Abstract
Encoding semantic relationships between items on word lists (semantic processing) enhances true memories, but also increases memory distortions. Episodic memory impairments in schizophrenia (SZ) are strongly driven by failures to process semantic relations, but the exact nature of these relational semantic processing deficits are not well understood. Here, we used a false memory paradigm to investigate the impact of implicit and explicit semantic processing manipulations on episodic memory in SZ. Thirty SZ and 30 demographically matched healthy controls (HC) studied Deese/Roediger-McDermott (DRM) lists of semantically associated words. Half of the lists had strong implicit semantic associations and the remainder had low strength associations. Similarly, half of the lists were presented under “standard” instructions and the other half under explicit “relational processing” instructions. After study, participants performed recall and old/new recognition tests composed of targets, critical lures, and unrelated lures. HC exhibited higher true memories and better discriminability between true and false memory compared to SZ. High, versus low, associative strength increased false memory rates in both groups. However, explicit “relational processing” instructions positively improved true memory rates only in HC. Finally, true and false memory rates were associated with severity of disorganized and negative symptoms in SZ. These results suggest that reduced processing of semantic relationships during encoding in SZ may stem from an inability to implement explicit relational processing strategies rather than a fundamental deficit in the implicit activation and retrieval of word meanings from patients’ semantic lexicon.
Keywords: Episodic memory, Relational processing, Semantic processing, Memory encoding
1. Introduction
Processing the semantic meaning of items during encoding increases memory strength and improves episodic retrieval (Craik & Lockhart, 1972; Kintch 1968). Investigators demonstrated that providing SZ patients with explicit task instructions to process items semantically improved item recognition (Bonner-Jackson et al., 2005; Paul et al., 2005; Ragland et al., 2003; 2005) and ventrolateral prefrontal brain activation (Bonner-Jackson et al., 2005; Ragland et al., 2005), and eliminated performance differences. However, successful episodic memory also depends upon processing semantic relationships between items, and there is evidence that relational memory processes are disproportionately impaired in SZ (Clare et al., 1993; Lepage et al., 2006; Ranganath, Minzenberg & Ragland, 2008; Titone et al., 2004). For example, on list-learning tasks such as the California Verbal Learning Test (CVLT; Delis et al., 1987; 2000), clustering of semantically-related words improves recall in healthy participants (Delis et al., 1987; 2000), but patients tend to rely on serial order (Gsottschneider et al., 2011) rather than semantic-clustering strategies, contributing to severe list-learning impairments (Brebion et al., 2004; Iddon et al., 1998; Stone et al., 1998).
Our ability to improve semantic relational processing deficits in SZ has not been clearly demonstrated. For example, several investigators manipulated relational processing by providing subjects with blocked versus unblocked lists of semantically related words (McClain, 1983; Gold et al., 1992), and found that performance improved only if patients were also provided with retrieval cues to guide performance (McClain, 1983). Likewise, Iddon and colleagues 1998 were unsuccessful training patients to employ relational semantic encoding strategies, and Ragland and colleagues (2012) found that familiarity-based recognition was impaired in SZ when patients were provided with explicit relational versus item-specific semantic encoding instructions. The current study utilizes a true and false memory list-learning paradigm allowing manipulation of implicit and explicit aspects of relational processing demands. Because activation and retrieval of semantic word meanings is generally intact in schizophrenia (Barch et al., 1996; Elvevåg et al., 2005; Boudewyn et al., 2012), we predicted that implicit manipulation of the semantic associative strength of list items would produce the same effects on task performance in HC and SZ. However, given previously noted evidence that SZ patients do not appear to benefit from relational encoding instructions and training, we predicted that explicit manipulations of task instructions would differentially affect HC and SZ memory performance.
To examine the ability of patients to benefit from implicit and explicit aspects of semantic processing at encoding, we conducted a study using the DRM paradigm (Roediger and McDermott, 1995). During DRM, participants study word lists converging on a semantic theme represented by a critical lure never presented during study. Participants perform recall and old/new recognition tests including studied words (targets), critical lures, and other lures that are not semantically associated (unrelated lures). This paradigm produces robust false memory for critical lures which are thought to stem from implicit processing of semantic associations (McDermott and Roediger, 1998; Roediger and McDermott, 1995).
The current study manipulated implicit associative strength of word lists to identify potential group differences (Stadler et al., 1999). High-strength lists differed from low strength lists in the probability that items within each list elicited other associates (i.e., critical lures) in healthy adults in free association tasks (Stadler et al., 1999; Roediger and McDermott, 1995). If associative strength within in the semantic lexicon is altered in SZ, impairments in patients’ ability to activate implicit semantic associations should be expected to lead to reduced false memory rates. Conversely, a lack of group differences in the effect of implicit manipulations of semantic strength would suggest that activation and retrieval of word meanings within the semantic lexicon of patients is relatively intact and is not responsible for their relational memory impairments.
