Abstract
Background
Functional ambulation requires concurrent performance of motor and cognitive tasks, which may create interference (degraded performance) in either or both tasks. People with essential tremor (ET) demonstrate impairments in gait and cognitive function. In this study we examined the extent of interference between gait and cognition in people with ET and controls during dual-task gait.
Methods
We tested 62 controls and 151 ET participants (age range: 72–102). ET participants were divided into two groups based on median score on the modified Mini Mental State Examination. Participants walked at their preferred speed, and performed a verbal fluency task while walking. We analyzed gait velocity, cadence, stride length, double support time, stride time, step width, step time difference, coefficient of variation (CV) of stride time and stride length.
Results
Verbal fluency performance during gait was similar across groups (p=0.68). Velocity, cadence and stride length was lowest whereas step time difference (p=0.003), double support time (p=0.009), stride time (p=0.002) and stride time CV (p=0.007) were highest for ET participants with lower cognitive scores (ETp-LCS), compared with ET participants with higher cognitive scores (ETp-HCS) and controls. ETp-LCS demonstrated greatest interference for double support time (p=0.005), step time difference (p=0.013) and stride time coefficient of variation (p=0.03).
Conclusions
ETp-LCS demonstrated high levels of cognitive motor interference. Gait impairments during complex tasks may increase risk for falls for this subgroup and underscore the importance of clinical assessment of gait under simple and dual-task conditions.
Keywords: Essential tremor, gait, dual-task, cognitive-motor interference
Introduction
Essential tremor (ET) is a common movement disorder presenting with both motor and non-motor impairments [1]. Gait and balance impairments have been observed, both on tandem walk [2] and on standardized clinical assessments of balance [3]. Gait impairments include decreased velocity and cadence, increased time in double support, and step time asymmetry [3, 4]. Gait and balance impairments are functionally significant because they may predispose people with ET to fear of falls, near falls or falls [5].
ET participants also present with cognitive deficits, including memory, executive function and visual attention, above and beyond what is seen in age-matched controls [6, 7]. Cognitive deficits are clinically relevant because they are associated with poor performance in activities of daily living [8]. While pathological changes underlying cognitive deficits in ET are unclear, factor analysis of motor and non-motor signs show that cognitive changes do not fall in the same domain as motor changes, suggesting that cognitive and motor signs may arise from independent pathological processes [9]. Population-based studies report that ET is associated with increased risk for dementia, suggesting cognitive changes in ET may be related to Alzheimer’s disease [10, 11]. However, the similarity of cognitive deficits in ET to those seen after cerebellar dysfunction indicate that cognitive deficits could arise from cerebellar-thalamo-cortical pathway dysfunction [12].
Functional ambulation in the community requires coordinated motor and cognitive skills. Cognitive demands of gait are typically evaluated with dual-task methodology, in which subjects perform a cognitive task concurrently with gait [13]. Changes in gait during performance of a concurrent cognitive task are indicative of cognitive-motor interference. Increased interference during dual-task conditions was reported in elderly participants [14] and stroke patients [15] and was associated with increased fall risk, highlighting the importance of studying dual-task gait.
While there is independent evidence of gait and cognitive impairments in ET, there are no studies of cognitive motor interference. In this study we examined if performance of a cognitive task during walking produced gait impairments in ET participants and controls. We used verbal fluency as the cognitive task because of its effectiveness in producing interference during gait [13]. Our aim was to examine if a sub-group of ET participants had greater cognitive-motor interference. We hypothesized that ET participants with lower cognitive scores would demonstrate greater cognitive-motor interference compared with ET participants with higher cognitive scores and controls. Therefore, we divided ET participants into two groups based on scores on the modified Mini Mental State Examination (mMMSE). Given the strong association of age with cognitive deficits [9], a second aim of the study was to examine the influence of age on cognitive-motor interference. We hypothesized that ET participants with low cognitive scores (ETp-LCS) would demonstrate cognitive-motor interference in excess of that seen in participants with higher cognitive scores and controls, even into advanced age.
Methods
Subjects
Participants were enrolled as future brain donors to the Essential Tremor Centralized Brain Repository (ETCBR) at Columbia University, a national repository for collection of ET brains. Recruitment was done through (1) advertisements in the International Essential Tremor Foundation website and newsletters, (2) advertisements on the Tremor Action Network website, and (3) an ETCBR study website (www.essentialtremor.us). The target population included people with ET and spousal controls living broadly across the United States (including 34 States). The diagnosis of ET was re-confirmed in each ET participant using published diagnostic criteria (moderate or greater amplitude kinetic tremor during three or more activities, or a head tremor, in the absence of Parkinson’s disease (PD)). Spousal controls were recruited if they did not have a diagnosis of ET. We excluded participants with dementia (mMMSE score < 40), other neurological disorders (such as stroke, PD or dystonia), orthopedic impairments that impair walking, or depression. We excluded participants with dementia because 1) they would have had difficulty completing the task, and 2) we wanted to maintain within-group homogeneity in ETp-LCS All participants signed a written informed consent form, approved by the institutional ethics committee.
