Summary
The synthesis of adiponectin, an adipokine with ill-defined functions in animals fed a normal diet, is enhanced by the osteoblast-derived hormone osteocalcin. Here we show that adiponectin signals back in osteoblasts to hamper their proliferation and favor their apoptosis, altogether decreasing bone mass and circulating osteocalcin levels. Adiponectin fulfills these functions, independently of its known receptors and signaling pathways, by decreasing FoxO1 activity in a PI3 kinase-dependent manner. Overtime however, these local effects are masked because adiponectin signals in neurons of the locus coeruleus, also through FoxO1, to decrease the sympathetic tone thereby increasing bone mass, and decreasing energy expenditure. This study reveals that adiponectin has the unusual ability to regulate the same function in two opposite manners depending on where it acts and that it opposes, partially, leptin’s influence on the sympathetic nervous system. It also proposes that adiponectin regulation of bone mass occurs through a PI3 kinase-FoxO1 pathway.
Introduction
The existence of a reciprocal regulation between bone and energy metabolisms is supported by a growing number of evidence (Karsenty and Oury, 2011). Two hormones, osteocalcin and leptin are the overarching determinants of this process. Osteocalcin, a bone-derived hormone, regulates energy metabolism, in part, by promoting insulin secretion by pancreatic β-cells (Lee et al., 2007). In a feed-forward loop, insulin signals back in osteoblasts to enhance osteocalcin activity and therefore insulin secretion (Ferron et al., 2010). Osteocalcin also signals in adipocytes where it favors the synthesis of the secreted molecule adiponectin (Lee et al., 2007). That insulin signals back to osteoblasts and influences osteocalcin activity raises the prospect that adiponectin might do the same.
The adipocyte-derived hormone leptin prevents bone mass accrual in all species in which this was tested. (Ducy et al., 2000; Elefteriou et al., 2004; Henry et al., 1999; Vaira et al., 2012). In the mouse, a mediator of leptin regulation of bone mass accrual is the sympathetic nervous system (Takeda et al., 2002). The development of high bone mass in the face of hypogonadism as seen in the absence of leptin signaling, is a unique situation that underscores the importance of the leptin-dependent sympathetic regulation of bone mass accrual. It also raises the prospect that, as it is the case for its regulation of appetite (Erickson et al., 1996), leptin stimulation of the sympathetic nervous system may be opposed by another secreted molecule. Such a molecule has not been identified yet.
Adiponectin, another adipocyte-derived secreted molecule, is best known for its insulin-sensitizing ability in animals fed a high fat diet (Kadowaki et al., 2006; Maeda et al., 2002), although its absence does not appear to overtly alter insulin sensitivity in animals fed a normal diet (Ma et al., 2002; Maeda et al., 2002). It seems unlikely that adiponectin would only function in animals fed a high fat diet since when it appeared during evolution, this diet did not exist nor were there any reasons to anticipate its eventual appearance. Moreover, even in present days, most animals never encounter this situation in the wild. A plausible explanation for this apparent lack of metabolic function in animals fed a normal diet is that adiponectin affects another biological processes than energy metabolism (Denzel et al., 2010; Sharma et al., 2008; Takemura et al., 2007). The appearance of adiponectin during evolution with bones (Figure 1A) and its regulation by osteocalcin are two reasons to suspect that bone could be a target tissue of this hormone. This hypothesis began to be tested in vivo through gain- and loss-of-function experiments. Some of these studies indicated that adiponectin affects bone mass, however, they did not provide molecular or cellular mechanisms for its action nor did they study whether adiponectin affects osteocalcin (Oshima et al., 2005; Shinoda et al., 2006).
Figure 1.
Adiponectin regulation of Rankl and bone mass. (A) Conservation of adiponectin, AdipoR1, AdipoR2 and T-cadherin sequences among vertebrate and invertebrate species. (B) Relative expression of Adiponectin in WT and Adiponectin−/− adipocytes, osteoblasts, and osteoclasts population. (C) Adiponectin regulation of gene expression analyzed by qPCR. Primary osteoblasts or osteoclasts cell populations treated with indicated concentration of adiponectin for 4 hours. (D) Bone histomorophometric analysis of 6 and 12 week-old WT (n>10) or Adiponectin−/− (n>10) mice. BV/TV: bone volume over tissue volume. Ob.N/T.Ar: number of osteoblasts per trabecular area. BFR: bone formation rate. OcS/BS: osteoclast surface per bone surface. ES/BS: Eroded surface per bone surface. (E) μCT analysis of 6 week-old WT (n=5) or Adiponectin−/− mice (n=5). Cort.Th: Cortical thickness. Conn-Den: connective density. Tb.N: trabecular number. (F–G) Three points bending test and Rankl expression in 6 week-old Adiponectin−/− bones. (H) Total and uncarboxylated osteocalcin serum levels in 6 week-old Adiponectin−/− mice. (I) Adiponectin serum levels in 12 week-old pLiv-Adiponectin (pLiv-Apn) mice. (J) Bone histomorphometric analysis of 12 week-old WT (n=10) and pLiv-Adiponectin mice (n=8). (K) Total and uncarboxylated osteocalcin serum levels in 12 week-old pLiv-Adiponectin mice. (L–M) Glucose tolerance test and glucose-stimulated insulin secretion test in 12 week-old Adiponectin−/− mice. For all panels, results are given as means ± standard error of mean (SEM). *, p < 0.05 by ANOVA and/or student t-test. NS: not significant. N.D: not determined. See also figure S1.
Adiponectin biology is further complicated by the fact that several receptors and more than one signaling pathway have been associated with this hormone. Expression cloning identified two atypical seven transmembrane proteins, AdipoR1 and AdipoR2, as receptors for adiponectin. Those receptors were subsequently shown to transduce adiponectin signal either in an AMP kinase (AMPK)-dependent or in an AMPK-independent but ceramide-dependent manner (Yamauchi et al., 2003; Holland et al., 2011). Adiponectin also binds to T-cadherin, a cell-surface molecule that lacks, however, any intracellular moiety (Hug et al., 2004). Collectively these observations suggest that, as it is the case for its function(s), the signaling mechanisms used by adiponectin may not be fully elucidated.
Testing whether bone is a target tissue of adiponectin revealed that this hormone exerts two opposite influences on this tissue. First, it signals in osteoblasts, prevents their proliferation and increases their apoptosis in a PI3 kinase-FoxO1-dependent manner, as a result it decreases bone formation, bone mass, and circulating osteocalcin levels. Yet these functions are rapidly masked, because, adiponectin also signals in the brain, through FoxO1, to inhibit the activity of the sympathetic nervous system, thereby increasing bone formation and bone mass, decreasing energy expenditure and blood pressure. These results identify target cells, functions and propose a novel signaling mechanism for adiponectin in animals fed a normal diet. They also indicate that adiponectin opposes, partially, leptin’s influence on the sympathetic nervous system and the physiological functions it regulates.