We also examined the effect of explicit instructions. Relational processing of similarities among to-be-remembered items can be enhanced if individuals are explicitly cued to use strategies intended to process the “gist” or semantic relations between words in each list (Dewhurst et al., 2007; Lampinen et al., 2006). If SZ memory deficits are due to reduced ability to self-initiate processing of semantic relations spontaneously, providing explicit semantic encoding instructions should lead to increases in true and false memory in SZ that are as large as or even larger than that of HC. In contrast, if patients are unable to engage relational processing following instruction, providing explicit relational encoding instructions should have little effect on performance. Finally, based on evidence that disorganized and negative symptoms predict cognitive performance in SZ (Delawalla et al., 2006), we examined associations with symptomatology.
2. Materials and Methods
2.1. Participants
The sample consisted of 60 English-speakers, 30 individuals with SZ and 30 HC. Patients were clinically stable outpatients within the first five years of illness. The Structured Clinical Interview for DSM-IV confirmed diagnosis of SZ, and confirmed that HC were free of Axis-I disorder. Symptoms were rated with the Scale for Assessment of Negative Symptoms, Scale for Assessment of Positive Symptoms, and Brief Psychiatric Rating Scale. Selected items from these scales were used to compute positive, disorganization and negative symptom scores. Exclusion criteria for all participants were: IQ < 70, drug/alcohol abuse or dependence in the previous three months, major medical or neurological illness, and significant head trauma. HC were also excluded for any first-degree relatives with a psychotic disorder. Groups were matched on age, gender distribution and parental education (Table 1). SZ had lower educational attainment than HC, and lower intellectual estimates on the Word Reading subtest of Wide Range Achievement Test, although both groups were estimated in the average range. After describing the study, written informed consent was obtained from all subjects prior to participation based on procedures approved by the UC Davis IRB.
Table 1.
Study Sample Demographics
| Patients (n = 30) | Control Subjects (n = 30) | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | |
| Age (years) | 29.97 | 9.56 | 31.30 | 8.95 | .58 |
| Gender (% male) | 57 | 60 | .79 | ||
| WRAT | 104.00 | 8.33 | 109.15 | 9.04 | .03* |
| Education (years) | 14.00 | 1.86 | 15.25 | 1.79 | .11 |
| Parental Education (years) | 14.41 | 2.51 | 14.19 | 2.37 | .53 |
| BPRS | 38.19 | 9.42 | |||
| SANS | 32.68 | 17.93 | |||
| SAPS | 11.77 | 15.05 | |||
WRAT, Wide Range Achievement Test; BPRS, Brief Psychiatric Rating Scale; SANS, Scale or the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms..
p < .05 statistically significant difference.
2.2. Materials
A total of 24 DRM lists of 12 words each were used. Lists were selected based on associative strength norms and effectiveness in producing false memories to critical lures during free recall and recognition (Stadler et al., 1999). Half of the lists were classified as high associative strength. Their rate of false recall ranged .43–.61 (.53±.07, mean±SD) and their rate of false recognition ranged .69–.84 (.78±.06), based on associative strength norms (Stadler et al.1999). The remaining 12 lists were classified as low associative strength, with false recall rates ranging .03–.37 (.22±.12) and false-recognition rates ranging .27–.60 (.47±.11). These high versus low lists differed statistically in their rates of false recall, t(11)=9.77, p<.001, and false recognition, t(11)=8.55, p<.001. The high lists also had a stronger mean backward association strength (BAS; .23±.10) than low lists (.14±.10), t(11)=9.81, p<.001 (see Roediger et al., 2001). BAS is the average probability that each member of a list will elicit critical lures in a free association task and is one of the most important predictors of the DRM false-memory effect.
DRM lists were studied under two instructions: “standard” (“Remember the words”) and “relational processing” (“Remember the words. These words are all related. Think about the relationship between them to help you remember”). Presentation of these lists during study was counterbalanced across association strength, but fixed for type of instruction, with standard instructions always occurring first to avoid carry over-effects (i.e., once instructed to attend to semantic relationships among items, it would be difficult to avoid doing so subsequently).
The recognition test included 144 words: 48 studied items (targets), 48 non-studied semantic associates (critical lures, CLs), and 48 new unrelated items (unrelated lures, ULs). Targets consisted of 2 items from each of the 24 studied lists (those in serial positions 1 and 8) and critical lures were the 1st and 3rd associate from each list, which were not presented during study. Unrelated lures (ULs) were selected from non-semantically related words based on psycholinguistic norms, matching CLs in frequency, familiarity, concreteness, and age of acquisition.
