Abstract
A delivery of collagenase at the islet-exocrine interface is crucial for successful human islet isolation. In this study, we investigated how the ductal preservation method at the procurement site affected collagenase distribution. At first, we analyzed human islet isolation data among groups using Serva collagenase with or without ductal injection (DI) or using new Liberase MTF with DI. Then, to assess the distribution of collagenase, human pancreata were classified into two groups: without DI (no DI, n = 5) and with DI at the procurement site (DI, n = 5). Collagenase with 1% marking dye was perfused in the same manner as in our clinical isolation. The distension of the pancreas and the microscopic distribution of the dyed collagenase in pancreas sections were examined. For microscopic analysis, islets were counted and classified into three criteria: unreached, dye didn’t reach the islet surface; surface, dye resided on the surface of the islet but not inside; and inside, dye was found inside the islet. As a result, DI groups substantially improved islet yields. In addition, Liberase MTF with DI significantly improved efficacy of pancreas digestion. All pancreata were well distended macroscopically. However, microscopically, the majority of islets in the no DI group were untouched by the dyed collagenase. Ductal preservation substantially improved dyed collagenase delivery on the surface of islets. In conclusion, delivery of collagenase on the surface of islets was unexpectedly insufficient without DI, which was substantially improved by DI. Thus, ductal preservation is a potent method to improve collagenase delivery and islet yields.
Keywords: ductal preservation, enzyme delivery, islet isolation, islet transplantation, pancreatic digestion
Introduction
For human islet transplantation to become more widely available and applicable, it is important to obtain adequate islets from a single donor pancreas to reverse diabetes.1 Despite recent progress, current islet isolation techniques usually require transplantation of islets from two or more donors to establish euglycemia,2,3 and a transplantable yield of islets is obtained from < 50% of all isolations even in advanced islet isolation centers.4-7 Successful islet isolation depends on a mechanically enhanced enzymatic digestion to dissociate the extracellular matrix of the islet-exocrine interface to release intact islets.8 Therefore, it is important to deliver the collagenase to the islet-exocrine interface area properly. For this purpose, collagenase is currently administered through the pancreatic ducts, a method reported to be superior to the simple injection method.9 Even when using collagenase with the optimized composition and the optimized delivery method, often many islets are still embedded in the surrounding exocrine tissue or over-digested after human islet isolation and the isolated islet yield is much less than expected. These facts indicate that the delivery of collagenase to the islet-exocrine interface area may not be adequate.
We have previously reported that pancreatic ductal preservation using a high-volume (1 mL/g pancreas weight) ductal injection (DI) technique with preservation solution substantially improved both the quality and quantity of isolated human islets.10-12 This resulted in a high success rate for clinical islet transplantation,10,12 even when using non–heart-beating donors.11 Ductal preservation prevents apoptotic cell death in islets,13 which can explain the mechanism of the effect on islet viability and function. However, the reason for the significantly increased islet yield achieved by ductal preservation has not been fully elucidated. We hypothesized that ductal injection immediately after pancreas procurement would enhance delivery of collagenase. To verify this hypothesis, we used a marking dye mixed with collagenase solution for collagenase perfusion in the same manner as clinical islet isolation to examine the distribution of collagenase solution with or without the DI.
Results
Effect of ductal preservation on human islet isolation
We previously reported that the ductal preservation method at the procurement site substantially improved islet yield and clinical outcomes of islet transplantation.10-12 Recently, new collagenase Liberase MTF has been implemented for clinical islet isolation with promising results.12,14 To verify a hypothesis that ductal preservation could digest the pancreas better, we analyzed our human islet isolation data among the groups with and without DI using Serva collagenase (DI Serva group, no DI Serva group) and with DI using Liberase MTF (DI MTF group). For this study, we used clinical grade pancreata and the criteria of clinical grade pancreata were shown in Table 1.
Table 1. Islet cell transplant—donor specific inclusion and exclusion criteria.
| Inclusion criteria |
Exclusion criteria |
| a. Multi-organ donor |
Pre-exisiting diseases: |
| b. Adequate in situ hypothermic perfusion |
a. Diabetes mellitus type 1 or 2 |
| c. Maximum 18 h cold ischemia in the above conditions |
b. Malignancies other than primary brain tumor |
| d. Minimum 18–70 y | c. Septicemia |
The donor and isolation variables are shown in Table 2. There was no significant difference in the donor characteristics among the groups. The undigested tissue volume in DI MTF group was significantly lower than in no DI Serva group. The islet yield (islet equivalent, IE) both prepurification and postpurification in the no DI Serva group was significantly lower than in the DI Serva group (prepurification: no DI Serva group, 474,333 ± 52,941 IE; DI Serva group, 1,007,458 ± 94,304 IE, postpurification: no DI Serva group, 371,741 ± 52,083 IE; DI Serva group, 727,777 ± 105,113 IE, Fig. 1A). There were no significant differences of islet yield between the DI Serva and the DI MTF groups in both pre and post purification (prepurification: DI MTF group, 1,157,354 ± 192,191 IE, postpurification: DI MTF group, 868,955 ± 158,053 IE, Fig. 1A).