Testing
Participants were tested at home on a single day by a trained tester, which allowed us to 1) examine performance in a familiar environment, and 2) recruit a large sample of subjects who would not have been able to travel the long distance to our hospital. In order to minimize differences in testing conditions across subjects, prior to testing, we ensured that subjects had access to a well-lit hallway long enough to accommodate the GAITRite® mat. Most participants had a hallway with wood flooring while few had pile carpeting. We placed corkboard under the GAITRite mat in order to make the support surface consistent (we established the reliability of measuring gait parameters with the cork board under the Gaitrite mat). Testing consisted of two parts, a clinical assessment and quantitative gait assessment. Participants were provided with rest, as needed, during testing.
Clinical assessment
All ET participants and controls underwent a clinical assessment that included collection of demographic and clinical data, which included age, gender, highest educational degree, and age at tremor onset. ET participants also underwent a standardized videotaped neurological examination [16] and a modified Mini Mental State Examination (mMMSE, range = 0–57, higher scores indicating better function) [17].
Quantitative gait assessment
The GAITRite, a 4.6 m long computerized mat (CIR Systems, Havertown, PA), was placed in the middle of a quiet hallway in the subjects’ home to collect gait data. The mat registers the location and timing of each footfall. Subjects began walking 3 meters from the beginning of the mat and stopped 3 meters beyond the end of the mat to record steady-state gait on the mat without the influence of gait initiation and termination. ET participants and controls performed three trials for each of two conditions: (a) standard walk, in which participants were asked to walk at their preferred speed; and (b) dual-task walk, during which participants performed verbal (category) fluency while walking. On each dual-task trial subjects were given a letter of the alphabet (“B”) and were requested to name aloud as many animals as they could that began with that letter (e.g., “Bear”, “Bat”), while walking at their preferred speed. The order of testing conditions was randomized. Participants were not given instructions regarding task priority and were requested not to use assistive devices during data collection.
Data were analyzed by AKR and JU, who were blinded to clinical diagnosis and age. We analyzed the following gait measures by computing the average of three trials per condition: velocity, stride length, cadence, stride time, double support time, step time difference, step length difference, step width, and coefficient of variation (CV) in stride time and stride length. On average, participants walked 10 steps per trial, enabling us to use 30 steps for computing variability. While some authors suggest that 30 steps may be adequate for computing variability [18], others recommend using hundreds of steps [19]. We used our data to provide an estimate of variability for comparison across groups, as seen in the literature (see [20] and [21]). Verbal fluency was only tested under dual-task conditions- we recorded the number of animals that each participant was able to name correctly across the three trials. Repetitions and incorrect responses were excluded.
Statistical Analysis
In order to examine if a sub-group of ET participants were at a greater risk of functional gait difficulty, we divided ET participants into two groups based on scores on the mMMSE (median value= 50). ET participants with scores ≥50 were classified as having higher cognitive test scores (ETp-HCS) and cases with scores <50 were classified as having lower cognitive test scores (ETp-LCS). Published data indicate that mMMSE scores <50 are associated with mild functional deficits [8].
Statistical analyses were performed in SPSS (version 18.0) by AKR. Clinical characteristics of ET participants and controls were compared with one-way analysis of variance (ANOVA) or Student t-test for continuous variables and χ2 tests for categorical variables. For gait measures, we conducted analysis of covariance (ANCOVA), with group (ETp-LCS, ETp-HCS, control) and condition (standard walk, dual-task walk) as factors. We used ANCOVA in order to correct for baseline differences between groups. Since age was different across groups, this was entered as a covariate. Gait measures that demonstrated a significant main effect of group and age, and significant group × condition interaction effect were subsequently entered into a linear regression analysis to examine the independent effects of age and condition on gait. We used age and group as independent predictors of gait in separate models (model 1: predictor= group; model 2: predictors= group, age). Outliers (n=2) were excluded from the analysis if they were > 2 standard deviations from the mean.
Results
Demographic and Clinical Characteristics
Sample size was estimated based on our previous study on tandem gait impairments [2]. We recruited 162 ET participants and 63 controls (Total= 225). One ET participant and one control were excluded because they could not perform the task without assistive devices. Ten ET participants were excluded prior to analysis because their score on the mMMSE was below 40/57. The final sample of 213 subjects included 61 ETp-LCS, 90 ETp-HCS and 62 controls.
Clinical characteristics of our sample are presented (Table 1). Both groups of ET participants (ETp-LCS and ETp-HCS) were of similar age but were older than controls (ETp-LCS, p = 0.0001 and ETp-HCS, p = 0.007). No differences were seen across groups in gender, highest educational degree or age at which tremor began (Table 1).
Table 1.