2.3. Procedures
The study phase was divided into two blocks of 12 lists each. Half of the visually presented lists within each study block were high associative strength, and half were low associative strength. Lists within the first study block were presented under standard instructions and, during the second block, were presented under relational instructions. Words within each list were presented in order of decreasing association strength at a rate of one word every 2500ms. After studying each list, participants performed a 30-second recall test of the studied words. They were then presented with a 30-second count-backwards filler task to prevent rehearsal. After each study block, participants were visually presented with an old/new recognition test, including 24 targets, 24 CLs, and 24 ULs corresponding to the 12 lists they just studied.
3. Data analysis
Data were analyzed using a 2 (Group: patients, controls) × 2 (Encoding instruction: standard, relational) × 2 (List strength: high, low) Analysis of Variance (ANOVA) design, with the last two factors varied within-subjects. Dependent measures included: 1) recall (mean number of items correctly recalled, falsely recalled, and perseverative repetitions in each condition: Standard-High, Standard-Low, Relational-High, Relational-Low); 2) old/new item recognition (hits, CL_FAs, UL_FAs); and, 3) corrected recognition scores calculated as Hits-CL_FAs and Hits-UL_FAs. We also used Spearman correlations to identify relationships with positive, negative, and disorganized clinical symptoms.
4. Results
4.1. Recall performance
4.1.1 True memory
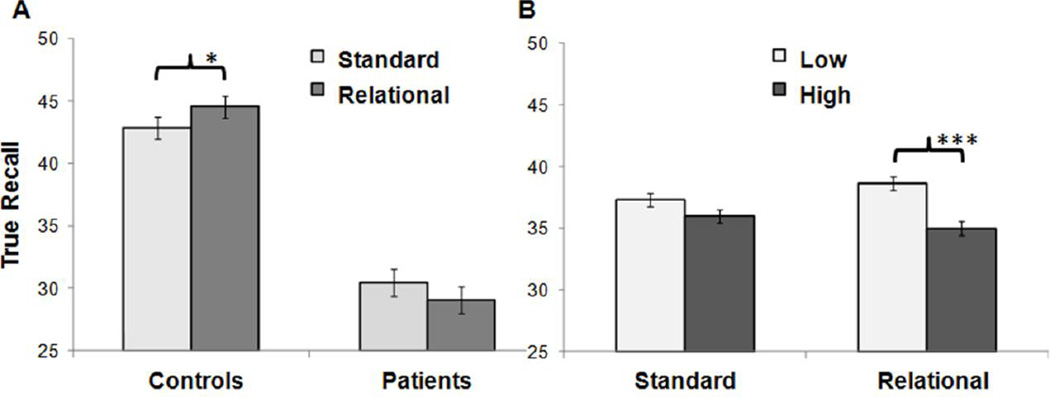
The ANOVA for the number of hits revealed that main effects of group, (F(1, 58)=54.43, p<.001), and strength, (F(1, 58)=20.71, p<.001), were qualified by significant group by instruction, (F(1, 58)=4.82, p<.05), and strength by instruction interactions, (F(1, 58)=4.04, p<.05). Simple-effects analysis for the group by instruction interaction revealed that, regardless of list strength, only HC were able to correctly recall more studied words when provided with relational encoding versus standard instructions, (F(1, 29)=3.70, p<.05). This effect was not evident in SZ, (F(1, 29)=1.60, p=.22) (Figure 1A). Post-hoc analyses of the strength by instruction interaction indicated that both groups had better recall of words from low versus high association strength lists under the relational encoding instructions, (F(1, 59)=19.59, p<.001). There was no effect of list strength under standard instructions for either group, (F(1, 59)=3.05, p=.09) (Figure 1B).
Figure 1.
Mean number of true recall as a function of (A) group and instruction, and (B) instruction and associative strength
4.1.2 False memory
In accord with previous findings in healthy participants (Stadler et al., 1999), high strength lists led to higher false memory rates in SZ and HC, (F(1, 58)=69.95, p<.001), with no group difference in false memory recall, (F(1, 58)=.85, p=.36). This same pattern held true for perseverative errors, which reflects retrieval monitoring difficulties, with a higher number of perseverative errors for high versus low strength lists, (F(1, 58)=4.22, p<.05), and no group difference, (F(1, 58)=.93, p=.34). No other main effects or interactions were significant, (F(1, 58)≤1.84, p≥.23).
In sum, examination of recall performance revealed that only HC were able to take advantage of explicit relational encoding instructions to improve true memory performance. In contrast, increasing the strength of semantic associations implicitly had the same impact on memory errors across participants.