Table 2. Donor and pancreas variables for human islet isolation data.
| Variables |
no DI Serva (n=3) |
DI Serva (n=8) |
DI MTF (n=7) |
| Age (years) |
41.3 ± 9.5 |
35.9 ± 3.9 |
43.0 ± 3.7 |
| Gender (F/M) |
0/3 |
2/6 |
4/3 |
| Body mass index (kg/m2) |
26.3 ± 3.1 |
32.2 ± 2.3 |
28.7 ± 1.2 |
| Cold ischemic time (min) |
299 ± 60 |
172 ± 24 |
199 ± 11 |
| Pancreas weight (g) |
104.4 ± 6.7 |
97.6 ± 10.1 |
101.7 ± 9.2 |
| Phase I (min) |
17.3 ± 1.4 |
12.9 ± 1.1 |
13.0 ± 1.0 |
| Phase II (min) |
52.7 ± 2.2 |
52.9 ± 3.7 |
47.6 ± 4.6 |
| Undigested tissue (g) |
27.7 ± 7.7* |
14.9 ± 3.4 |
5.6 ± 0.9* |
| Embedded islet rate (%) |
40.0 ± 20.5 |
31.4 ± 7.4 |
28.1 ± 4.1 |
| Post-purification Purity (%) |
48.2 ± 10.3 |
56.6 ± 5.7 |
60.7 ± 3.0 |
| Post-purification Viability (%) |
98.5 ± 0.2 |
97.8 ± 0.5 |
96.2 ± 0.5 |
| Recovery rate (%) |
81.3 ± 12.9 |
74.5 ± 9.8 |
76.1 ± 6.8 |
| Final pellet volume (ml) |
18.6 ± 6.5 |
8.2 ± 0.9 |
10.6 ± 3.3 |
| Stimulation Index | 1.1 ± 0.2 | 7.8 ± 2.8 | 13.7 ± 2.9 |
Data are expressed as mean ± SE.* P < 0.05.
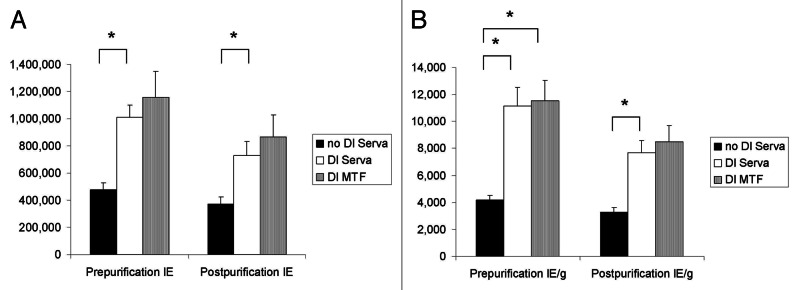
Figure 1. The effects of ductal preservation on human islet isolation outcome. (A) Prepurification and postpurification islet yields in the no DI Serva group, the DI Serva group and the DI MTF group. Islet yields were significantly higher in the DI Serva group compared with the no DI Serva group. (B) Prepurification and postpurification islet yield per pancreas weight (IE/g) in the three groups. Prepurification and postpurification IE/g was significantly higher in the DI Serva group compared with the no DI Serva group. Prepurification IE/g in the DI MTF group was significantly higher than in the no DI group. Islet yield in the DI MTF group was higher than in the DI Serva, but the difference was not significant. IE, islet equivalent. *p < 0.05.
The islet yield per pancreas weight (IE/g) both prepurification and postpurification in the no DI Serva group was also significantly lower than in the DI Serva group (prepurification: no DI Serva group, 4,159 ± 353 IE/g; DI Serva group, 11,155 ± 1,388 IE/g, postpurification: no DI Serva group, 3,255 ± 339 IE/g; DI Serva group, 7,698 ± 887 IE/g, Fig. 1B). There were no significant differences of islet yield per pancreas weight between the DI Serva and the DI MTF groups in both pre and post purification (prepurification: DI MTF group, 11,523 ± 1,537 IE/g, postpurification: DI MTF group 8,475 ± 1,206 IE/g, Fig. 1B).