Results
Adiponectin inhibits bone mass accrual in young mice
Proteins with significant homology to adiponectin are found only in bony vertebrates (Figure 1A). This could mean that adiponectin is expressed in, or signals in bone cells. To test the first possibility, we measured Adiponectin expression in adipocytes, and bone cells obtained from wildtype (WT) and Adiponectin−/− mice. Adiponectin expression was 10000-fold higher in WT adipocytes than in bone cells, moreover, there was no difference in Adiponectin expression between WT and Adiponectin−/− osteoblasts and osteoclasts (Figure 1B). Likewise, qPCR and in situ hybridization analyses failed to detect Adiponectin expression in osteocytes (Figure S1E–G). Thus, the appearance of adiponectin with bone during evolution cannot be explained by its expression in bone cells.
To address the second possibility, we measured the expression of multiple genes in cell populations enriched with calvarial osteoblasts or in osteoclasts derived in vitro from bone marrow-derived cells. These cells were then treated with recombinant mouse full-length adiponectin 4 hours. Among all the genes tested, only Rankl, a gene expressed in osteoblasts and encoding an osteoclast differentiation factor (Lacey et al., 1998), saw its expression increased more than 10-fold after stimulation by even low amounts of adiponectin or its globular domain (Figures 1C and S1A, S1H). The same stimulation of Rankl expression was observed when using enriched populations of osteoblasts derived from bone marrow cells treated with adiponectin for 2, 4 and 8 hours (McCulloch et al., 1991) (Fig. S1H). These results identifying the osteoblast and Rankl as an adiponectin target cell and gene implied that this hormone should inhibit bone mass accrual by favoring bone resorption. This hypothesis was tested through the study of Adiponectin−/− mice fed a normal diet. Since Adiponectin−/− mice of either gender displayed the same bone mass abnormality at a later age (Figures 3A and S2A), we used only male mice for the rest of this study.
Figure 3.
Analysis of older Adiponectin−/− mice. (A) Bone histomorophometric analysis of 24 and 36 week-old WT (n>10) and Adiponectin−/− (n>10) mice. (B–C) Cyclin expression, Cyclin D1 accumulation in 36 week-old Adiponectin−/− bones. (D–F) Serum CTx levels, Rankl expression, Energy expenditure in 36 week-old Adiponectin−/− mice. (G–J) Fat pad weight, body weight, blood pressure and heart rate of 12 and/or 36 week-old Adiponectin−/− mice. (K) Adiponectin accumulation in brain. 6 week-old Adiponectin−/− mice received 5 μg/day of adiponectin peripherally for 7 days, adiponectin levels in hypothalamus, brainstem, cortex, and cerebellum were then measured. ND: not detectable. (L) Adiponectin binding to locus coeruleus (LC), visualized with anti-biotin antibody (red) together with locus coeruleus-specific marker DBH using anti-DBH antibody (green). (M) Norepinephrine content in the brainstem of 36 week-old Adiponectin−/− mice. (N) Ucp1 expression in brown fat of 6, 12 and 36 week-old Adiponectin−/− and 12 week-old pLib-Adiponectin mice. (O) Urinary epinephrine elimination of 6, 12 and 36 week-old Adiponectin−/− mice. (P) Ucp1 expression in 36 week-old Adiponectin−/− subcutaneous fat (sub. fat). See also figure S2.
At 6 and to a lesser extent, 12 weeks of age Adiponectin−/− mice fed a normal or high fat diet showed the expected high bone mass phenotype affecting axial and appendicular skeleton, trabecular and cortical bones (Figures 1D–E, S1B, S2I). Consequently, Adiponectin−/− bones had better biomechanical properties than WT ones (Figure 1F). Surprisingly however, this high bone mass was not caused by a decrease in bone resorption since Rankl expression was mildly affected and the osteoclast number was not significantly changed in Adiponectin−/− bones. What explained, instead, this high bone mass phenotype in Adiponectin−/− mice was a massive increase in the osteoblast number (Figures 1D and 1G), that resulted in an increase in the rate of bone formation and in circulating levels of total and undercarboxylated osteocalcin (Figure 1H). This high bone mass phenotype could not be explained by abnormal insulin sensitivity since insulin tolerance tests were normal throughout life in Adiponectin−/− mice fed a normal diet (Figure S1C). In contrast, 12 week-old Adiponectin−/− mice fed a normal diet, showed a significant decrease in insulin secretion as measured by a glucose-stimulated insulin secretion test (GSIS) and an intolerance to glucose as determined by a glucose tolerance test (GTT) (Figure 1L–M). A mechanism explaining these abnormalities was later identified (see below).
We also analyzed transgenic mice harboring a 5-fold increase in adiponectin circulating levels (pLiv-Adiponectin) (Figure 1I). Mirroring what was seen in Adiponectin−/− mice, 12 week-old pLiv-Adiponectin mice showed a low bone mass phenotype due to a significant decrease in osteoblast number (Figure 1J). Consequently, total and undercarboxylated circulating osteocalcin levels were significantly decreased in these mice compared to WT littermates (Figure 1K). Hence, both loss- and gain-of-function models identify adiponectin as a regulator of osteoblast number, bone formation and circulating osteocalcin levels.
Adiponectin inhibits proliferation and favors apoptosis of osteoblasts
Since it is the basis of the bone phenotypes of Adiponectin−/− and pLiv-Adiponectin mice, we investigated how adiponectin regulates the osteoblast number. While expression of osteoblast differentiation markers such as Runx2 and Osterix was unaffected in Adiponectin−/− bones and in adiponectin-treated osteoblasts (Figures 1C and S1D), three cellular abnormalities could explain the elevated osteoblast number of Adiponectin−/− mice.
First, proliferation of osteoblast progenitor cells, as measured by BrdU incorporation and CyclinD1 accumulation, was increased in Adiponectin−/− bones, and adiponectin treatment of mouse osteoblast progenitor cells decreased their proliferation and CyclinD1 accumulation (Figures 2A–D). Second, there was a decrease in osteoblasts apoptosis in Adiponectin−/− bones as measured by a TUNEL assay and by the number of Annexin V-positive osteoblasts (Figures 2E and 2G). Conversely, adiponectin treatment of osteoblast progenitor cells increased the number of TUNEL-positive cells and the production of cleaved caspase-3 by osteoblasts (Figures 2F and 2H). Third, there was a decrease in oxidative stress defined by the production of malondialdehyde and 4-hydroxynonenal and accumulation of reactive oxygen species in Adiponectin−/− osteoblasts; this may also trigger apoptosis (Finkel and Holbrook, 2000) (Figures 2I and 2J). That none of these abnormalities were observed when hepatocytes and myoblasts were treated with adiponectin suggests that the influence of this hormone on osteoblasts is, to an extent, specific to this cell type (Figures 2B and 2F). In summary, by signaling in osteoblasts adiponectin inhibits bone formation and decreases circulating osteocalcin levels because it decreases proliferation and favors apoptosis of osteoblasts.