Clinical Characteristics of Participants
| ET participants with Lower Cognitive Scores |
ET participants with Higher Cognitive Scores |
Controls | p Value | ||
|---|---|---|---|---|---|
| Number of subjects | 61 | 90 | 62 | - | |
| Mean Modified | 44.7 (2.29) | 53.3 (1.90) | NT | 0.0001a | |
| MMSE score (SD) | 40–49 | 50–57 | |||
| Range | |||||
| Mean Age, years (SD) | 84.4 (5.9) | 82.6 (5.6) | 79.6 (6.6) * | 0.0001a | |
| Range | 72–102 | 72–97 | 68–94 | ||
| Female Gender (percent) | 42 (68%) | 55 (61%) | 40 (65%) | 0.79b | |
| Highest Education category (SD) |
|||||
| 1=No degree | 3 | 2 | NT | 0.19b | |
| 2= High-school diploma | 23 | 29 | |||
| 3= Associates degree | 5 | 9 | |||
| 4= Bachelor degree | 13 | 25 | |||
| 5=Masters degree | 11 | 17 | |||
| 6= Doctorate or equivalent | 6 | 8 | |||
| 7= Trade school | 0 | 0 | |||
| Age of Tremor Onset, Years (SD) |
43.3 (23.42) | 40.1 (21.84) | N/A | 0.43a | |
| Dual-task Verbal fluency score |
1.85 (1.00) | 1.85 (0.93) | 1.61 (0.77) | 0.68c | |
= Student’s t-test;
= Chi square test;
=One-way ANOVA;
NT= not tested; N/A= Not applicable;
= Tukey Post-hoc analysis revealed that controls were younger than ET participants with lower cognitive scores (p=0.0001) and ET participants with higher cognitive scores (p=0.007). There was no age difference between the two ET groups (p=0.173); Statistically significant values in bold
Quantitative Gait Analysis
Mean, standard deviation and significance values are presented in Table 2. Gait velocity, stride length and cadence demonstrated a main effect of group. Post-hoc analysis indicated that ETp-LCS had significantly lower velocity, stride length and cadence compared with ETp-HCS and controls. No difference was seen between ETp-HCS and controls. Under dual task walk, velocity, stride length and cadence decreased for all groups (main effect of condition). Group × condition interaction was not seen, indicating that performance under dual task walk worsened similarly for all groups. Finally, velocity, stride length and cadence decreased with increasing age for all groups (main effect of age).
Table 2.
Mean (Standard Deviation) for Gait Measures Across Groups and Conditions (Standard Walk and Dual-Task Walk) and results of statistical analysis
| ETp-LCS | ETp-HCS | Controls | Group | Group post-hoc analysis | Condition | Age | Group × Condition |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SW | DTW | SW | DTW | SW | DTW | ETp-LCS Vs ETp- HCS |
ETp- LCS vs C |
ETp- HCS vs C |
|||||
| Velocity, m/sec (SD) |
0.72 (0.24) |
0.46 (0.21) |
0.89 (0.31) |