These data confirmed the benefit of ductal preservation to increase human islet yield substantially. Our data showed that Liberase MTF was equivalent to or even better than Serva collagenase. Indeed, the success rate of islet isolation (defined as > 300,000 IE/pancreas) using MTF in our institute is more than 90% (data not shown). Therefore, we conducted the following studies using Liberase MTF.
Distribution of collagenase study, pancreas distension after collagenase delivery
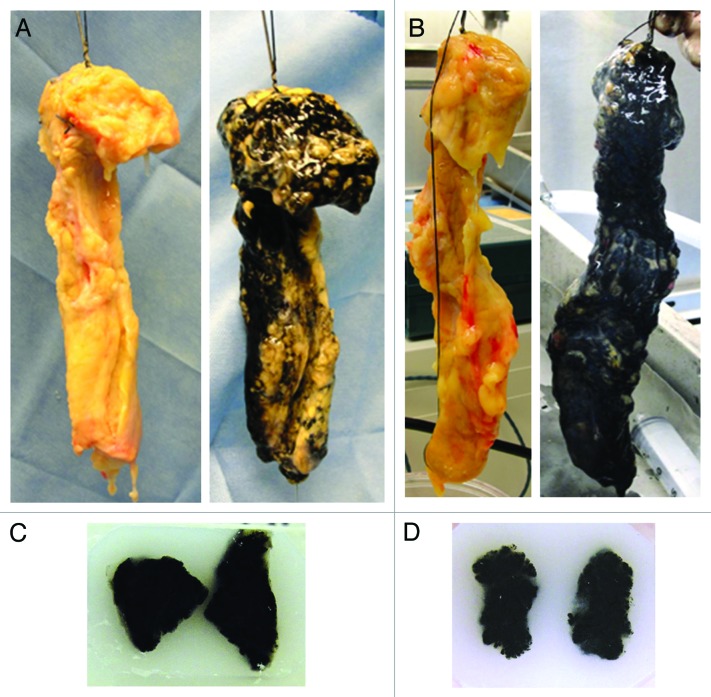
The study schema of the effect of ductal preservation on the delivery of collagenase is shown in Fig. 2. The characteristics of the used pancreata are shown in Table 3. There were no significant differences in donor characteristics between the two groups. An average of 112 ± 4 mL of ET-Kyoto solution (Otsuka Pharmaceutical Factory Inc.) was infused for ductal preservation in the DI group. The peak pressure observed during DI ranged from 300–400 mmHg. Cold ischemic time was less than 10 h in all cases. After continuous perfusion of collagenase solution with dye, pancreata were well distended macroscopically in both the no DI (Fig. 3A, right) and the DI groups (Fig. 3B, right). However, some non-dyed spots were seen in the pancreas in the no DI group (Fig. 3A, right) whereas dye spread more thoroughly in the DI group (Fig. 3B, right). For a quantitative assessment of the distention, the weight of pancreata was measured before and after the collagenase perfusion (Table 4). The rate of weight increase was not different between the two groups.
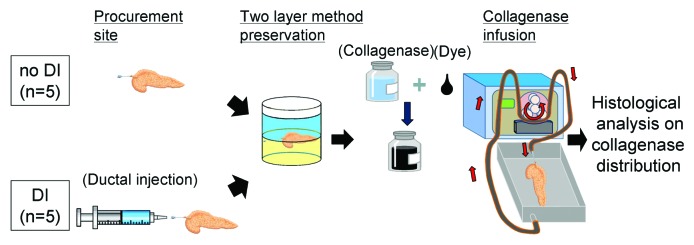
Figure 2. Schematic of the study of collagenase distribution with or without ductal preservation. In the no DI group, after procurement the pancreas was preserved with the two layer method (upper layer: ET-Kyoto solution, lower layer: oxygenated perfluorocarbon). Meanwhile, ET-Kyoto solution was infused through the main pancreatic duct at the procurement site in the DI group before the two layer method. After preservation, collagenase solution with the marking dye was perfused as described in the Methods. Then the pancreas was fixed and analyzed. Dye: a marking dye.
Table 3. Donor and pancreas variables for the distribution of collagenase study.
| Variable |
no DI group (n = 5) |
DI group (n = 5) |
P value |
| Gender (F/M) |
2/3 |
1/4 |
N/A |
| Age (years) |
47 ± 4 |
50 ± 2 |
0.56 |
| Height (cm) |
175 ± 3 |
174 ± 4 |
0.78 |
| Body weight (kg) |
94 ± 7 |
93 ± 15 |
0.95 |
| Body mass index (kg/m2) |
30.1 ± 1.7 |
30.8 ± 4.5 |
0.90 |
| Cold ischemic time (min) |
323 ± 65 |
261 ± 48 |
0.51 |
| Pancreas weight (g) |
108 ± 12 |
114 ± 14 |
0.79 |
| Volume of ductal injection (ml) | N/A | 112 ± 4 | N/A |
DI, ductal injection; N/A, not applicable. Data are expressed as mean ± SE.