Figure 2.
Adiponectin regulates osteoblast proliferation and apoptosis. (A, B) BrdU incorporation in 10 day-old Adiponectin−/− bones, or WT osteoblasts, myoblasts or hepatocytes treated with vehicle or adiponectin for 24 hours. (C–D) Cyclins accumulation in 6 week-old Adiponectin−/− bones, and WT osteoblasts treated with vehicle or adiponectin for 24 hours. (E–F) TUNEL assay in 10 day-old Adiponectin−/− bones, and WT osteoblasts, myoblasts or hepatocytes treated with vehicle or adiponectin for 24 hours. (G) Annexin-V positive 6 week-old Adiponectin−/− osteoblasts. (H) Cleaved-caspase 3 accumulation in WT osteoblasts treated with vehicle, adiponectin, or H2O2 (100μM) treated for 24 hours. (I, J) Quantification of malondialdehyde, 4-hydroxynonenal and reactive oxygen species (ROS) in 6 week-old Adiponectin−/− osteoblasts.
Adiponectin favors bone mass accrual in older mice
A singular feature of the high bone mass phenotype displayed by Adiponectin−/− mice is that it peaked at an early age. Indeed, the two-fold increase in osteoblast number seen in 6 week-old mutant mice had largely vanished once these mice reached 3 months of age. This dynamic influence of adiponectin on bone mass accrual led us to analyze Adiponectin−/− mice at multiple time points.
Bone formation, bone resorption parameters and bone mass were normal, if not slightly decreased, in 6 month-old male and female Adiponectin−/− mice, even more striking was the fact that 9 month-old Adiponectin−/− mice demonstrated a severe low bone mass phenotype affecting all skeletal elements tested (Figures 3A and S2A–B). This was due to the conjunction of a decrease in the number and proliferation ability of osteoblasts (Figures 3A–C) and an increase in bone resorption parameters such as osteoclast surface, CTx serum levels and Rankl expression (Figures 3A, 3D–E). In other words, the bone phenotype of older Adiponectin−/− mice and the cellular abnormalities explaining it were exactly opposite to those seen in younger ones.
Two other physiological functions were also affected in 9 month-old Adiponectin−/− mice fed a normal diet. First, there was a marked increase in energy expenditure during both dark and light cycles (Figure 3F). As a result, fat pad weight did not increase overtime in Adiponectin−/− as it did in WT mice (Figure 3G). Paradoxically given this massive increase in energy expenditure, appetite was not increased and in fact was slightly decreased in older Adiponectin−/− mice. This explained why the body weight of 9 month-old Adiponectin−/− mice was significantly lower than the one of WT littermates (Figures 3H and S2E). Expression of genes affecting appetite, such Mc4r, Npy and Cart, was similar between Adiponectin−/− and WT mice (Figure S2D). Second, blood pressure and heart rate were significantly increased in Adiponectin−/− mice compared to WT littermates (Figures 3I and 3J).
Adiponectin inhibits the activity of the sympathetic nervous system
Two observations suggested that the phenotype observed in older Adiponectin−/− mice could be due to an increase in the sympathetic tone. The first one is that, adiponectin crosses the blood brain barrier (Figure 3K)(Kusminski et al., 2007; Qi et al., 2004). To determine if adiponectin accumulates in the locus coeruleus where the sympathetic nervous system originates from (Rush and Geffen, 1980), we delivered it in Adiponectin−/− mice and analyzed where it binds. In the conditions of this experiment, adiponectin bound to neurons expressing DBH, a specific marker of neurons of the locus coeruleus (Thomas et al., 1997). That an excess of non-labeled adiponectin abolished biotinylated-adiponectin binding to DBH-expressing neurons while biotinylated-GST did not, verified the specificity of this binding (Figure 3L). A second reason to test whether adiponectin regulates the sympathetic tone is that the sympathetic nervous system is known to inhibit bone formation, to favor bone resorption, to increase energy expenditure and blood pressure, to promote liver gluconeogenesis and to inhibit insulin secretion (Elefteriou et al., 2005; Nonogaki, 2000; Takeda et al., 2002; Tentolouris et al., 2006).
As hypothesized, whether we measured norepinephrine content in the brain, Ucp1 expression in brown fat or urinary epinephrine elimination, there was, throughout life, a nearly two-fold increase in the sympathetic tone in Adiponectin−/− mice compared to WT littermates fed a normal or a high fat diet (Figures 3M–O, S2H). As a result, Ucp1 expression was higher in white adipose tissue of 9 month-old Adiponectin−/− mice reflecting a “beiging” of white fat (Figure 3P) (Wu et al., 2012). Conversely, Ucp1 expression in brown fat was lower in pLiv-Adiponectin than in WT mice (Figure 3N), Since the sympathetic tone enhances bone resorption by increasing Rankl expression (Elefteriou et al., 2005), we speculate that the high sympathetic activity seen in Adiponectin−/− mice antagonizes the consequence on Rankl expression of the absence of adiponectin signaling in osteoblasts. This may explain the overall normal bone resorption noted in young Adiponectin−/− mice.
If the increase in sympathetic activity explains the low bone mass of older Adiponectin−/− mice, normalizing it should result in a high bone mass phenotype because of the absence of adiponectin signaling in osteoblasts. This assumption was tested by removing one allele of Dopamine β-hydroxylase (Dbh), the gene encoding the initial enzyme in catecholamine biosynthesis from Adiponectin−/− mice (Thomas et al., 1995). That Ucp1 expression in brown fat, norepinephrine content in the brain and urinary epinephrine elimination were similar in Adiponectin−/−;Dbh+/− and control littermates verified that the sympathetic activity was normalized in the compound mutant mice (Figures S3A–C). Nine month-old Adiponectin−/−;Dbh+/− mice presented an increase in bone mass secondary to an increase in osteoblast numbers and bone formation rate (Figures 4A–C, and S3E). At the same time energy expenditure, fat pad and body weights were normal in these compound mutant mice (Figures 4E–F and S3D). That normalizing sympathetic tone in 12 week-old Adiponectin−/− mice normalized also GTT and GSIS tests indicates that the decrease in insulin secretion seen in the absence of adiponectin is secondary to the increase in sympathetic activity (S3F–G).
Figure 4.