4.2. Recognition performance
4.2.1. True recognition
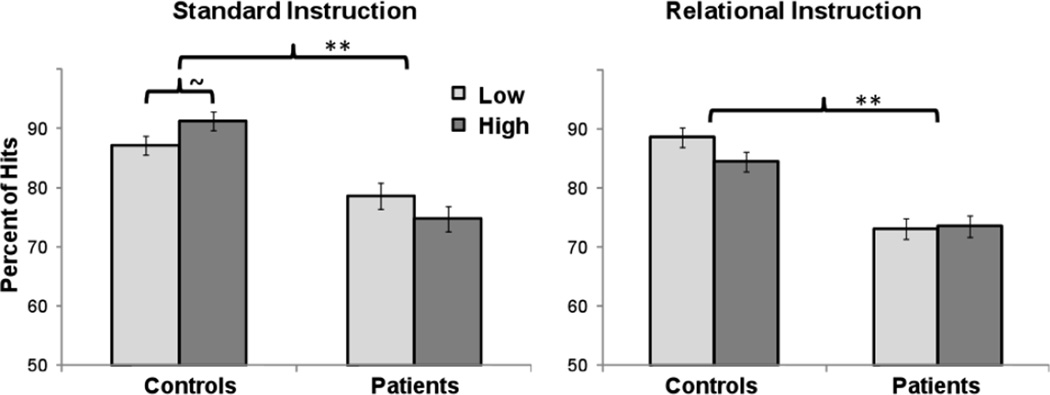
Examination of hits revealed a significant main effect of group, (F(1, 58)=11.22, p<.01), and a group by instruction by strength interaction, (F(1, 58)=6.87, p<.01). Post-hoc comparisons were performed separately for standard and relational instructions. Under standard instructions, there was a main effect of group, (F(1, 58)=10.19, p<.01), and a group by strength interaction, (F(1, 58)=4.31, p<.05). HC showed a statistical trend of improved recognition for the high versus low associative strength lists, (F(1, 29)=3.24, p=.08). In contrast, there was no effect of list strength in SZ, (F(1, 29)=1.57, p=.22) (Figure 2). Under the relational encoding instructions, there was, again, a main effect of group, (F(1, 58)=8.34, p<.01), but the group by list strength was not significant (F(1, 58)=1.76, p=.19). Overall recognition was better in HC than in SZ (Figure 2), and the proportion of hits was greater for high-strength than low-strength lists under standard instructions in HC only, with no main/interactive effects involving list strength in patients, (F(1, 29)≤1.24, p≥.27).
Figure 2.
Mean percent of true recognition (Hits) as a function of instruction, group, and associative strength
4.2.2. False recognition
The pattern of false recognition results largely paralleled recall results. The proportion of CL_FAs was higher for strong versus weak lists (53.72±2.22 versus 39.55±2.42, respectively), across groups and encoding conditions, (F(1, 58)=88.31, p<.001). There were no effects of group, (F(1, 58)=.01, p=.96) or instruction on false recognition, (F(1, 58)=1.05, p=.31). Thus, false recognition was similarly influenced by implicit associative strength in both groups, and was unaffected by encoding instructions.
4.2.3. Differences between True and False memory
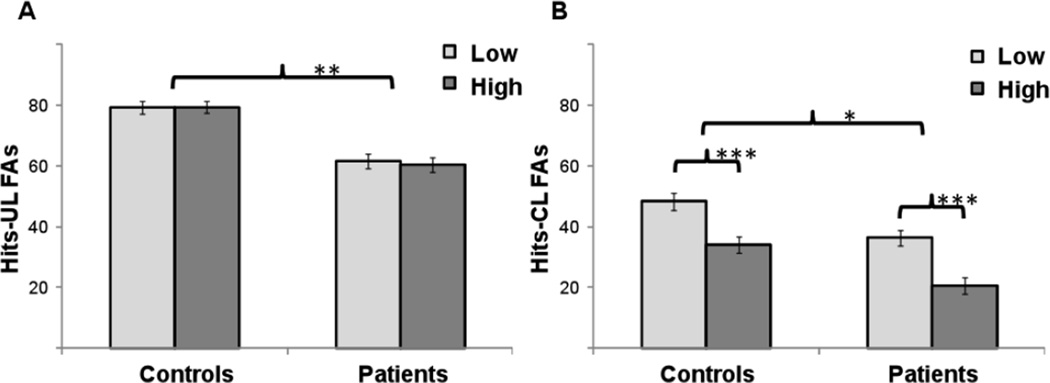
To identify group differences in the ability to discriminate between true and false memories, recognition performance was examined based on two difference scores: Hits-UL_FAs and Hits-CL_FAs. For both Hits-UL_FAs and for Hits-CL_FAs there were main effects of group, (F(1, 58)=9.40, p<.01; and F(1, 58)=6.30, p<.05; respectively), indicating that HC were better than SZ at discriminating true from false memories (Figure 3). For the Hits-CL_FAs analysis, there was also a main effect of strength, (F(1, 58)=61.79, p<.001), such that the capacity to discriminate between true and false memories was better for low versus high association strength lists across groups.