Figure 3. Macroscopic appearance of the pancreas before and after collagenase mixed with 1% dye perfusion: the representative pancreas without DI (A) and with DI (B) before (left) and after (right) infusion. Tissue samples of the (C) no DI group and (D) DI group for histologic analysis. The dye was well distributed macroscopically in all samples.
Table 4. Pancreas weight change after collagenase perfusion.
| Variable |
no DI group (n = 5) |
DI group (n=5) |
P value |
| Postperfusion pancreas weight (g) |
157 ± 14 |
161 ± 19 |
0.88 |
| Increase of weight (g)* |
51 ± 9 |
44 ± 7 |
0.65 |
| Ratio of weight increase (%)† | 45 ± 10 | 60 ± 13 | 0.47 |
Postperfusion pancreas weight – preperfusion pancreas weight. †Increase of weight/preperfusion pancreas weight ×100 (%). DI, ductal injection. Data are expressed as mean ± SE.
Distribution of the collagenase after delivery
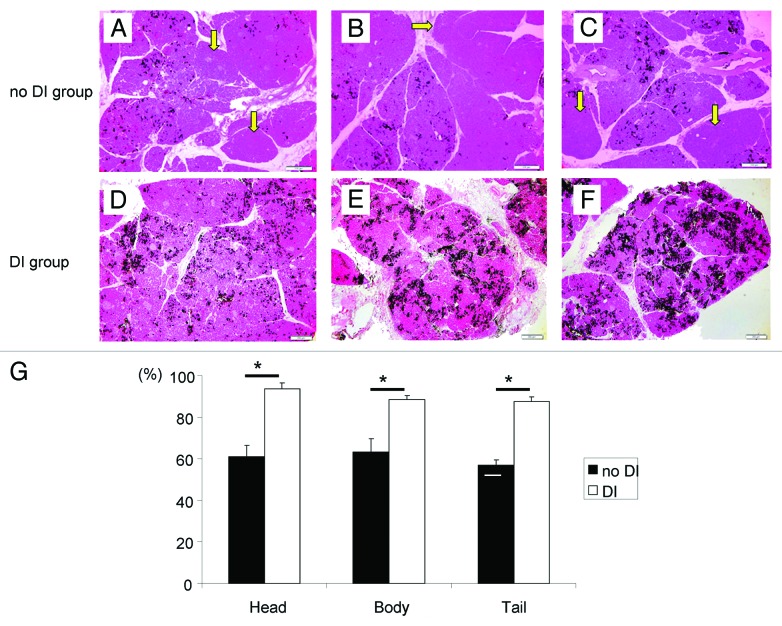
The dye spread through pancreatic tissue macroscopically (Fig. 3C and D). Microscopically, the distribution of the dye was unequal in the tissues. The dye was not detected in some lobules, which were often found in the no DI group (Fig. 4A–C, yellow arrows) whereas the dye-positive lobules were well observed in the DI group (Fig. 4D–F).
Figure 4. Histologic appearance of the distended pancreas of the no DI group (A–C) and the DI group (D–F) after collagenase solution and 1% dye infusion. In the DI group, dye was found in almost all lobules. However, in the no DI group, the dye was not found in some lobules. Arrows indicate dye-negative lobule (A–C). Magnification: × 40. Scale bars: 200 μm. (G) The ratio of the dye-positive lobules to all lobules in Head, Body and Tail parts of the pancreas. The DI group had a significantly higher rate in all parts. *p < 0.05.
We measured the ratio of dye-positive lobules to all lobules in the head, body, tail of the pancreas, and the DI group showed a significantly higher rate in all parts (Head: no DI, 61.2 ± 5.1%; DI, 93.7 ± 2.8%, Body: no DI, 63.4 ± 6.3%; DI, 88.3 ± 1.9%, Tail: no DI, 56.9 ± 2.7%; DI, 87.4 ± 2.4%; p < 0.05 in all parts between two groups, Fig. 4G). These findings indicate that ductal preservation makes the dye distribution more uniform and through.