0.61 (0.26) |
0.99 (0.33) |
0.71 (0.24) |
0.0001 | 0.001 | 0.0001 | 0.11 | 0.003 | 0.0001 | 0.81 |
| Stride length, m (SD) |
0.91 (0.23) |
0.73 (0.21) |
1.03 (0.23) |
0.85 (0.24) |
1.08 (0.21) |
0.93 (0.20) |
0.0001 | 0.003 | 0.0001 | 0.06 | 0.021 | 0.005 | 0.2 |
| Cadence, steps/min (SD) |
89.11 (15.31) |
70.30 (20.07) |
97.04 (14.46) |
78.27 (17.78) |
101.55 (11.98) |
86.01 15.46) |
0.004 | 0.004 | 0.003 | 0.57 | 0.035 | 0.0001 | 0.26 |
| Stride time, sec (SD) |
0.56 (0.26) |
0.99 (0.77) |
0.49 (0.32) |
0.74 (0.43) |
0.42 (0.29) |
0.56 (0.21) |
0.002 | 0.01 | 0.001 | 0.18 | 0.465 | 0.006 | 0.03 |
| Double support time, sec (SD) |
1.39 (0.32) |
1.89 (0.83) |
1.27 (0.28) |
1.63 (0.48) |
1.21 (0.21) |
1.46 (0.32) |
0.009 | 0.029 | 0.003 | 0.29 | 0.46 | 0.0001 | 0.005 |
| Step width, m (SD) |
0.12 (0.04) |
0.14 (0.05) |
0.11 (0.03) |
0.12 (0.04) |
0.01 (0.41) |
0.11 (0.46) |
0.05 | 0.04 | 0.03 | 0.71 | 0.21 | 0.002 | 0.4 |
| Step length difference, m (SD) |
0.06 (0.08) |
0.16 (0.24) |
0.04 (0.06) |
0.06 (0.07) |
0.03 (0.05) |
0.05 (0.07) |
0.15 | 0.1 | 0.07 | 0.7 | 0.11 | 0.23 | 0.22 |
| Step time Difference, sec (SD) |
0.11 (0.58) |
0.04 (0.08) |
0.03 (0.02) |
0.04 (0.08) |
0.03 (0.05) |
0.03 (0.03) |
0.003 | 0.001 | 0.01 | 0.8 | 0.02 | 0.0001 | 0.013 |
| CV stride length, % (SD) |
5.66 (5.77) |
13.28 (16.17) |
4.59 (8.40) |
7.33 (4.84) |
3.24 (4.8) |
6.12 (4.76) |
0.15 | 0.14 | 0.06 | 0.54 | 0.34 | 0.0001 | 0.44 |
| CV Stride time, % (SD) |
5.85 (4.93) |
8.55 (8.68) |
4.59 (4.83) |
6.91 (4.93) |
4 (4.54) |
5.34 (2.94) |
0.007 | 0.005 | 0.006 | 0.76 | 0.33 | 0.0001 | 0.03 |
ETp-LCS = ET participants with lower cognitive test scores; ETp-HCS = ET participants with higher cognitive test scores; C= control; SW= Standard walk; DTW= Dual-task walk; SD= Standard deviation; CV= Coefficient of variation; Statistically significant values in bold
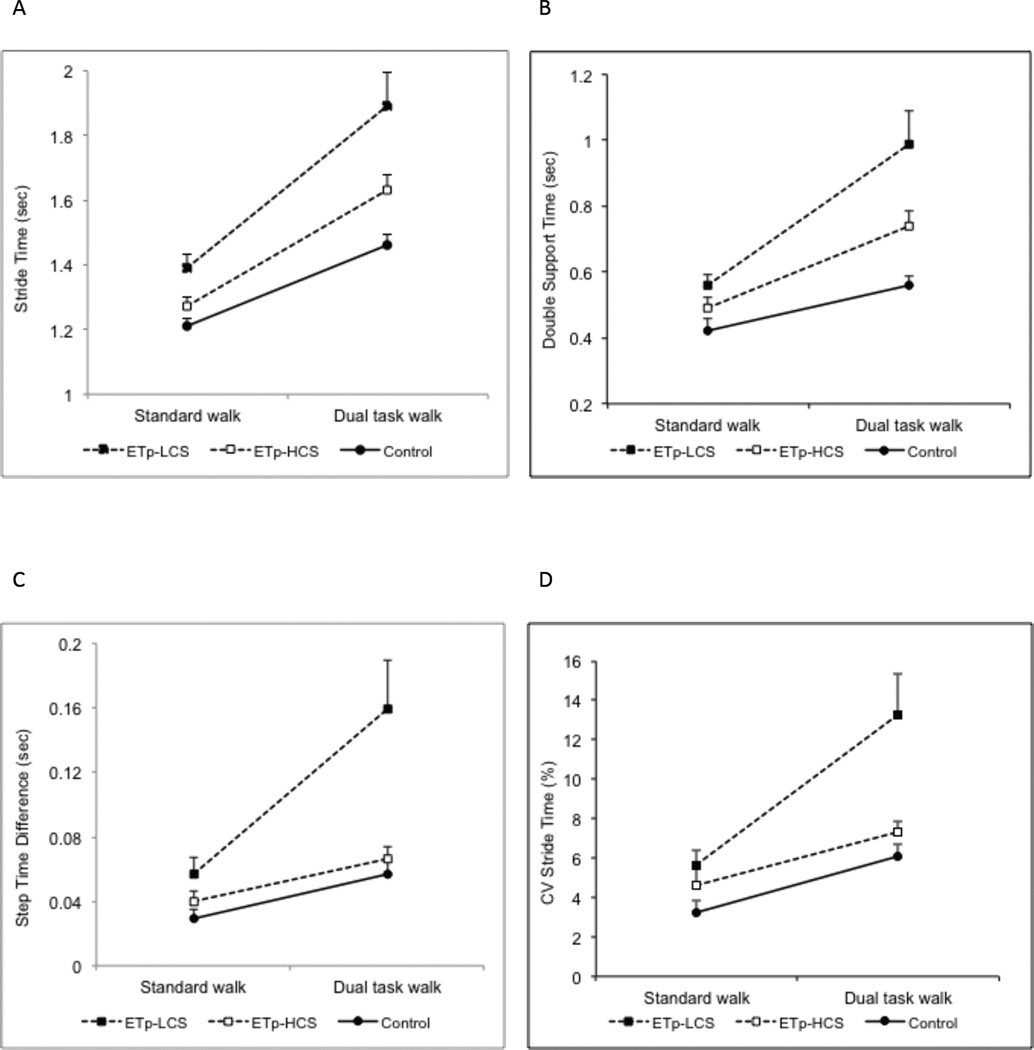
Stride time and double support time also demonstrated a main effect of group. Post hoc analysis demonstrated that ETp-LCS had the highest stride time and double support time compared with ETp-HCS and controls. No difference was seen between ETp-HCS and controls. Both variables demonstrated a significant group × condition interaction, as seen in Figure 1. While all three groups worsened under dual task walk, the increase in stride time (Fig.1A) and double support time (Fig. 1B) was highest for ETp-LCS. Step width was higher in ETp-LCS compared with ETp-HCS and controls, as seen on post-hoc analysis, and increased with age for all groups, as seen by a main effect of age.