Figure 3.
Mean difference percent between true and false memories for (A) Hits-UL_FAs and (B) Hits-CL_FAs as a function of group and associative strength
In sum, although all participants successfully discriminated between true and false, controls did this more successfully regardless of the nature of the distracters. Consistent with recall results, all participants exhibited higher false recognition for high association versus low association lists, indicating sensitivity to implicit associations among list items.
4.3. Relationship with clinical symptoms
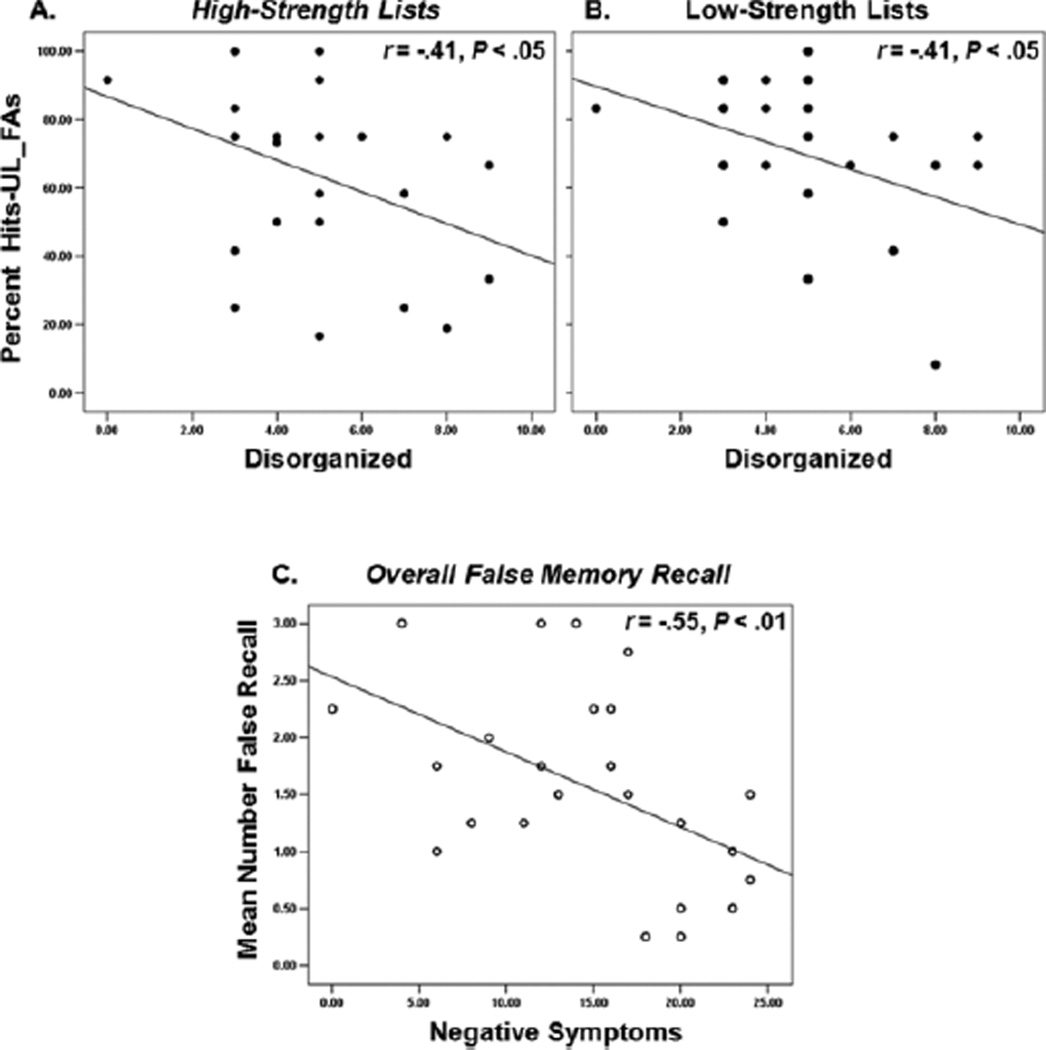
Under standard instructions, patients who were less disorganized better discriminated hits from ULs on both low-strength and high-strength lists (both r=−.41, p<.05; Figure 4A&B). In addition, more severe negative symptoms were associated with lower false memory. This was captured by negative correlations with overall false memory recall (r=−.55, p<.01; Figure 4C); and with false memory recall examined separately for high-strength lists (r=−.45), low-strength lists (r=−.53), relational-encoding instructions (r=−.39), and standard-encoding instructions (r=−.47; ps<.05). No correlations were obtained with positive symptoms.