Localization of the collagenase in the pancreas in relation to the islets
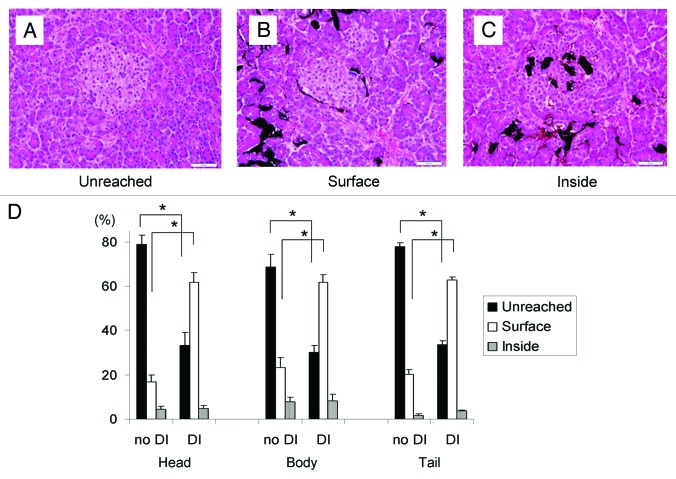
The islets in the sections were counted and classified into three criteria based upon the distribution of dye around the islet: unreached, the dye didn’t reach the islet surface (Fig. 5A); surface, the dye resided on the surface of the islet or in the peri-insular space but not inside it (Fig. 5B); and inside, the dye was found inside the islet (Fig. 5C).
Figure 5. A ratio of microscopic distribution of the collagenase. (A–C) The representative figures of the islet in relation to dye delivery: (A) dye did not reach the islet (unreached); (B) dye was on the surface of the islet but not inside (surface); (C) dye was inside the islet (inside). Magnification: × 400. Scale bars: 20 μm. (D) The percentage of islets of three classifications (unreached, surface, and inside) in both groups in the three parts of the pancreas. The DI group showed a significantly lower rate of ‘unreached’ islets and higher rate of ‘surface’ islets in all parts. The percentage of ‘inside’ islets was not statistically different; *p < 0.05.
The distribution of the dye around the islets was unexpectedly poor in the no DI group, although after perfusion the pancreas appeared well distended macroscopically. The majority of islets in the no DI group were untouched by the dye in all parts (Head: unreached, 78.7 ± 4.1%; surface, 16.8 ± 3.1%; inside, 4.5 ± 1.2%, Body: unreached, 68.7 ± 5.6%; surface, 23.3 ± 4.5%; inside, 8.0 ± 2.1%, Tail: unreached, 77.8 ± 1.6%; surface, 20.3 ± 2.1%; inside, 1.8 ± 0.6%, Fig. 5D). On the other hand, in the DI group, dye delivery to the islets was significantly improved (Head: unreached, 33.3 ± 5.6%; surface, 61.8 ± 4.4%; inside, 4.8 ± 1.3%, Body: unreached, 30.1 ± 3.0%; surface, 61.7 ± 3.2%; inside, 8.2 ± 3.0%, Tail: unreached, 33.5 ± 1.7%; surface, 62.7 ± 1.5%; inside, 3.8 ± 0.3%; p < 0.05 in unreached and surface between the no DI and the DI group, respectively; Fig. 5D).
Discussion
We have demonstrated that the ductal injection substantially improved islet isolation and clinical outcomes using both Serva collagenase and Liberase HI enzymes.10 In this study, we demonstrated that ductal injection significantly improved islet yield when we used Serva collagenase. In addition, the islet yield was similar between the Serva DI group and the Liberase MTF DI group. Indeed, efficacy of pancreas digestion assessed by undigested tissue volume was significantly improved with Liberase MTF. With these positive results, currently we use Liberase MTF for all clinical islet isolations. Therefore we performed histology study using only Liberase MTF.
Since we did not have no DI group using Liberase MTF for islet isolation study, the improvement of islet yield might be due to simply Liberase MTF. However, previously we demonstrated that the DI significantly improved islet yield using Liberase HI.13 Liberase HI and Liberase MTF are similar strong enzymes, therefore, we can expect that high islet yield with DI using Liberase MTF should be mainly due to the DI.
