individuals with no history of PCP, whereas the level of IFN-γ was similar between these two groups (38). This suggests that in patients able to clear P. jiroveci, memory CD4+ T cell responses are predominantly Th2 driven. In agreement with this study and confirming our observations in mice, lower IL-4, IL-5 and IL-13 levels in plasma from HIV-positive individuals correlated with Pneumocystis colonization, suggesting that Th2 responses are associated with enhanced fungal host defense.
In summary, our study establishes that local and systemic Th2-mediated immunity contributes to multiple aspects of host defense and correlates with resistance against Pneumocystis lung infection. An unexpected finding from this work was the impairment of local and systemic Th2 responses in the absence of Stat4, primarily in Balb/c mice and to a lesser extent in BL/6 mice. Future studies are required to probe this observation more thoroughly to determine whether this is a strain-specific phenomenon during P. murina infection as well as to identify specific Stat4-dependent mechanisms critical for Th2 development. Although the mechanisms of how local and systemic Th2 and type-2 responses are generated and maintained during experimental Pneumocystis lung infection are not currently known, data presented here suggests that Th2 responses in humans may be protective, thus understanding the development of the Th2 response may lead to better immunotherapeutics to target Pneumocystis lung infection.
Footnotes
This work was supported by PHS grants T32 HL105346 (R.M.), R01 HL083461 (A.M.) and R21 HL110023 and R21 HL117090 (both to C.S.).
References
- 1.Ives NJ, Gazzard BG, Easterbrook PJ. The changing pattern of aids-defining illnesses with the introduction of highly active antiretroviral therapy (haart)in a london clinic. J Infect. 2001;42:134–139. doi: 10.1053/jinf.2001.0810. [DOI] [PubMed] [Google Scholar]
- 2.Hardak E, Oren I, Dann EJ, Yigla M, Faibish T, Rowe JM, Avivi I. The increased risk for pneumocystis pneumonia in patients receiving rituximab-CHOP-14 can be prevented by the administration of trimethoprim/sulfamethoxazole: a single-center experience. Acta Haematol. 2012;127:110–114. doi: 10.1159/000334113. [DOI] [PubMed] [Google Scholar]
- 3.Morris A, Sciurba FC, Lebedeva IP, Githaiga A, Elliott WM, Hogg JC, Huang L, Norris KA. Association of chronic obstructive pulmonary disease severity and Pneumocystis colonization. Am J Respir Crit Care Med. 2004;170:408–413. doi: 10.1164/rccm.200401-094OC. [DOI] [PubMed] [Google Scholar]
- 4.Vargas SL, Ponce CA, Gallo M, Perez F, Astorga JF, Bustamante R, Chabe M, Durand-Joly I, Iturra P, Miller RF, Aliouat e. M., Dei-Cas E. Near-universal prevalence of Pneumocystis and associated increase in mucus in the lungs of infants with sudden unexpected death. Clin Infect Dis. 2013;56:171–179. doi: 10.1093/cid/cis870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris A, Wachter RM, Luce J, Turner J, Huang L. Improved survival with highly active antiretroviral therapy in HIV-infected patients with severe Pneumocystis carinii pneumonia. AIDS. 2003;17:73–80. doi: 10.1097/00002030-200301030-00010. [DOI] [PubMed] [Google Scholar]
- 6.Radhi S, Alexander T, Ukwu M, Saleh S, Morris A. Outcome of HIV-associated Pneumocystis pneumonia in hospitalized patients from 2000 through 2003. BMC Infect Dis. 2008;16:118–127. doi: 10.1186/1471-2334-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masur H. CD4 counts as predictors of opportunistic pneumonias in human immunodeficiency virus (HIV) infection. Ann Intern Med. 1989;111:223–231. doi: 10.7326/0003-4819-111-3-223. [DOI] [PubMed] [Google Scholar]
- 8.Garvy BA, Wiley JA, Gigliotti F, Harmsen AG. Protection against Pneumocystis carinii pneumonia by antibodies generated from either T helper 1 or T helper 2 responses. Infection & Immunity. 1997;65:5052–5056. doi: 10.1128/iai.65.12.5052-5056.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shellito JE, Tate C, Ruan S, Kolls J. Murine CD4+ T Lymphocyte Subsets and Host Defense against Pneumocystis carinii. J. Infect. Dis. 2000;181:2011–2017. doi: 10.1086/315487. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi MH, Harmsen AG, Garvy BA. IL-10 modulates host responses and lung damage induced by Pneumocystis carinii infection. J. Immunol. 2003;170:1002–1009. doi: 10.4049/jimmunol.170.2.1002. [DOI] [PubMed] [Google Scholar]
- 11.McKinley L, Logar AJ, McAllister F, Zheng M, Steele C, Kolls JK. Regulatory T cells dampen pulmonary inflammation and lung injury in an animal model of pneumocystis pneumonia. J Immunol. 2006;177:6215–6226. doi: 10.4049/jimmunol.177.9.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudner XL, Happel KI, Young EA, Shellito JE. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect Immun. 2007;75:3055–3061. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thieu VT, Yu Q, Chang HC, Yeh N, Nguyen ET, Sehra S, Kaplan MH. Signal transducer and activator of transcription 4 is required for the transcription factor T-bet to promote T helper cell-fate determination. Immunity. 2008;29:679–690. doi: 10.1016/j.immuni.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 16.Morris A, Alexander T, Radhi S, Lucht L, Sciurba FC, Kolls JK, Srivastava R, Steele C, Norris KA. Airway obstruction is increased in Pneumocystis-colonized human immunodeficiency virus-infected outpatients. J Clin Microbiol. 