Figure 4.
Negative associations between patients’ memory performance and clinical symptoms: Less disorganized patients showed better discrimination between hits and UL_FAs for low-strength lists (A) and for high-strength lists (B). Having more negative symptoms was associated with lower false memory recall (C)
5 Discussion
We used the DRM paradigm to investigate true and false memory in SZ, while manipulating semantic processing using implicit (list associative strength) and explicit task manipulations (encoding instructions). In support of our predictions, patients were impaired, relative to HC, in their ability to explicitly engage semantic relational processing strategies to improve true memory, but showed the same increase in false memory as HC when the implicit semantic strength of the word lists was increased. Performance was related to severity of disorganized and negative symptoms. Less disorganized patients were better at discriminating hits from unrelated false alarms, and patients with more severe negative symptoms were better at avoiding false alarms.
Similar increases in false recall and recognition for strongly versus weakly associated word lists across subjects suggests that the structure of the lexical-semantic system and automatic spread-of-activation within this network is intact in SZ. This finding is consistent with prior levels-of-processing studies, which found that patients benefit from incidental encoding instructions that automatically engage semantic processing of individual items (Bonner-Jackson et al., 2005; Ragland et al., 2003, 2005). Although priming studies utilizing lexical decision tasks under automatic conditions raised concern about increased spread-of-activation in SZ, a recent meta-analysis clarified that aberrant hyperpriming was restricted to prominent thought disorder (Pomarol-Clotet et al., 2008).
Although patients in the current study did not have severe thought disorder, the correlations with disorganized symptoms were with true memory discriminability and not with false memory rates, suggesting that increased thought disorder would not have altered current findings. On the other hand, false memory was inversely associated with severity of negative symptoms, suggesting that more prominent negative symptoms may be associated with a more conservative response bias. The absence of correlations with positive symptoms is consistent with prior research indicating that cognition is most tightly linked with disorganized and negative symptom dimensions (Delawalla et al., 2006).
Current findings of group differences in true but not false memories replicate some (Moritz et al., 2004, 2006; Weiss et al., 2002), but not all previous DRM studies of SZ (Eleväg et al., 2003; Huron and Danion, 2002). Both these conflicting studies also found reduced true memory rates in SZ, but Huron & Danion (2002) additionally found elevated false memory rates, and Eleväg and colleagues (2003) found group differences in false memory rates that were no longer significant once true memory rates were taken into account. Even in the absence of increased false memory rates, several studies argued that different processes contributed to false memory formation in SZ (i.e., adoption of gist-based strategies at retrieval) and HC (i.e., spreading of activation) (Moritz et al., 2004, 2006; Weiss et al., 2002). Although not designed to test retrieval strategies, current results are not consistent with this alternative strategy explanation. That is, when patients were explicitly instructed to process the semantic relationship among items, which should enhance awareness of the semantic gist and lead to over-use of the strategy, there was no increase in false memory rates in SZ – contrary to a gist-based explanation. Conversely, when the implicit semantic strength of the list items was increased, false memory recall and recognition increased in HC and SZ, supporting the conclusion that spreading activation within the semantic-lexical network contributed to false memory in both groups.
The failure of patients to utilize relational encoding instructions to improve true memory differs from developmental studies utilizing the DRM paradigm in children (Brainerd et al., 2008). In those studies, providing explicit relational semantic processing instructions increased true and false memory in children as they reached middle childhood (Dewhurst et al., 2007; Lampinen et al., 2006). The failure of patients to show this same benefit suggests that their ability to engage in relational memory processes and conceptual understanding of semantic relations during encoding may not be fully functional, consistent with previous findings indicating that relational processing represents one of the more severe and enduring aspects of episodic memory dysfunction in schizophrenia (Clare et al., 1993; Lepage et al., 2006; Ranganath, Minzenberg & Ragland, 2008; Titone et al., 2004). Patients’ successful completion of practice tasks and overall acceptable performance levels discount the possibility of a generalized deficit in following instructions.
In sum, our results have theoretical and applied implications. Automatic semantic associative processes involved in episodic memory encoding appear to be intact in patients with schizophrenia. In contrast, patients appear to have a fundamental deficit in their ability to form explicit conceptual representations of relationships between items during episodic encoding to support veridical recall. Development of behavioral and pharmacological interventions to remediate these relational memory processes and associated medial temporal and prefrontal brain networks can be viewed as an important target for development of cognitive enhancing medications and cognitive training procedures.
Acknowledgements
We thank controls and individuals with schizophrenia who participated in the present research. We also thank Silvia A. Bunge for initial guidance and encouragement.