Figure 1.
Mean ± standard error for stride time (A), double support time (B), step time difference (C) and stride time CV (D) for standard walk and dual-task walk for ETp-LCS (filled square), ETp-HCS (empty square) and controls (filled circle).
Step time difference (Fig. 1C) and stride time CV (Fig. 1D) were higher (i.e. more impaired) for ETp-LCS compared with ETp-HCS and with controls, as seen by a main effect of group and post-hoc comparisons. During dual-task walk, step time difference and stride time CV were higher for all groups (main effect of condition) though the increase in variability was highest for ETp-LCS group (group × condition interaction). With increasing age, all groups demonstrate higher step time difference and stride time CV. In contrast, stride length CV was not different across groups, or conditions, though there was an effect of age (p=0.0001).
In summary, dual-task walk impaired gait for all three groups. However, for some variables (stride time, double support time, step time difference and stride time CV) the influence of the dual-task walk was most pronounced for ETp-LCS.
Effect of Age on Cognitive-Motor Interference
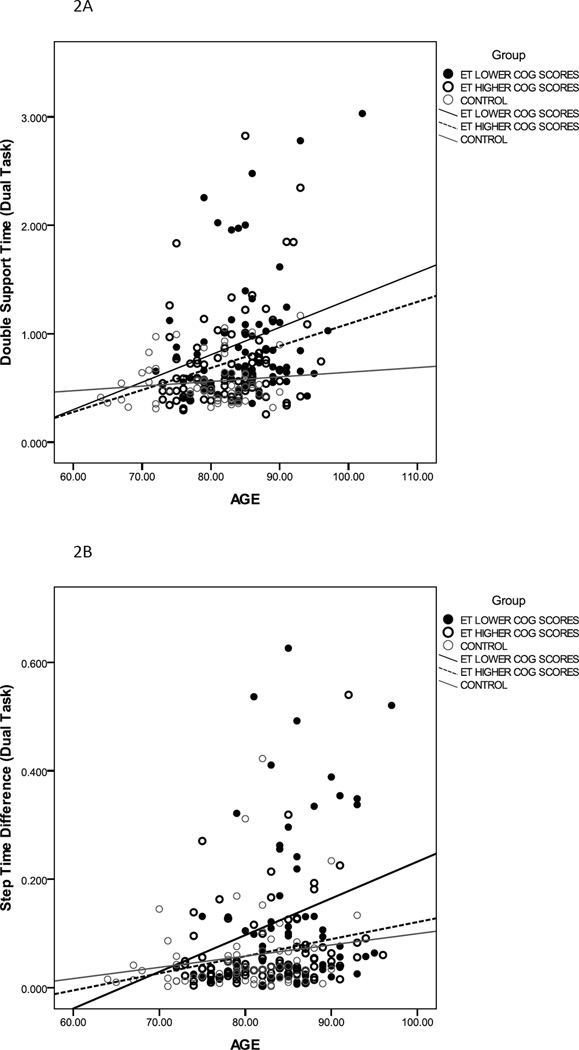
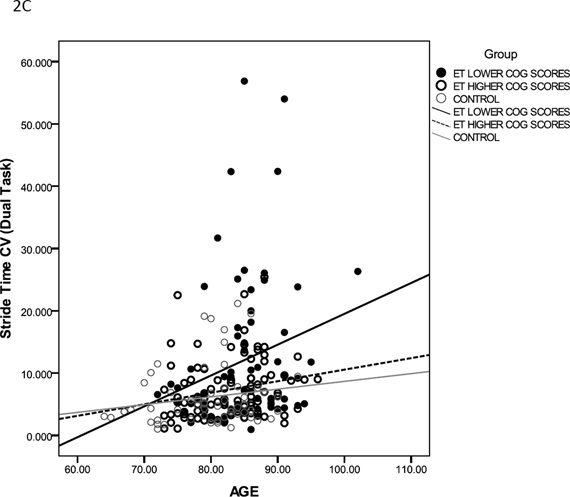
Gait variables that demonstrated significant group and condition main effects, and group × condition interaction effect were further analyzed to examine the interaction of age on cognitive-motor interference (Table 3). In the simple model, diagnostic group was a significant predictor of stride time, double support time, step time difference and stride time CV under both standard walk and dual-task walk conditions. In the complex model, group and age were significant predictors of double support time, step time difference and stride time CV under dual-task conditions. Figure 2 shows results for double support time (2A), step time difference (2B) and stride time CV (2C) under dual-task conditions. The slope of the linear regression fit is clearly different for ETp-LCS, indicating that performance of these participants worsened under dual task gait with increasing age. These results indicate that under dual-task conditions, group was an independent predictor of gait impairments above and beyond the effect of age.
Table 3.