Analysis of Adiponectin−/−;Dbh+/−, Adiponectin−/−;Adrb2osb+/− and Adipoenctin−/−;ob/ob mice. (A) Bone histomorophometric analysis of 36 week-old Adiponectin−/−;Dbh+/− mice. WT (n>10), Dbh+/− (n>10), Adiponectin−/− (n>10), or Adiponectin−/−;Dbh+/− (n>10). *, P<0.05 between WT and Adiponectin−/−. #, P<0.05 between Adiponectin−/− and Adiponectin−/−;Dbh+/−. **, P<0.05 between WT and Adiponectin−/−;Dbh+/−. (B–F) Serum CTx levels, Rankl expression, Cyclin D1 accumulation, energy expenditure, and fat pad weight in 36 week-old Adiponectin−/−;Dbh+/− mice. *, P<0.05 between WT and Adiponectin−/−. #, P<0.05 between Adiponectin−/− and Adiponectin−/−;Dbh+/−. (G) Bone mass of 12 week-old Adiponectin−/−;Adrb2osb+/− mice. (H) Ucp1 expression in 6 week-old Adiponectin−/−;ob/ob brown fat. (I–L) Energy expenditure, fat pad weight, blood pressure, heart rate in 6 week-old Adiponectin−/−;ob/ob mice. (M–N) Bone histomorphometric analysis of 10 week-old Adiponectin−/−;ob/ob vertebrae and femora. WT (n=9), ob/ob (n=8), Adiponectin−/− (n=7), Adiponectin−/−;ob/ob (n=7) *, P<0.05 between WT and Adiponectin−/−, **, P<0.05 between WT and ob/ob, ***, P<0.05 between WT and Adiponectin−/−;ob/ob. # P<0.05 between Adiponectin−/− and ob/ob. ##, P<0.05 between Adiponectin−/− and Adiponectin−/−;ob/ob. $, P<0.05 between Adiponectin−/−;ob/ob and ob/ob. See also figure S3.
To determine if it is by signaling in osteoblasts that the sympathetic nervous system antagonizes the local effect of adiponectin we generated Adiponectin−/− mice lacking one copy of Adrb2, the adrenergic receptor mediating sympathetic signaling in osteoblasts (Takeda et al., 2002), in these cells only. Three month-old Adiponectin−/−;Adrb2osb+/− mice had significantly higher bone mass than Adiponectin−/− mice (Figure 4G). Taken together, these experiments support the notion that adiponectin favors bone mass accrual and decreases energy expenditure by inhibiting sympathetic signaling in osteoblasts. This mechanism of action eventually masks the consequence of the absence of signaling of this hormone on osteoblasts.
Adiponectin antagonizes leptin regulation of the sympathetic tone
Since adiponectin and leptin exert opposite influences on sympathetic activity, we wondered what might be the consequences of removing Adiponectin from ob/ob mice (Adiponectin−/−;ob/ob). Although not normalized, Ucp1 expression and urinary epinephrine levels were increased two-fold in 6 and 10 week-old Adiponectin−/−;ob/ob compared to ob/ob mice (Figures 4H, S3I and S3L). As a result, energy expenditure was significantly higher in Adiponectin−/−;ob/ob than in ob/ob mice at both 6 and 10 week-old (Figures 4I and S3M) and these mutant mice gained less weight, had lighter fat pad weight, than ob/ob mice as long as this was tested (Figures 4J, S3H and S3K). Moreover, blood pressure and heart rate were normal in 6 week-old Adiponectin−/−;ob/ob mice (Figures 4K–L). Since the correction of the low sympathetic tone observed in ob/ob mice by Adiponectin deletion is only partial, the glucose metabolism abnormalities of ob/ob mice at 10 week-old remained unaffected (Figure S3J). These results indicate that adiponectin antagonizes, partially, the functions of leptin that are mediated by the sympathetic nervous system. Ten week-old Adiponectin−/−;ob/ob mice also exhibited a lower bone mass, in both axial and peripheral, compared to ob/ob mice, a result consistent with the fact that adiponectin and leptin exert an opposite influence on the sympathetic tone (Figures 4M–N).
Adiponectin does not use known signaling pathways in osteoblasts
To identify molecular mechanisms used by adiponectin to mediate its functions in animals fed a normal diet we focused on osteoblasts, a cell type that can be cultured, and first asked whether any of its known receptors were involved.
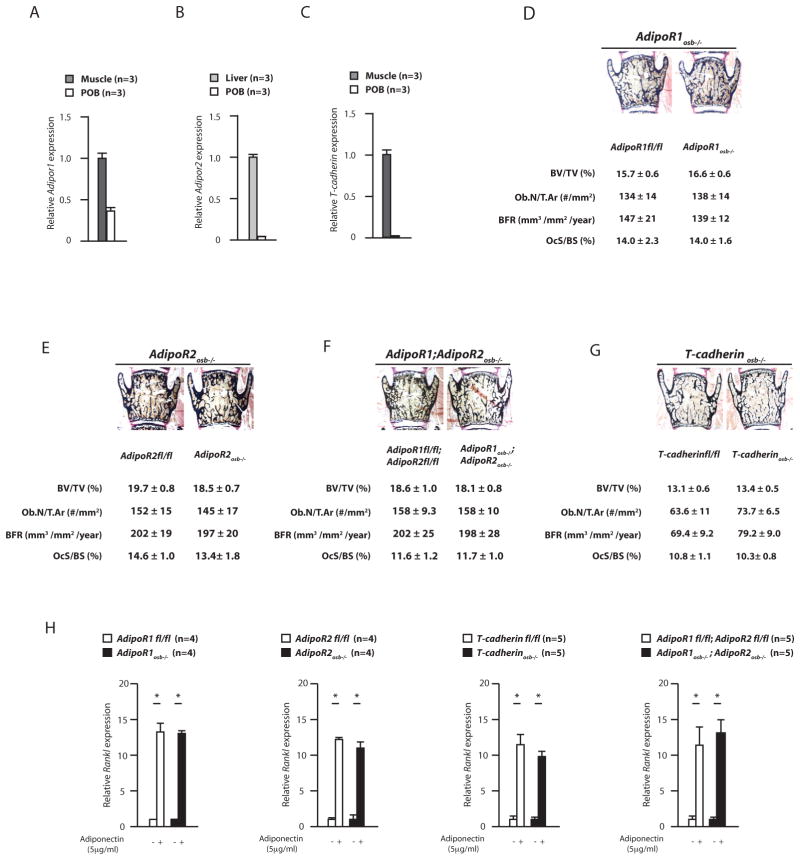
AdipoR1 was expressed at similar levels in osteoblasts and skeletal muscle while expression of AdipoR2 and T-cadherin was 10-fold lower in osteoblasts than in tissues where they are abundantly expressed (Hug et al., 2004; Yamauchi et al., 2003) (Figures 5A–C). We next generated mutant mouse strains lacking AdipoR1, AdipoR2, T-cadherin, or a combination of these genes, in osteoblasts only and verified that we had efficiently deleted the gene(s) of interest in osteoblasts but not in other cell types (Figures S4C–D, S4G–H and S4K–L). Unlike what was the case for Adiponectin−/− mice of the same age and maintained on the same genetic background, 12 week-old AdipoR1osb−/−, AdipoR2osb−/−, T-cadherinosb−/−, and AdipoR1osb−/−;AdipoR2osb−/− mice displayed normal bone mass, osteoblast number, osteoclast number, circulating osteocalcin levels and Rankl expression (Figures 5D–G, S4M and S4N). Moreover in cell culture, adiponectin induced Rankl expression equally well in fl/fl, AdipoR1−/−, AdipoR2−/−, AdipoR1−/−;AdipoR2−/− and T-cadherin−/− osteoblasts (Figure 5H).