Funding
This work was supported by the National Institutes of Health (R01 MH084895 to J.D.R), grants from the Spanish Ministry of Innovation and Science (Juan de la Cierva and Consolider-Ingenio 2010 CSD2008-00048 to P.P.), and a James S. McDonnell Foundation Scholar Award (to S.G.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declared no conflicts of interest.
Contributors
Dr. Paz-Alonso participated in the design of the study, prepared materials and procedures, conducted the analysis, and participated in manuscript drafting. Dr. Ghetti participated in the design the study, developed the analysis plan and participated in manuscript drafting. Ian Ramsay partiticipated in data collection and assisted in data analysis. Dr. Solomon, Dr. Yoon, and Dr. Carter assited with clinical population recruitment and provided helpful comments on the manuscript draft. Dr. Ragland designed the study, developed the analysis plan, supervised data collection and participated in manuscript drafting. All authors contributed to and have approved the final manuscript.
References
- Barch DM, Servan-Schreiber D, Steingard S, Cohen JD, Steinhauer SS, Van Kammen DP. Semantic priming in schizophrenia: an examination of spreading activation using word pronunciation and multiple SOAs. J. Abnorm. Psychology. 1996;105:592–601. doi: 10.1037//0021-843x.105.4.592. [DOI] [PubMed] [Google Scholar]
- Bonner-Jackson A, Haut K, Csernansky JG, Barch DM. The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biol. Psychiatry. 2005;58:47–55. doi: 10.1016/j.biopsych.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudewyn MA, Carter CS, Swaab TY. Cognitive control and discourse comprehension in schizophrenia. Schiz. Res. and Treatment. 2012 doi: 10.1155/2012/484502. (Epub, April 8, 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF, Ceci SJ. Developmental reversals in false memory: a review of data and theory. Psychol. Bull. 2008;134:343–382. doi: 10.1037/0033-2909.134.3.343. [DOI] [PubMed] [Google Scholar]
- Brebion G, David AS, Jones H, Pilowsky LS. Semantic organization and verbal memory efficiency in patients with schizophrenia. Neuropsychology. 2004;18:378–383. doi: 10.1037/0894-4105.18.2.378. [DOI] [PubMed] [Google Scholar]
- Clare L, McKenna PJ, Mortimer AM, Baddeley AD. Memory in schizophrenia: what is impaired and what is preserved? Neuropsychologia. 1993;31 doi: 10.1016/0028-3932(93)90070-g. 1225–1124. [DOI] [PubMed] [Google Scholar]
- Craik F, Lockhart R. Levels of processing: A framework for memory research. J. Verbal Learn. Verbal Behav. 1972;11:671–684. [Google Scholar]
- Delawalla Z, Barch DM, Eastep JLF, Thomason ES, Hanewinked MJ, Thomposon PA, et al. Factors mediating cognitive deficits and psychopathology among siblings of individuals with schizophrenia. Schizophr. Bull. 2006;32:525–537. doi: 10.1093/schbul/sbj082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Second Edition. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Dewhurst SA, Pursglove RC, Lewis C. Story contexts increase susceptibility to the DRM illusion in 5-year-olds. Dev. Sci. 2007;10:374–378. doi: 10.1111/j.1467-7687.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- Elveväg B, Fisher JE, Weickert TW, Weinberger DR, Goldberg TE. Lack of false recognition in schizophrenia: a consequence of poor memory? Neuropsychologia. 2003;42:546–554. doi: 10.1016/j.neuropsychologia.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Elvevåg B, Heit E, Storms G, Goldberg T. Category content and structure in schizophrenia: an evaluation using the instantiation principle. Neuropsychology. 2005;19:371–380. doi: 10.1037/0894-4105.19.3.371. [DOI] [PubMed] [Google Scholar]
- Gold JM, Randolf C, Carpenter CJ, Goldberg TE, Weinberger DR. Forms of memory failure in schizophrenia. J Abnorm Psychol. 1992;101:487–494. doi: 10.1037//0021-843x.101.3.487. [DOI] [PubMed] [Google Scholar]
- Gsottschneider A, Keller Z, Pitschel-Walz G, Frobose T, Bauml J, Jahn T. The role of encoding strategies in the verbal memory performance in patients with schizophrenia. J. Neuropsychol. 2011;5:56–72. doi: 10.1348/174866410X497382. [DOI] [PubMed] [Google Scholar]