Regression Model and Coefficients for Select Gait Measures
| Regression Model: Group | Regression Model: Group and Age | |||||
|---|---|---|---|---|---|---|
| Coefficient | P Value | Coefficient Group |
P Value | Coefficient Age |
P Value | |
|
Stride Time Standard walk |
−0.214 | 0.002 | −0.14 | 0.03 | 0.243 | 0.0001 |
|
Stride Time Dual-task walk |
−0.27 | 0.0001 | −0.24 | 0.001 | 0.102 | 0.13 |
|
Double Support Time Standard walk |
−0.146 | 0.03 | −0.069 | 0.32 | 0.26 | 0.0001 |
|
Double Support Time Dual-task walk |
−0.31 | 0.0001 | −0.24 | 0.0001 | 0.222 | 0.001 |
|
Step Time difference Standard walk |
−0.14 | 0.03 | −0.068 | 0.33 | 0.257 | 0.0001 |
|
Step Time difference Dual-task walk |
−0.25 | 0.0001 | −0.19 | 0.005 | 0.21 | 0.003 |
|
Stride Time CV % Standard walk |
−0.114 | 0.09 | −0.048 | 0.49 | 0.222 | 0.002 |
|
Stride Time CV % Dual-task walk |
−0.28 | 0.0001 | −0.22 | 0.001 | 0.19 | 0.005 |
CV= Coefficient of variation; Statistically significant values in bold
Figure 2.
Relationship between age and double support time (A) step time difference (B) and Stride time CV (C) for ETp-LCS (filled black circles), ETp-HCS (empty black circles) and controls (empty grey circles) under dual-task conditions. Linear regression fit is shown for all three groups (ETp-LCS: thick black line; ETp-HCS: black dashed line; Controls: grey line).
Cognitive Function
The mMMSE was administered only to ET participants. Item analysis of the mMMSE indicated that ETp-LCS performed worse on two items, orientation to place (p=0.04) and calculation (p=0.01) compared with ETp-HCS. As seen in Table 1, all groups performed comparably on verbal fluency during dual task walk. Thus, our results indicate that verbal fluency during dual-task performance was not impaired in ET participants. However, control of gait under dual-task performance was impaired, particularly for ETp-LCS.
Discussion
Gait and balance impairments are commonly seen in ET participants. These problems are not merely a subclinical phenomenon observed in the laboratory; emerging data indicate that people with ET experience more fear of falls, near falls and associated morbidities [22]. Some subgroups of ET patients could be at increased risk of these difficulties, and it would be important for clinicians to be aware of this.
In this study, ET participants with lower scores on the mMMSE (ETp-LCS) had more gait impairments during dual-task walk as compared with ET participants with higher cognitive test scores (ETp-HCS) and controls. In contrast, all three groups (ETp-LCS, ETp-HCS and controls) performed comparably on the cognitive task (verbal fluency). While all groups demonstrated some worsening of gait under dual-task walk, ETp-LCS demonstrated the greatest cognitive motor interference. This was particularly true for stride time, double support time, step time difference and stride time CV.
Functional ambulation often requires concurrent performance of walking and cognitive tasks (such as remembering items on shopping lists). Increased cognitive motor interference during complex dual-task conditions may be associated with increased risk for falls and decreased quality of life. Our study is the first to demonstrate cognitive motor interference in a particular subgroup of ET participants (i.e., those with lower cognitive scores), who could be at a greater risk for functional gait difficulty.
Increase in stride time and stride time CV under dual-task conditions has previously been reported in elderly people with mild cognitive dysfunction and dementia [23], and people with PD [24]. For elderly people with cognitive or neurological dysfunction, increase in stride time and stride time variability under dual-task conditions is associated with increased risk for falls [24, 25]. In contrast, a recent study reported that dual task walk was not predictive of falls in people with PD [26]. However, the subjects in that study were younger than participants in our study, as well as elderly people with mild cognitive dysfunction [23] and elderly people with dementia. It is possible that the predictive value of dual task walk for falls increases with age, though this remains to be tested.
The relationship of cognitive motor interference and fall risk has not been studied in ET. Our results indicate that ETp-LCS have greater impairments in gait. Whether greater gait impairments result in increased fall risk needs to be tested. Assessment of cognitive-motor interference is not currently part of routine clinical examination of ET participants [7]. Our results support inclusion of dual-task gait to identify ET participants who may be at a greater risk for functional gait difficulty [5].
While ETp-LCS had lower gait velocity, stride length and cadence compared with ETp-HCS and controls, all groups worsened similarly under dual-task conditions. Slowing of gait speed with increasing task complexity in elderly subjects is well-documented [13], and may be associated with structural changes in the pre-frontal cortex, suggested to be involved with performance of both cognitive tasks and control of gait speed [27].