Figure 5.
Known adiponectin receptors and signaling pathways do not mediate adiponectin function in osteoblasts. (A–C) AdipoR1, AdipoR2, and T-cadherin expression in osteoblasts, muscle or liver. (D–G) Bone histomorophometric analyses of 12 week-old AdipoR1osb−/−, AdipoR2 osb−/−, AdipoR1osb−/−; AdipoR2osb−/−, or T-cadherin osb−/− mice. (H) Rankl expression in 12 week-old AdipoR1osb−/−, AdipoR2 osb−/−, T-cadherin osb−/− and AdipoR1osb−/−; AdipoR2osb−/− or fl/fl osteoblasts treated with vehicle or adiponectin. See also figure S4.
We also studied signaling pathways reported to mediate adiponectin functions. Adiponectin can induce AMP kinase (AMPK) phosphorylation in some settings (Yamauchi et al., 2002), yet, it failed to do so in cultured osteoblasts (Figure 6A). This was not due to a poor quality of the osteoblast preparation since adiponectin induced Rankl expression and AICAR, the positive control in this experiment, induced robust AMPK phosphorylation in these cells (Figure 6A). Adiponectin has also been proposed to enhance ceramidase activity in some target cells (Holland et al., 2011) but there was no significant increase in ceramide content in bones of Adiponectin−/− mice fed a normal diet (Figure 6B). Thus, results presented here indicate that, in osteoblasts, adiponectin may signal through distinct signaling pathways.
Figure 6.
FoxO1 mediates adiponectin functions in osteoblasts and neurons. (A) Western blot analysis of phospho-AMPK in WT osteoblass treated with vehicle, 15 μg/ml adiponectin or AICAR (0.5 mM) for 5 or 30 min. (B) Ceramide contents in 6 week-old Adiponectin−/− bones. (C–D) Western blot analysis of phospho-FoxO1 in WT and 6 week-old Adiponectin−/− bones, and osteoblasts treated with vehicle, adiponectin or insulin for 15 min. (E) FoxO1 luciferase assay in ROS 17/6.2 cells treated with adiponectin for 24 hours. (F) Expression of FoxO1 target genes in 12 week-old Adiponectin−/− or pLiv-Adiponectin bones. (G) Bone histomorphometric analysis of 12 week-old Adiponectin−/−;FoxO1osb+/− bones. Controls (n=8), Adiponectin−/− (n=8), Adiponectin−/−;FoxO1osb+/− (n=6). *, P<0.05 between Controls and Adiponectin−/−. **, P<0.05 between Adiponectin−/− and Adiponectin−/−;FoxO1osb+/−. (H) BrdU incorporation in 10 day-old Adiponectin−/−;FoxO1osb+/− bones. (I) CyclinD1 accumulation in 12 week-old Adiponectin−/−;FoxO1osb+/− bones. (J) Energy expenditure of 36 week-old Adiponectin−/−;FoxO1LC+/− mice. (K) Ucp1 expression in 36 week-old Adiponectin−/−;FoxO1LC+/− brown fat. (L) Fat pad weight of 36 week-old Adiponectin−/−;FoxO1LC+/− mice. (M) Bone histomorphometric analysis of 36 week-old Adiponectin−/−;FoxO1LC+/− mice. Controls (n=5), Adiponectin−/− (n=6), Adiponectin−/−;FoxO1LC+/− (n=5). *, P<0.05 between Controls and Adiponectin−/−. **, P<0.05 between Adiponectin−/− and Adiponectin−/−;FoxO1LC+/−. (N) Rankl expression in 36 week-old Adiponectin−/−;FoxO1LC+/− bones. (O) CTx levels of 36 week-old Adiponectin−/−;FoxO1LC+/− mice. See also figure S5.
Adiponectin dual regulation of bone mass accrual occurs by decreasing FoxO1 activity
To understand how adiponectin could regulates bone mass we searched for a single signaling molecule regulating all three cellular events affected by adiponectin in osteoblasts, i.e., proliferation, apoptosis and oxidative stress. The transcription factor FoxO1 affects these three cellular events in the opposite direction than adiponectin (Rached et al., 2010) and five different lines of evidence indicate that it lies downstream of adiponectin signaling in osteoblasts.
First, adiponectin, and insulin, a positive control, increases phosphorylation of FoxO1 in osteoblasts, an event that decreases its transcriptional activity (Burgering and Kops, 2002) (Figure 6C). Conversely, FoxO1 is less phosphorylated, i.e. more active in Adiponectin−/− than in WT bones (Figure 6D). Second, adiponectin, like insulin, decreases the quantity of FoxO1 present in the nuclear compartment of osteoblasts (Figure S5A). Third, adiponectin treatment of ROS17/2.8 osteoblastic cells decreases activity of a Luciferase gene driven by an artificial promoter containing multiple FoxO1 binding sites (Figure 6E). Fourth, expression of P16 and P19, two FoxO1 target genes in osteoblasts (Rached et al., 2010), was increased in Adiponectin−/− and decreased in pLiv-Adiponectin bones (Figure 6F). Fifth, we generated Adiponectin−/− mice lacking one allele of FoxO1 in osteoblasts only (Adiponectin−/−;FoxO1osb+/− mice). P16 and P19 expression was similar in Adiponectin−/−;FoxO1osb+/− and control littermate (Adiponectin+/+;FoxO1fl/+) bones indicating that we had normalized FoxO1 activity in osteoblasts through this manipulation (Figure S5B). Bone mass, bone formation rate, osteoblast and osteoclast numbers were all indistinguishable between controls and 3 month-old Adiponectin−/−;FoxO1osb+/− mice (Figures 6G). BrdU incorporation and cyclin accumulation verified that osteoblast proliferation was decreased in Adiponectin−/−;FoxO1osb+/− compared to Adiponectin−/− bones (Figures 6H and 6I), as a result, osteocalcin circulating levels were normal in Adiponectin−/−;FoxO1osb+/− mice (Figures S5D and S5E). Hence, adiponectin affects osteoblast proliferation, apoptosis and bone formation by decreasing FoxO1 activity in osteoblasts.