- Huron C, Danion JM. Impairment of constructive memory in schizophrenia. Int. Clin. Psychopharmacol. 2002;17:127–133. doi: 10.1097/00004850-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Iddon JL, McKenna PJ, Sahakian BJ, Robbins TW. Impaired generation and use of strategy in schizophrenia: evidence from visuospatial and verbal tasks. Psychol. Med. 1998;28:1049–1062. doi: 10.1017/s0033291798006758. [DOI] [PubMed] [Google Scholar]
- Kintsch W. Recognition and free recall of organized lists. J. Exp. Psychol. Gen. 1968;78:481–487. [Google Scholar]
- Lampinen JM, Leding JK, Reed KB, Odegard TN. Global gist extraction in children and adults. Memory. 2006;14:952–964. doi: 10.1080/09658210601008957. [DOI] [PubMed] [Google Scholar]
- Lepage M, Montoya A, Pelletier M, Achim AM, Menear M, Lal S. Associative memory encoding and recognition in schizophrenia: an event-related fMRI study. Biol. Psychiatry. 2006;60:1215–1223. doi: 10.1016/j.biopsych.2006.03.043. [DOI] [PubMed] [Google Scholar]
- McClain L. Encoding and retrieval in schizophrenics’ free recall. J. Nerv. Ment. Dis. 1983;171:471–479. doi: 10.1097/00005053-198308000-00004. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Roediger HL. Attempting to avoid illusory memories: robust false recognition of associates persists under conditions of explicit warnings and immediate testing. J. Mem. Lang. 1998;39:508–520. [Google Scholar]
- Moritz S, Woodward TS, Cuttler C, Whitman JC, Watson JM. False memories in schizophrenia. Neuropsychology. 2004;18:276–283. doi: 10.1037/0894-4105.18.2.276. [DOI] [PubMed] [Google Scholar]
- Moritz S, Woodward TS, Rodriguez-Raecke R. Patients with schizophrenia do not produce more false memories than controls but are more confident in them. Psychol. Med. 2006;36:659–667. doi: 10.1017/S0033291706007252. [DOI] [PubMed] [Google Scholar]
- Paul BM, Elvevag B, Bokat CE, Weinberger DR, Goldberg TE. Levels of processing effects on recognition memory in patients with schizophrenia. Schizophr. Res. 2005;74:101–110. doi: 10.1016/j.schres.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Oh TM, Laws KR, McKenna PJ. Semantic priming in schizophrenia: systematic review and meta-analysis. Br. J. Psychiatry. 2008;192:92–97. doi: 10.1192/bjp.bp.106.032102. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez JN, Loughead J, Elliott M, Kohler C, et al. Levels-of-processing effect on frontotemporal function in schizophrenia during word encoding and recognition. Am. J. Psychiatry. 2005;162:1840–1848. doi: 10.1176/appi.ajp.162.10.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Moelter ST, McGrath C, Hill SK, Gur RE, Bilker WB, et al. Levels-of-processing effect on word recognition in schizophrenia. Biol. Psychiatry. 2003;54:1154–1161. doi: 10.1016/s0006-3223(03)00235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Ranganath C, Barch DM, Gold JM, Haley B, MacDonald AW, 3rd, Silverstein SM, Strauss ME, Yonelinas AP, Carter CS. Relational and Item-Specific Encoding (RISE): task development and psychometric characteristics. Schizophr. Bull. 2012;38:114–124. doi: 10.1093/schbul/sbr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Minzenberg MJ, Ragland JD. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol. Psychiatry. 2008;64:18–25. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger HL, McDermott KB. Creating false memories: Remembering words not presented in lists. J. Exp. Psychol.: Learn., Mem., Cog. 1995;21:803–814. [Google Scholar]
- Roediger HL, Watson JM, McDermott KB, Gallo DA. Factors that determine false recall: a multiple regression analysis. Psychonom. Bul. Rev. 2001;8:385–407. doi: 10.3758/bf03196177. [DOI] [PubMed] [Google Scholar]
- Stadler MA, Roediger HL, 3rd, McDermott KB. Norms for word lists that create false memories. Mem. Cognit. 1999;27:494–500. doi: 10.3758/bf03211543. [DOI] [PubMed] [Google Scholar]
- Stone M, Gabrieli JDE, Stebbins GT, Sullivan EV. Working and strategic memory deficits in schizophrenia. Neuropsychology. 1998;12:278–288. doi: 10.1037//0894-4105.12.2.278. [DOI] [PubMed] [Google Scholar]
- Titone D, Ditman T, Holzman PS, Eichenbaum H, Levy DL. Transitive inference in schizophrenia: impairments in relational memory organization. Schizophr. Res. 2004;68:235–247. doi: 10.1016/S0920-9964(03)00152-X. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Dodson CS, Goff DC, Schacter DL, Heckers S. Intact suppression of increased false recognition in schizophrenia. Am. J. Psychiatry. 2002;159:1506–1513. doi: 10.1176/appi.ajp.159.9.1506. [DOI] [PubMed] [Google Scholar]