2009;47:3773–3776. doi: 10.1128/JCM.01712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson MP, Metz AE, Li S, Lowell CA, Steele C. The absence of Hck, Fgr and Lyn tyrosine kinases augments lung innate immune responses to Pneumocystis murina. Infect Immun. 2009;77:1790–1797. doi: 10.1128/IAI.01441-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson MP, Christmann BS, Werner JL, Metz AE, Trevor JL, Lowell CA, Steele C. IL-33 and M2a alveolar macrophages promote lung defense against the atypical fungal pathogen Pneumocystis murina. J Immunol. 2011;186:2372–2381. doi: 10.4049/jimmunol.1002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng M, Ramsay AJ, Robichaux MB, Norris KA, Kliment C, Crowe C, Rapaka RR, Steele C, McAllister F, Shellito JE, Marrero L, Schwarzenberger P, Zhong Q, Kolls JK. CD4+ T cell-independent DNA vaccination against opportunistic infections. J Clin Invest. 2005;115:3536–3544. doi: 10.1172/JCI26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapaka RR, Ricks DM, Alcorn JF, Chen K, Khader SA, Zheng M, Plevy S, Bengten E, Kolls JK. Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina. J Exp Med. 2010;207:2907–2919. doi: 10.1084/jem.20100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meissner NN, Swain S, Tighe M, Harmsen A, Harmsen A. Role of type I IFNs in pulmonary complications of Pneumocystis murina infection. J Immunol. 2005;174:5462–5471. doi: 10.4049/jimmunol.174.9.5462. [DOI] [PubMed] [Google Scholar]
- 22.Meissner N, Swain S, McInnerney K, Han S, Harmsen AG. Type-I IFN signaling suppresses an excessive IFN-{gamma} response and thus prevents lung damage and chronic inflammation during Pneumocystis (PC) clearance in CD4 T cell-competent cice. Am J Pathol. 2010;176:2818. doi: 10.2353/ajpath.2010.091158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruan S, McKinley L, Zheng M, Rudner XL, D'Souza S, Kolls JK, Shellito JE. Interleukin-12 and host defense against murine Pneumocystis pneumonia. Infect Immun. 2008;76:2130–2137. doi: 10.1128/IAI.00065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 25.Furuta S, Kagami S, Tamachi T, Ikeda K, Fujiwara M, Suto A, Hirose K, Watanabe N, Saito Y, Iwamoto I, Nakajima H. Overlapping and distinct roles of STAT4 and T-bet in the regulation of T cell differentiation and allergic airway inflammation. J Immunol. 2008;180:6656–6662. doi: 10.4049/jimmunol.180.10.6656. [DOI] [PubMed] [Google Scholar]
- 26.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limper AH, Hoyte JS, Standing JE. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J. Clin. Invest. 1997;99:2110–2117. doi: 10.1172/JCI119384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2012;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 30.Roths JB, Sidman CL. Both immunity and hyperresponsiveness to Pneumocystis carinii result from transfer of CD4+ but not CD8+ T cells into severe combined immunodeficiency mice. J. Clin. Invest. 1992;90:673–678. doi: 10.1172/JCI115910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcotte H, Levesque D, Delanay K, Bourgeault A, de la DR, Brochu S, Lavoie MC. Pneumocystis carinii infection in transgenic B cell-deficient mice. J Infect. Dis. 1996;173:1034–1037. doi: 10.1093/infdis/173.4.1034. [DOI] [PubMed] [Google Scholar]
- 32.Lund FE, Schuer K, Hollifield M, Randall TD, Garvy BA. Clearance of Pneumocystis carinii in miceis dependent on B cells but not on P. carinii-specific antibody. J. Immunol. 2003;171:1423–1430. doi: 10.4049/jimmunol.171.3.1423. [DOI] [PubMed] [Google Scholar]
- 33.Levy J, Espanol-Boren T, Thomas C, Fischer A, Tovo P, Bordigoni P, Resnick I, Fasth A, Baer M, Gomez L, Sanders EA, Tabone MD, Plantaz D, Etzioni A, Monafo V, Abinun M, Hammarstrom L, Abrahamsen T, Jones A, Finn A, Klemola T, DeVries E, Sanal O, Peitsch MC, Notarangelo LD. Clinical spectrum of X-linked hyper-IgM syndrome. J Pediatr. 1997;131:47–54. doi: 10.1016/s0022-3476(97)70123-9. [DOI] [PubMed] [Google Scholar]
- 34.Boom WH, Liano D, Abbas AK. Heterogeneity of helper/inducer T lymphocytes. II. Effects of interleukin 4- and interleukin 2-producing T cell clones on resting B lymphocytes. J Exp Med. 1988;167:1350–1363. doi: 10.1084/jem.167.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haggqvist B, Hultman P. Effects of deviating the Th2-response in murine mercury-induced autoimmunity towards a Th1-response. Clin Exp Immunol. 2003;134:202–209. doi: 10.1046/j.1365-2249.2003.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapoval SP, Nabozny GH, Marietta EV, Raymond EL, Krco CJ, Andrews AG, David CS. Short ragweed allergen induces eosinophilic lung disease in HLA-DQ transgenic mice. J Clin Invest. 1999;103:1707–1717. doi: 10.1172/JCI6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barr TA, Brown S, Mastroeni P, Gray D. B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c. J Immunol. 2009;183:1005–1012. doi: 10.4049/jimmunol.0803706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theus SA, Linke MJ, Andrews RP, Walzer PD. Proliferative and cytokine responses to a major surface glycoprotein of Pneumocystis carinii. Infect. Immun. 1993;61:4703–4709. doi: 10.1128/iai.61.11.4703-4709.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]