Since FoxO1 is expressed in neurons of the locus coeruleus, we asked if it also lies downstream of adiponectin signaling in the brain, by generating Adiponectin−/− mice lacking one allele of FoxO1 only in neurons of the locus coeruleus (Figure S5F). Adiponectin−/−;FoxO1LC+/− mice had normal sympathetic activity (Figure S5G), normal energy expenditure, Ucp1 expression in brown fat, fat pad and body weights (Figures 6J–L and S5H). Bone mass, osteoblast number, and bone formation rate were significantly increased while osteoclast number, Rankl expression, and serum CTx levels were lower in Adiponectin−/−;FoxO1LC+/− than in Adiponectin−/− mice (Figures 6M–O and S5I), indicating that FoxO1 is also part of the adiponectin signaling pathway in neurons of the locus coeruleus.
Adiponectin affects FoxO1 phosphorylation in a PI3 kinase-dependent manner in osteoblasts
In the last set of experiments, we used information presented above in an effort to identify a signaling cascade used by adiponectin in osteoblasts. Adiponectin did not induce cAMP production in osteoblasts and inhibitors of signaling through G-protein coupled receptor (GPCR) did not prevent adiponectin to induce Rankl expression in osteoblasts (Figures 7D and S6A–B), thus suggesting that the putative adiponectin receptor in osteoblasts may not be a GPCR. On the other hand, that FoxO1 phosphorylation is often regulated by PI-3 kinase dependent events (Taniguchi et al., 2006) led us to test whether adiponectin signaling in osteoblasts rely on signaling events that can be elicited by PI-3 kinase–dependent mechanisms.
Figure 7.
Adiponectin signaling in osteoblasts. (A) Accumulation of phosphatidylinositol 3-phosphate in osteoblasts treated with vehicle, adiponectin or insulin (10 nM) in the presence PI3 kinase inhibitor of LY294002 (10 μM) for 5 min. (B) Western blot analysis of phospho-AKT in osteoblasts treated with vehicle, adiponectin, insulin (10 nM) or PTH (10 nM) for 5 min. (C) Western blot analysis of phospho-AKT and phospho-FoxO1 in the presence of LY294002 (10 μM) for 5 min (P-AKT) and 15 min (P-FoxO1). (D–E) Rankl induction and phosphorylation of AKT by adiponectin in the presence of Gs inhibitor (NF449), Gi inhibitor (NF023), Gii inhibitor (Gallein), or PLC inhibitor (ET-18-OCH3). (F) Western blot analysis of phospho-tyrosine in osteoblasts treated with vehicle, adiponectin or PTH (10 nM) for 2 min. Arrowhead, the phosphorylated band around 170 kDa. (G) Evolution of the bone phenotype of Adiponectin−/− mice overtime. (H) Schematic representation of the diverse functions exerted by adiponectin in mice fed a normal diet and of its two sites of action. See also figure S6.
In support of this hypothesis we note that adiponectin, like insulin, could increase PI3 kinase activity in osteoblasts as measured by the accumulation of phosphatidylinositol 3-phosphate (Figure 7A) and induced phosphorylation of AKT (Figure 7B), a signaling molecule acting downstream of PI3 kinase (Engelman, 2009). Adiponectin-dependent phosphorylation of AKT and FoxO1 were both abolished when osteoblasts were pre-treated with LY294002, a PI3 kinase inihibitor, but not by inhibitors of signaling through GPCR (Figures 7C–E, S6B). Furthermore, adiponectin treatment of osteoblasts induced tyrosine phosphorylation of a protein with a molecular weight of close to 170 kDa (Figure 7F). These results indicate that adiponectin ability to regulate proliferation and apoptosis of osteoblasts in a FoxO1-dependent manner is determined, at least in part, by PI3 kinase activity.
Discussion
This study, prompted by the regulation of Adiponectin expression by osteocalcin showed that adiponectin regulates bone mass accrual through two opposite mechanisms, and counteracts, partially, some of leptin’s functions. It also points toward a novel signaling mechanism for this hormone. We should emphasize that our results do not affect in any way conclusions reached by analyzing the function of this molecule in mice fed a high fat diet and in other cell types.
Adiponectin exerts two opposite influences on bone mass accrual
By showing that the inactivation of Adiponectin in otherwise unchallenged mice results in significant perturbations of their bone mass, our results identify the regulation of bone mass accrual by adiponectin as physiologically relevant. This regulation, consistent with the appearance of adiponectin with bone during evolution, is unusual because it relies on two mechanisms, one local and one central, that antagonize each other. On the one hand, adiponectin acts directly in osteoblasts to prevent their proliferation and increase their apoptosis; this is why young Adiponectin−/− mice display a high bone mass caused by an increase in bone formation parameters. Bone may not be the only tissue affected by peripheral actions of adiponectin in animals fed a normal diet, however, we note that the influence that adiponectin exerts on proliferation and apoptosis of osteoblasts was not observed in other cell types such as myoblasts and hepatocytes.
Overtime however, this local effect is obscured by a second and more powerful mode of action of adiponectin, its ability to decrease activity of the sympathetic nervous system. This is why mice lacking Adiponectin develop, as they age, a severe low bone mass phenotype explained by the deleterious influence of a high sympathetic tone on bone mass accrual that is never seen in young mutant mice (Elefteriou et al., 2005; Takeda et al., 2002). This latter mode of action illustrates the importance of the sympathetic nervous system as a regulator of bone mass accrual (Guntur and Rosen, 2012) and suggests that increasing adiponectin signaling through pharmacological means may prevent age-related bone loss by decreasing the sympathetic tone.
Remarkably, the two opposite modes of action of adiponectin target the same molecules. Through it signaling in osteoblasts, adiponectin inhibits osteoblast proliferation and CyclinD1 accumulation, through its central signaling it favors osteoblast proliferation and CyclinD1 accumulation in osteoblasts. Likewise, adiponectin increases Rankl expression in osteoblasts but through its signaling in the brain and its effect on the sympathetic tone, it inhibits Rankl expression. The fact that the same hormone exerts opposite influences on the same physiological function as adiponectin does, is to the best of our knowledge, a rare future. The availability of a growing number of mutant mouse strains each lacking a specific hormone will allow to test if this is a more universal rule of endocrinology.
Adipocytes secrete two hormones exerting opposite influence on the same physiological process
Adiponectin defines with leptin a group of adipokines that exert a significant regulation of bone mass in vivo. As a matter of fact, uncovering adiponectin regulation of bone mass accrual shows how this hormone counteracts, partially, leptin functions that are mediated by the sympathetic nervous system. Although we had hypothesized that hormone(s) opposing leptin’s influence on bone mass accrual would exist, the fact that it is also synthesized by adipocytes was unexpected. Thus it appears that the adipocyte is a rare if not unique example of an endocrine cell secreting two hormones exerting exactly opposite influences on the same physiological functions. Again, the fact that many mutant mouse strains are now available to study the biology of most hormones should allow one to test whether this aspect of adipocyte biology is a specific feature of this cell type, or if it applies to other endocrine cells. In any case, the results presented in this study underscore the importance of adipocytes in the control of bone mass accrual.