Next, we examined the effect of ductal injection on collagenase distribution using dyed collagenase solution. Ductal injection significantly improved collagenase distribution. Surprisingly, most of the dye-marked collagenase did not reach the surface of islets in the no DI group, even though the pancreas was well distended macroscopically. Although this finding can provide a reason for the low success rate of islet isolation, it contradicts another study conducted by Cross et al. showing that infused collagenase reached 100% of islets and penetrated into more than half of the islets’ interior.15 We speculate several possible reasons for this discrepancy. First, Cross et al. used anti-collagenase binding antibody to detect collagenase, which might, by its high sensitivity and inevitable non-specific binding, overestimate a low-level collagenase that would be negligible for purposes of isolation. In contrast, our method using a marking dye should represent real distribution of collagenase because the dye was mixed with collagenase immediately before collagenase delivery. In addition, our data in this study better reflect the outcome of human islet isolation, in which only few islets were usually over-digested. Furthermore, our method in this study was exactly the same as in practical human islet isolation. Insufficient delivery of collagenase (“unreached” islets) is most likely to lead to embedded islets or even a loss of islets because the tissue may not be digested. Such embedded islets are known to be difficult to separate from exocrine tissue and might be lost during the purification step. Therefore, improvement of microscopic enzyme delivery to the surface of islets might be the key mechanism to increase islet yield by ductal injection. There is a concern that the delivered enzymes to the surface of islets might cause over digestion. However, previously we demonstrated that the DI improved not only islet yield but also islet in vitro and in vivo viability.13 In addition, islets have extra cellular matrix on their surface, which can work as a barrier against enzymes delivered through pancreatic ducts. Indeed, most dye was detected as spreading on the surface of islets and few islets had ink inside. It suggests that delivered enzyme to the surface of islets may not be harmful to inner islet cells. Excellent clinical outcomes with DI could also support that the DI should not be harmful to the isolated islets.10-12
Ductal preservation (DI at the procurement site) with large amount of ET-Kyoto solution could have several effects: first, the infused preservation solution could dilate the ductal system at every terminal and keep it open. Well-preserved pancreatic ducts might facilitate good distribution of the collagenase solution in the human pancreas. In fact, we use only single cannula for collagenase injection instead of two cannulas, which should be indirect evidence of excellent preservation of pancreatic ductal system. Second, a large amount of solution could dilute the endogenous proteases in the duct to prevent autolysis. Third, cell protective additives including trehalose in ET-Kyoto solution could distribute throughout the pancreas. Fourth, a large amount of DI could wash out general preservation solution such as University of Wisconsin (UW) solution, which is widely used for organ preservation but was shown to have a collagenase inhibitory effect.13,16
Meanwhile, DI using large amount of solution with high pressure might damage pancreatic ducts. Especially, when donor pancreata were young (less than 30 y old), it was difficult to isolate islets even with DI.17 In our preliminary study, when donor age was young, low pressure (< 100 mmHg) and less volume (0.5 mL/g pancreas weight) of DI solution improved islet isolation outcomes. Therefore, optimal pressure and volume of solution is an important research target for the DI.
The optimal solution for ductal preservation and its optimal volume are still unknown. In this study, we used a relatively large amount (1 mL/g pancreas weight) of ET-Kyoto solution, which was reported to have less collagenase inhibitory activity than UW solution.13 Indeed, we have shown good isolation outcomes using this solution for DI.10,11 Previously we compared modified ET-Kyoto solution and Celsior solution for pancreas preservation before islet isolation and ET-Kyoto solution showed significant better outcomes.18 However, the ET-Kyoto solution still has some inhibitory effect on collagenase;13 therefore, further studies might yield a better ductal preservation solution.
In conclusion, ductal preservation immediately after pancreas procurement could improve the collagenase distribution. This seems the mechanism of ductal preservation for the increasing islet yield. Improvement of collagenase distribution could be one of the research targets for improving islet isolation.
Materials and Methods
Ethical Guidelines
This study and the islet isolation protocol were approved by the institutional review board of the Baylor Health Care System.
Human pancreas
To mimic clinical situation, donor selections were performed based on the Edmonton protocol for a clinical-grade pancreas (Table 1).2 Pancreata from brain-dead donors were procured through either Southwest Transplant Alliance or LifeGift between August 2007 and September 2011. Pancreata which were not used for clinical for some reasons were randomly allocated for this study. Of note, currently approximately 7,000 organ donations were occurred every year in the United States and only less than 20% of donated pancreata have been clinically used.19 In our local areas, approximately 500 organ donations occurred every year and we obtained 90 pancreata for research purposes during this period (4 y). Therefore, we were fortunate to use clinical grade pancreata for this research.