ET participants and controls performed similarly on verbal fluency during gait despite differences in cognitive ability (scores on mMMSE). The phenomenon of worsening gait control while maintaining performance of a cognitive dual-task has been demonstrated previously in PD [28]. In these studies, healthy young subjects prioritized gait control at the cost of performance on the cognitive task [28]. PD participants, on the other hand, prioritized performance of the cognitive task at the cost of postural stability during gait [28]. Similar results have been reported in elderly subjects. The prioritization of the cognitive task has the effect of worsening gait impairments and increasing the risk for falls. Our cohort of ETp-LCS did not prioritize gait control, which may place them at a higher risk for falls. It remains to be tested whether ET participants can prioritize their gait performance on explicit instructions, as has been demonstrated in the elderly [29] and in PD [30].
Our study has some limitations. Since we did not administer the mMMSE to control subjects, it is unclear whether they had a similar range of scores compared with ET participants. However, even if some of the controls had had lower scores on the mMMSE, this would have biased our results towards the null hypothesis. Second, we did not collect data on falls, which would allow us to examine the relationship between gait impairments under dual-task conditions and falls. Third, we did not test verbal fluency as a single task. However, since we were primarily interested in the influence of a cognitive dual task on gait, any differences in verbal fluency performance as a single task would not influence our conclusions.
To summarize, our results show that ET participants demonstrate gait impairments above and beyond those seen in the healthy elderly. These gait impairments worsen during the performance of a concurrent cognitive task during gait, particularly in ET participants with lower scores on a cognitive test. The results underscore the importance of clinical assessment of gait under simple and dual-task conditions in ET [5].
Research Highligts.
We examined if performance of a cognitive task (verbal fluency) during walking produced gait impairments (i.e. cognitive-motor interference) in ET participants and controls
No differences were seen in the performance of the cognitive task
ET participants with low scores on a cognitive test demonstrated the highest interference, particularly for double support time, step time difference and stride time variability
Clinical assessment of gait in ET participants should include testing under simple and dual-task conditions.
Acknowledgements
We thank all ET subjects, their families and control subjects for participating in the study. We did not receive any writing assistance for preparing this manuscript.
Sources of Funding:
This work was supported by the National Institutes of Health (Bethesda, MD) under grant number 5R01NS042859. The funding source did not have a role in the design and implementation of the project, or in the writing of the manuscript. Ashwini K Rao conducted the statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosure:
None of the authors report a conflict of interest with respect to financial or personal relationships with organizations that may have an influence on the work.
Author Roles
Ashwini K. Rao: Research project: conception, organization and execution; Statistical Analysis: Design, execution, review and critique; Manuscript preparation: writing the first draft, review and critique
Jasim Uddin: Research project: organization, execution; Statistical Analysis: execution and review and critique; Manuscript Preparation: Review and critique
Arthur Gillman: Research project: organization, execution; Statistical Analysis: execution and review and critique; Manuscript Preparation: Review and critique
Elan D. Louis: Research project: conception, organization and execution; Statistical Analysis: Review and critique; Manuscript preparation: Review and critique
Contributor Information
Ashwini K Rao, Program in Physical Therapy and G.H. Sergievsky Center. Columbia University College of Physicians and Surgeons. New York. USA.
Jasim Uddin, Program in Physical Therapy. Columbia University College of Physicians and Surgeons. New York. USA.
Arthur Gillman, G.H. Sergievsky Center. Columbia University College of Physicians and Surgeons. New York. USA.
Elan D Louis, G.H. Sergievsky Center, Taub Institute for Alzheimer’s Disease and the Aging Brain and Department of Neurology, Columbia University College of Physicians and Surgeons; Department of Epidemiology, Mailman School of Public Health, Columbia University, New York. USA.