Mediation of adiponectin regulation of bone mass accrual
The identification of adiponectin as a regulator of bone mass accrual begged the question of the nature of the signaling pathway(s) this hormone used in osteoblasts and neurons. Because this cell can be studied ex vivo more easily we focused most of our work on osteoblasts.
A first surprising result was that deletion of the known receptors for adiponectin did not recapitulate the phenotype caused by the absence of ligand nor did they prevent adiponectin to enhance Rankl expression in osteoblasts. These results could have two explanations; either adiponectin signals through a different receptor, or through the adiponectin receptor in osteoblasts, a complex made of known receptors such as AdipoR1 and a novel one. Of note, AdipoR1 and T-cahderin are also expressed in neurons of the locus coeruleus where their functions will need to be investigated (Lein et al., 2007). A second surprising result was that adiponectin signaling does not rely on AMPK phosphorylation or ceramidase catabolism to fulfill its functions in osteoblasts. Instead, our analyses reveal that one event triggered by adiponectin in osteoblasts is to decrease the activity of the transcription factor FoxO1. That removing one allele of FoxO1 in osteoblasts or neurons of the locus coeruleus from Adiponectin−/− mice sufficed to correct the bone phenotype or their high sympathetic activity, increased energy expenditure indicated that this molecular event is also critical for the local and central modes of action of adiponectin.
Using this information to identify an adiponectin-dependent signaling cascade in osteoblasts we noticed the frequent involvement of FoxO1 downstream of PI3 kinase signaling (Engelman, 2009; Taniguchi et al., 2006). In agreement with this notion, adiponectin treatment of osteoblasts increases PI3 kinase activity and phosphorylates AKT in addition to FoxO1. Moreover, adiponectin-induced AKT phosphorylation was prevented by inhibiting PI3 kinase activity. Lastly, adiponectin phosphorylates at least one membrane protein on tyrosine residues. We are fully aware that, in absence of a bona fide receptor, these results are only suggestive. This being acknowledged, they nevertheless point toward similarities between adiponectin signaling in osteoblasts and signaling through a receptor tyrosine kinase that deserve further study.
In summary this work provides evidence that adiponectin has specific functions in animals fed a normal diet. Whether the functions describe here are the only ones of adiponectin or not remains to be determined. These functions, however, identify adiponectin as a major regulator of bone mass and energy metabolism, and in both cases, as an antagonist of leptin. Hence, they reveal an unanticipated complexity in the endocrine function of the adipocytes and illustrate how tight is the central control of bone mass and energy expenditure (Figure 7H).
Experimental procedures
Mice generation
All analyses were performed with male mice except the bone histomorphometric analysis performed in 24 week-old Adiponectin−/− mice that included both males and females. Adiponectin−/−, AdipoR1fl/fl, AdipoR2fl/fl, and T-cadherinfl/fl mice were generated using 129/Sv ES cells. The estimated percentage of each genetic background was based on the number of backcrossing with C57BL/6J WT, α1(I) collagen-cre, or Dbh-cre mice. Chimeric mice harboring a mutant allele of Adiponectin were crossed with C57BL/6J WT, and Adiponectin+/− progenies (estimated as C57BL/6J:129/Sv; 50%:50%) backcrossed with C57BL/6J WT to obtain F2 generation Adiponectin+/− mice (C57BL/6J:129/Sv; 75%:25%). Analyses were performed with Adiponectin−/− and WT littermate mice obtained by intercrossing between F2 generation Adiponectin+/− mice. pLiv-Adiponectin construct was obtained by inserting full-length mouse Adiponectin cDNA into pLiv-7 plasmid (Fan et al., 1994). Transgenic mice were generated by injecting the pLiv-Adiponectin construct into 129/Sv embryos. pLiv-Adiponectin founder mice were crossed with C57BL/6J WT mice, and F1 generation pLiv-Adiponectin mice (C57BL/6J:129/Sv; 50%:50%) crossed with C57BL/6J WT to obtain pLiv-Adiponectin and WT littermates (C57BL/6J:129/Sv; 75%:25%) for analyses. Chimeric mice harboring a mutant allele of AdipoR1, AdipoR2, or T-cahderin (Figures S4A, S4E and S4I) were crossed with C57BL/6J WT mice, AdipoR1fl/+, AdipoR2fl/+, T-cadherinfl/+ mice (C57BL/6J: 129/Sv; 50%:50%), then crossed with C57BL/6J α1(I) collagen-cre mice to obtain F2 generation (C57BL/6J:129/Sv; 75%:25%). Analyses were performed with AdipoR1osb−/− and littermate AdipoR1fl/fl mice, AdipoR2osb−/− and littermate AdipoR2fl/fl mice, T-cadherinosb−/− and littermate T-cadherinfl/fl mice obtained from crosses between F2 generation AdipoR1osb+/− and AdipoR1fl/+, AdipoR2osb+/− and AdipoR2fl/+, T-cadherinosb+/− and T-cadherin fl/+ mice. Analyses of AdipoR1osb−/−;AdipoR2osb−/− were performed with AdipoR1osb−/−;AdipoR2osb−/− and littermate AdipoR1fl/fl;AdipoR2fl/fl obtained by intercrosses between F2 generation AdipoR1osb+/−;AdipoR2osb+/− mice (C57BL/6J:129/Sv; 75%:25%). Dbh+/− mice were previously reported (Thomas et al., 1995). Analyses of Adiponectin−/−; Dbh+/− mice were performed with littermate WT, Dbh+/−, Adiponectin−/− mice as controls generated by intercrosses between Adiponectin+/−; Dbh+/− mice (C57BL/6J:129/Sv; 87.5%:12.5%). ob/ob mice were obtained from Jackson laboratory. Analyses of Adiponectin−/−;ob/ob mice were performed with littermate WT, Adiponectin−/−, ob/ob mice as controls generated by intercrosses between Adiponectin+/−; ob/+ mice (C57BL/6J:129/Sv; 93.75%:6.25%). FoxO1osb+/− mice were previously reported (Rached et al., 2010). Analyses of Adiponectin−/−; FoxO1osb+/− mice were performed using littermate FoxO1fl/+ (Controls in figures), Adiponectin−/−;FoxO1fl/+ (Adiponectin−/− in the figures and manuscript) (C57BL/6J:129/Sv; 93.75%:6.25%) as controls. Analysis of FoxO1osb+/− mice were performed using FoxO1fl/+ littermates as controls (Pure C57BL/6J). FoxO1LC+/− mice were generated by crossing FoxO1fl/+ mice with Dbh-Cre transgenic mice (Kobayashi et al., 2004). Analyses of Adiponectin−/−; FoxO1LC+/− mice were performed using FoxO1fl/+ littermates and FoxO1LC+/−, Adiponectin−/−;FoxO1fl/+ (presented as Adiponectin−/− in the figure and manuscript) as controls. Normal diet and high-fat diet are obtained from LabDiet (Calories, Protein 24%, Fat 13%, Carbohydrates 62%) and Research Diets (Calories, Protein 16%, Fat 58%, Carbohydrate 25%) respectively. All procedures involving animals were approved by CUMC IACUC and conform to the relevant regulatory standards.