Human islet isolation data with or without ductal preservation
When we were notified to obtain research pancreata for islet isolation using Serva collagenase, we randomly allocated the pancreata into with DI and without DI groups. Before pancreas procurement, University of Wisconsin (UW) solution or SPS-1 (Organ Recovery System) was used for general organ perfusion through the aorta in all cases. After the pancreas was procured, we immediately removed the duodenum and spleen from the pancreas and inserted a cannula into the pancreas through the main pancreatic duct from the direction of the pancreatic head at the procurement site. These processes were performed by the Baylor islet team. For the group with ductal preservation, approximately 1 mL/g pancreas weight of ET-Kyoto solution (Otsuka Pharmaceutical Factory Inc.) was administered intraductally (DI).11,20 The pressure during ductal injection was measured. After completion of ductal injection, the orifice of cannula kept open to avoid overpressure to pancreatic duct during preservation. For the group without ductal preservation, the ductal injection process was not performed. All pancreata were preserved by the oxygen-charged static two-layer (oxygenated perfluorocarbon/preservation solution) method for less than 10 h.21
Islet preparations were performed according to good manufacturing practice at the Baylor Research Institute cell processing facility in Dallas, TX USA. Islets were isolated according to the Ricordi method with our modifications.2,10,12,22
After the pancreas arrived at our islet processing facility, the duct was perfused in a controlled fashion with a cold enzyme solution. Collagenase NB with neutral proteases (Serva Electrophoresis GmbH) was used without DI (no DI Serva group, n = 3) or with DI (DI Serva group, n = 8). These two groups were conducted during the same period. At the moment, DI Serva group showed significantly improved isolation outcomes. Therefore when a new enzyme blend, Liberase MTF (Roche Molecular Biochemicals) was introduced afterward, we used it with DI (DI MTF group, n = 7). After the subsequent digestion and purification steps, isolated islets were assessed based on the Edmonton protocol.2
The study of collagenase distribution with or without ductal preservation
In this study, we performed pancreas procurement, preservation, transportation, and collagenase perfusion steps using the same processes as for our clinical islet isolation.10,12,20 The pancreata were classified into ductal injection (DI) and no ductal injection (no DI) groups (Fig. 2). For the DI group (n = 5), approximately 1 mL/g pancreas weight of ET-Kyoto solution was administered intraductally at the procurement site. The pressure during ductal injection was measured. For the no DI group (n = 5), the ductal injection process was not performed. After preservation, the collagenase solution (1 vial of Liberase MTF and 1 vial of Thermolysin MTF; Roche) was prepared in 200 mL of perfusion solution (Mediatech) and mixed with 1% marking dye. Infusions were conducted by the recirculation method as the speed was adjusted, aiming to maintain pressures of 60–80 mmHg for 5 min, increasing to 160–180 mmHg for the subsequent 5 min as described previously.9 The temperature was kept at 4°C in all procedures. Immediately after infusion, the pancreas was weighed and the quality of pancreas distension and extent of dye distribution was observed macroscopically. The tissue samples (0.5 cm3) were taken from each part of pancreatic head, body and tail, where the dye appeared to be excellently distributed and fixed with 10% formalin. The paraffin-embedded tissue sections (5 µm) were stained with hematoxylin and eosin. For quantitative analysis of microscopic dye distribution, slides were examined under a microscope; an average of 109 ± 13 islets were assessed per section, with 12 different sections examined for each part of the pancreas. Images were captured using an Olympus BX61 microscope and analyzed with cellSense digital imaging software (Olympus).
Statistical analysis
All results are expressed as mean ± standard error (SE). Statistically significant differences among the groups were determined by ANOVA followed by Student’s t-test with Bonferroni correction. A p value < 0.05 was considered significant.
Acknowledgments
This study was partially supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (1R21DK090513–019) (to SM), and Roche diagnostics corp. (to SM). The authors thank Ms. Yoshiko Tamura, Ms. Ana M. Rahman, and Ms. Anne-Marie Brun for technical support and Ms. Cynthia Orticio for editing this manuscript.