References
- 1.Benito-Leon J, Louis ED. Essential tremor: emerging views of a common disorder. Nat Clin Pract Neurol. 2006;2(12):666–678. doi: 10.1038/ncpneuro0347. quiz 2p following 691. [DOI] [PubMed] [Google Scholar]
- 2.Louis ED, Rios E, Rao AK. Tandem gait performance in essential tremor: clinical correlates and association with midline tremors. Mov Disord. 2010;25(11):1633–1638. doi: 10.1002/mds.23144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Earhart GM, et al. Gait and balance in essential tremor: variable effects of bilateral thalamic stimulation. Mov Disord. 2009;24(3):386–391. doi: 10.1002/mds.22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao AK, Gillman A, Louis ED. Quantitative gait analysis in essential tremor reveals impairments that are maintained into advanced age. Gait Posture. 2011;34(1):65–70. doi: 10.1016/j.gaitpost.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis ED, Rao AK, Gerbin M. Functional correlates of gait and balance difficulty in essential tremor: balance confidence, near misses and falls. Gait Posture. 2012;35(1):43–47. doi: 10.1016/j.gaitpost.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benito-Leon J, Louis ED, Bermejo-Pareja F. Population-based case-control study of cognitive function in essential tremor. Neurology. 2006;66(1):69–74. doi: 10.1212/01.wnl.0000192393.05850.ec. [DOI] [PubMed] [Google Scholar]
- 7.Louis ED. Essential tremor as a neuropsychiatric disorder. J Neurol Sci. 2010;289(1–2):144–148. doi: 10.1016/j.jns.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis ED. Functional correlates of lower cognitive test scores in essential tremor. Mov Disord. 2010;25(4):481–485. doi: 10.1002/mds.22920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis ED. Factor analysis of motor and nonmotor signs in essential tremor: are these signs all part of the same underlying pathogenic process? Neuroepidemiology. 2009;33(1):41–46. doi: 10.1159/000211952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thawani SP, Schupf N, Louis ED. Essential tremor is associated with dementia: prospective population-based study in New York. Neurology. 2009;73(8):621–625. doi: 10.1212/WNL.0b013e3181b389f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bermejo-Pareja F, Louis ED, Benito-Leon J. Risk of incident dementia in essential tremor: a population-based study. Mov Disord. 2007;22(11):1573–1580. doi: 10.1002/mds.21553. [DOI] [PubMed] [Google Scholar]
- 12.Troster AI, et al. Neuropsychological deficits in essential tremor: an expression of cerebello-thalamo-cortical pathophysiology? Eur J Neurol. 2002;9(2):143–151. doi: 10.1046/j.1468-1331.2002.00341.x. [DOI] [PubMed] [Google Scholar]
- 13.Al-Yahya E, et al. Cognitive motor interference while walking: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2011;35(3):715–728. doi: 10.1016/j.neubiorev.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Beauchet O, et al. Dual task-related changes in gait performance in older adults: a new way of predicting recurrent falls? J Am Geriatr Soc. 2008;56(1):181–182. doi: 10.1111/j.1532-5415.2007.01464.x. [DOI] [PubMed] [Google Scholar]
- 15.Plummer-D'Amato P, et al. Interactions between cognitive tasks and gait after stroke: a dual task study. Gait Posture. 2008;27(4):683–688. doi: 10.1016/j.gaitpost.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis ED, et al. Correlates of functional disability in essential tremor. Mov Disord. 2001;16(5):914–920. doi: 10.1002/mds.1184. [DOI] [PubMed] [Google Scholar]
- 17.Stern YSM, Paulsen J, Mayeux R. Modified mini-mental state examination: validity and reliability. Neurology. 1987;37(Supplement 1):179. [Google Scholar]
- 18.Galna B, Lord S, Rochester L. Is gait variability reliable in older adults and Parkinson's disease? Towards an optimal testing protocol. Gait Posture. 2012 doi: 10.1016/j.gaitpost.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Hollman JH, et al. Number of strides required for reliable measurements of pace, rhythm and variability parameters of gait during normal and dual task walking in older individuals. Gait Posture. 2010;32(1):23–28. doi: 10.1016/j.gaitpost.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerman ME, et al. MRI- and MRS-derived hippocampal correlates of quantitative locomotor function in older adults. Brain Res. 2009;1291:73–81. doi: 10.1016/j.brainres.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muir SW, et al. Gait assessment in mild cognitive impairment and Alzheimer's disease: The effect of dual-task challenges across the cognitive spectrum. Gait Posture. 2011 doi: 10.1016/j.gaitpost.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Louis ED, Rao AK, Gerbin M. Functional correlates of gait and balance difficulty in essential tremor: Balance confidence, near misses and falls. Gait Posture. 2011 doi: 10.1016/j.gaitpost.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verghese J, et al. Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc. 2008;56(7):1244–1251. doi: 10.1111/j.1532-5415.2008.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly VE, Eusterbrock AJ, Shumway-Cook A. A review of dual-task walking deficits in people with Parkinson's disease: motor and cognitive contributions, mechanisms, and clinical implications. Parkinsons Dis. 2012;2012 doi: 10.1155/2012/918719. p. 918719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beauchet O, et al. Relationship between dual-task related gait changes and intrinsic risk factors for falls among transitional frail older adults. Aging Clin Exp Res. 2005;17(4):270–275. doi: 10.1007/BF03324609. [DOI] [PubMed] [Google Scholar]
- 26.Smulders K, et al. Assessment of dual tasking has no clinical value for fall prediction in Parkinson's disease. J Neurol. 2012 doi: 10.1007/s00415-012-6419-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosano C, et al. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci. 2008;63(12):1380–1388. doi: 10.1093/gerona/63.12.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloem BR, et al. The multiple tasks test. Strategies in Parkinson's disease. Exp Brain Res. 2001;137(3–4):478–486. doi: 10.1007/s002210000672. [DOI] [PubMed] [Google Scholar]
- 29.Verghese J, et al. Walking while talking: effect of task prioritization in the elderly. Arch Phys Med Rehabil. 2007;88(1):50–53. doi: 10.1016/j.apmr.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canning CG. The effect of directing attention during walking under dual-task conditions in Parkinson's disease. Parkinsonism Relat Disord. 2005;11(2):95–99. doi: 10.1016/j.parkreldis.2004.09.006. [DOI] [PubMed] [Google Scholar]