Binding assay
Adiponectin−/− mice were implanted subcutaneously with 14-day osmotic pump (Alzet) filled with a solution of recombinant full-length adiponectin or vehicle (5 μg/day). After 7 days of infusion, blood vessels were injected with PBS for extensive wash in order to remove remaining blood and brain regions were dissected. Tissues were homogenized in ice-cold PBS, soluble fractions were used to measure adiponectin. For binding assay, Adiponectin−/− brains were snap frozen in liquid nitrogen and 20 μm-thick section were prepared and desiccated overnight at 4C under vacuum. On the following day, sections were rehydrated in ice cold binding buffer (50 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 0.1mM EDTA and 0.1% BSA) for 15 min and incubated for 1 hour at room temperature in the presence of biotinylated adiponectin (2 μg/ml) or biotynylated GST (2 μg/ml) and in the presence of 50-fold excess of non-biotinylated adiponectin (100 μg/ml) or non-biotinylated GST (100 μg/ml). After washing in harvesting buffer (50 mM Tris-HCl, pH 7.4), samples were fixed in 4% paraformaldehyde for 15 min, washed in PBS and incubated with goat anti-biotin antibody (1:1000, Vector laboratories) and anti-DBH antibody (1:4000, Abcam) over night at 4 °C. Signal was visualized by incubating anti-goat IgG Cy-3 (1:200, Jackson immuno research) and anti-rabbit-Alexa488 (1:500, Invitrogen) using Leica DM 5000B microscope (Leica).
Statistical analyses
Results are given as means ± standard error of the mean (SEM). Statistical analyses were performed ANOVA and/or student t-test.
Supplementary Material
Highlights.
Adiponectin regulates bone mass through two mechanisms that oppose each other.
By acting centrally adiponectin opposes several functions of leptin.
The adipocyte synthesizes two hormones with opposite functions.
Adiponectin signals by decreasing FoxO1 activity in osteoblasts and neurons.
Acknowledgments
The authors thank Dr. S. Thomas and Dr. K. Kobayashi for Dbh−/− mice and Dbh-Cre mice respectively, Dr. J. Wei for its generous help. This work was supported by grant DK58883 from National Institutes of Health (G.K.).
Footnotes
Supplemental information includes six figures, additional experimental procedures and references.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci. 2002;27:352–360. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120:4342–4352. doi: 10.1172/JCI43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, Ogawa Y, Liu X, Ware SM, Craigen WJ, et al. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci U S A. 2004;101:3258–3263. doi: 10.1073/pnas.0308744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nature reviews Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Fan J, Wang J, Bensadoun A, Lauer SJ, Dang Q, Mahley RW, Taylor JM. Overexpression of hepatic lipase in transgenic rabbits leads to a marked reduction of plasma high density lipoproteins and intermediate density lipoproteins. Proc Natl Acad Sci U S A. 1994;91:8724–8728. doi: 10.1073/pnas.91.18.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Guntur AR, Rosen CJ. Bone as an endocrine organ. Endocr Pract. 2012;18:758–762. doi: 10.4158/EP12141.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BA, Goding JW, Alexander WS, Tilbrook AJ, Canny BJ, Dunshea F, Rao A, Mansell A, Clarke IJ. Central administration of leptin to ovariectomized ewes inhibits food intake without affecting the secretion of hormones from the pituitary gland: evidence for a dissociation of effects on appetite and neuroendocrine function. Endocrinology. 1999;140:1175–1182. doi: 10.1210/endo.140.3.6604. [DOI] [PubMed] [Google Scholar]
- Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty G, Oury F. Biology without walls: the novel endocrinology of bone. Annu Rev Physiol. 2011;74:87–105. doi: 10.1146/annurev-physiol-020911-153233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi M, Matsushita N, Miyazaki J, Koike M, Yaginuma H, Osumi N, Kaibuchi K. Survival of developing motor neurons mediated by Rho GTPase signaling pathway through Rho-kinase. J Neurosci. 2004;24:3480–3488. doi: 10.1523/JNEUROSCI.0295-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusminski CM, McTernan PG, Schraw T, Kos K, O’Hare JP, Ahima R, Kumar S, Scherer PE. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia. 2007;50:634–642. doi: 10.1007/s00125-006-0577-9. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Ma K, Cabrero A, Saha PK, Kojima H, Li L, Chang BH, Paul A, Chan L. Increased beta -oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem. 2002;277:34658–34661. doi: 10.1074/jbc.C200362200. [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- McCulloch CA, Strugurescu M, Hughes F, Melcher AH, Aubin JE. Osteogenic progenitor cells in rat bone marrow stromal populations exhibit self-renewal in culture. Blood. 1991;77:1906–1911. [PubMed] [Google Scholar]
- Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43:533–549. doi: 10.1007/s001250051341. [DOI] [PubMed] [Google Scholar]
- Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun. 2005;331:520–526. doi: 10.1016/j.bbrc.2005.03.210. [DOI] [PubMed] [Google Scholar]
- Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- Rached MT, Kode A, Xu L, Yoshikawa Y, Paik JH, Depinho RA, Kousteni S. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 2010;11:147–160. doi: 10.1016/j.cmet.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush RA, Geffen LB. Dopamine beta-hydroxylase in health and disease. Crit Rev Clin Lab Sci. 1980;12:241–277. doi: 10.3109/10408368009108731. [DOI] [PubMed] [Google Scholar]
- Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda Y, Yamaguchi M, Ogata N, Akune T, Kubota N, Yamauchi T, Terauchi Y, Kadowaki T, Takeuchi Y, Fukumoto S, et al. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem. 2006;99:196–208. doi: 10.1002/jcb.20890. [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117:375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nature reviews Molecular cell biology. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Tentolouris N, Liatis S, Katsilambros N. Sympathetic system activity in obesity and metabolic syndrome. Ann N Y Acad Sci. 2006;1083:129–152. doi: 10.1196/annals.1367.010. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- Vaira S, Yang C, McCoy A, Keys K, Xue S, Weinstein EJ, Novack DV, Cui X. Creation and Preliminary Characterization of a Leptin Knockout Rat. Endocrinology. 2012 doi: 10.1210/en.2012-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.