Glossary
Abbreviations:
- ANOVA
analysis of variance
- DI
ductal injection
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/19255
REFERENCES
- 1.Matsumoto S. Islet cell transplantation for Type 1 diabetes. Diabetes. 2010;2:16–22. doi: 10.1111/j.1753-0407.2009.00048.x. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–30. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 4.Goto M, Eich TM, Felldin M, Foss A, Källen R, Salmela K, et al. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation. 2004;78:1367–75. doi: 10.1097/01.TP.0000140882.53773.DC. [DOI] [PubMed] [Google Scholar]
- 5.Ichii H, Wang X, Messinger S, Alvarez A, Fraker C, Khan A, et al. Improved human islet isolation using nicotinamide. Am J Transplant. 2006;6:2060–8. doi: 10.1111/j.1600-6143.2006.01452.x. [DOI] [PubMed] [Google Scholar]
- 6.Kin T, Zhai X, Murdoch TB, Salam A, Shapiro AM, Lakey JR. Enhancing the success of human islet isolation through optimization and characterization of pancreas dissociation enzyme. Am J Transplant. 2007;7:1233–41. doi: 10.1111/j.1600-6143.2007.01760.x. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto I, Sawada T, Nakano M, Sakai T, Liu B, Ansite JD, et al. Improvement in islet yield from obese donors for human islet transplants. Transplantation. 2004;78:880–5. doi: 10.1097/01.TP.0000134396.03440.1E. [DOI] [PubMed] [Google Scholar]
- 8.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–20. doi: 10.2337/diabetes.37.4.413. [DOI] [PubMed] [Google Scholar]
- 9.Lakey JR, Warnock GL, Shapiro AM, Korbutt GS, Ao Z, Kneteman NM, et al. Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell Transplant. 1999;8:285–92. doi: 10.1177/096368979900800309. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto S, Noguichi H, Shimoda M, Ikemoto T, Naziruddin B, Jackson A, et al. Seven consecutive successful clinical islet isolations with pancreatic ductal injection. Cell Transplant. 2010;19:291–7. doi: 10.3727/096368909X481773. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto S, Okitsu T, Iwanaga Y, Noguchi H, Nagata H, Yonekawa Y, et al. Successful islet transplantation from nonheartbeating donor pancreata using modified Ricordi islet isolation method. Transplantation. 2006;82:460–5. doi: 10.1097/01.tp.0000231710.37981.64. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto S, Takita M, Chaussabel D, Noguchi H, Shimoda M, Sugimoto K, et al. Improving efficacy of clinical islet transplantation with iodixanol based islet purification, thymoglobulin induction and blockage of IL-1 beta and TNF alpha. Cell Transplant. 2011;20:1641–7. doi: 10.3727/096368910X564058. [DOI] [PubMed] [Google Scholar]
- 13.Noguchi H, Ueda M, Hayashi S, Kobayashi N, Okitsu T, Iwanaga Y, et al. Ductal injection of preservation solution increases islet yields in islet isolation and improves islet graft function. Cell Transplant. 2008;17:69–81. doi: 10.3727/000000008783907062. [DOI] [PubMed] [Google Scholar]
- 14.Shimoda M, Noguchi H, Naziruddin B, Fujita Y, Chujo D, Takita M, et al. Assessment of human islet isolation with four different collagenases. Transplant Proc. 2010;42:2049–51. doi: 10.1016/j.transproceed.2010.05.093. [DOI] [PubMed] [Google Scholar]
- 15.Cross SE, Hughes SJ, Partridge CJ, Clark A, Gray DW, Johnson PR. Collagenase penetrates human pancreatic islets following standard intraductal administration. Transplantation. 2008;86:907–11. doi: 10.1097/TP.0b013e318186df87. [DOI] [PubMed] [Google Scholar]
- 16.Contractor HH, Johnson PR, Chadwick DR, Robertson GS, London NJ. The effect of UW solution and its components on the collagenase digestion of human and porcine pancreas. Cell Transplant. 1995;4:615–9. doi: 10.1016/0963-6897(95)00039-Z. [DOI] [PubMed] [Google Scholar]
- 17.Shimoda M, Noguchi H, Naziruddin B, Fujita Y, Chujo D, Takita M, et al. Improved method of human islet isolation for young donors. Transplant Proc. 2010;42:2024–6. doi: 10.1016/j.transproceed.2010.05.094. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi H, Naziruddin B, Onaca N, Jackson A, Shimoda M, Ikemoto T, et al. Comparison of modified Celsior solution and M-kyoto solution for pancreas preservation in human islet isolation. Cell Transplant. 2010 doi: 10.3727/096368909x508852. In press. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto S, Noguchi H, Hatanaka N, Shimoda M, Kobayashi N, Jackson A, et al. Estimation of donor usability for islet transplantation in the United States with the kyoto islet isolation method. Cell Transplant. 2009;18:549–56. doi: 10.1177/096368970901805-610. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto S, Noguchi H, Naziruddin B, Onaca N, Jackson A, Nobuyo H, et al. Improvement of pancreatic islet cell isolation for transplantation. Proc (Bayl Univ Med Cent) 2007;20:357–62. doi: 10.1080/08998280.2007.11928323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto S, Rigley TH, Qualley SA, Kuroda Y, Reems JA, Stevens RB. Efficacy of the oxygen-charged static two-layer method for short-term pancreas preservation and islet isolation from nonhuman primate and human pancreata. Cell Transplant. 2002;11:769–77. [PubMed] [Google Scholar]
- 22.Matsumoto S, Qualley SA, Goel S, Hagman DK, Sweet IR, Poitout V, et al. Effect of the two-layer (University of Wisconsin solution-perfluorochemical plus O2) method of pancreas preservation on human islet isolation, as assessed by the Edmonton Isolation Protocol. Transplantation. 2002;74:1414–9. doi: 10.1097/00007890-200211270-00013. [DOI] [PubMed] [Google Scholar]