Abstract
Ambulatory locomotion in the rodent is robustly activated by unilateral infusions into the basal forebrain of type A gamma-aminobutyric acid (GABAA) receptor antagonists, such as bicuculline and picrotoxin. The present study was carried out to better localize the neuroanatomical substrate(s) underlying this effect. To accomplish this, differences in total locomotion accumulated during a 20 minute test period following bicuculline versus saline infusions in male Sprague-Dawley rats were calculated, rank ordered and mapped on a diagram of basal forebrain transposed from immunoprocessed sections. The most robust locomotor activation was elicited by bicuculline infusions clustered in rostral parts of the preoptic area. Unilateral infusions of bicuculline into the ventral pallidum produced an unanticipatedly diminutive activation of locomotion, which led us to evaluate bilateral ventral pallidal infusions, and these also produced only a small activation of locomotion, and, interestingly, a non-significant trend toward suppression of rearing. Subjects with bicuculline infused bilaterally into the ventral pallidum also exhibited persistent bouts of abnormal movements. Bicuculline infused unilaterally into other forebrain structures, including the bed nucleus of stria terminalis, caudate-putamen, globus pallidus, sublenticular extended amygdala and sublenticular substantia innominata, did not produce significant locomotor activation. Our data identify the rostral preoptic area as the main substrate for the locomotor activating effects of basal forebrain bicuculline infusions. In contrast, slight activation of locomotion and no effect on rearing accompanied unilateral and bilateral ventral pallidal infusions. Implications of these findings for forebrain processing of reward are discussed.
Keywords: lateral preoptic area, ventral pallidum, substantia innominata, dopamine, reward, GABAA receptor antagonist
Introduction
It was shown years ago that ambulatory locomotion in the rodent is robustly activated by unilateral infusions of picrotoxin, a type A gamma-aminobutyric acid (GABAA) receptor antagonist, into the basal forebrain (Mogenson and Nielsen, 1983; Mogenson et al., 1985). Whereas much of the ensuing literature attributed this and related effects of various GABAergic, glutamatergic and peptide receptor ligands to actions in the lateral preoptic area, ‘subpallidal region’ and substantia innominata (Mogenson et al., 1983;1985; 1993; Shreve and Uretsky, 1988; 1989; 1991; Mogenson and Yang, 1991), other influential papers (Austin and Kalivas, 1989; 1990; 1991; Kalivas et al., 1991; Johnson et al., 1996) appear to have led to a consensus view (see, e.g., Willens et al., 1992; Gong et al., 1999; Johnson and Napier, 2000; Chen et al., 2001; June et al., 2003; Hubert et al., 2010) that the effective structure is the then relatively recently described ventral pallidum (Heimer et al., 1972; Heimer and Wilson, 1975; Switzer et al., 1982). Ventral pallidum (VP) since has been implicated in reward mechanisms (Johnson et al., 1996; Tindell et al., 2004; 2005; 2006), control of population activity of midbrain dopaminergic neurons (Floresco et al., 2003) and transmission to midbrain dopaminergic neurons of signals originating in the hippocampus (Lodge and Grace, 2006). Almost exclusively bilateral drug infusions have been used to elicit locomotion from the VP, such that subsequent to the earlier reports of Mogenson and his colleagues (Mogenson and Nielsen, 1983; Mogenson et al., 1985) the literature seems to convey that forebrain-elicited locomotor activation requires bilateral infusions.
During the process of formulating our recent report on robust locomotor activations elicited by unilateral infusions of bicuculline, another GABAA receptor antagonist, into the preoptic area (Reynolds et al., 2006) we noticed that a number of early studies also mapped their effective infusion sites more prominently to the preoptic area than ventral pallidum (Mogenson and Nielsen, 1983; Shreve and Uretsky, 1988; 1989; 1991; Willens et al., 1992). Consequently, we did the present study in order to revisit the localization of basal forebrain site(s) most effective in producing locomotor activation.
Materials and Methods
Animals
Male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 225–250g were used in accordance with policy mandated in the Public Health Service Policy on Humane Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/references/phspol.htm) provided by the U.S. Department of Health and Human Services. The rats were housed on a 12-hour light-dark cycle in groups of two to four until surgeries were done, after which all were singly housed. Access to food and water was provided ad libitum.
Immunohistochemistry
Rats were anesthetized with a 1.6 ml/kg i.p. injection of a solution containing ketamine (100 mg/ml), xylazine (20 mg/ml) and saline (0.9%) at a volume ratio of 9:7:4, resulting in final doses of ketamine and xylazine of 72 and 11.2 mg/kg, respectively. Under deep anesthesia, the rats then were killed by vascular perfusion with a solution of 0.1 M Sorenson’s phosphate buffer (SPB, pH 7.4) containing 4.0% paraformaldehyde and 2.5% sucrose, preceded by a brief rinse with 0.01 M SPB-buffered saline containing 0.5% procaine and 2.5% sucrose. The brains were removed immediately and stored for at least four hours in the fixative solution, and then transferred to SPB and, after at least an hour, were sunk in SPB containing 30% sucrose. Then, frozen sections at 50 μm were taken with a sliding microtome. The sections were stored at −20° C in a solution of 0.1 M SPB containing 30% ethylene glycol and 30% sucrose pending further processing.
Sections were retrieved from cryoprotectant, rinsed in SPB and treated for 15 minutes in 1% sodium borohydride after which they were subjected to additional multiple rinses in SPB to remove all traces of sodium borohydride. The sections were then immersed at room temperature with agitation into SPB containing 0.1% triton X-100 (SPB/triton) and antibodies against parvalbumin (PV) or nitric oxide synthase (NOS), which in previoius studies done in our laboratory were revealed as excellent markers of basal forebrain neuroanatomical organization (Zahm et al., 2003; 2011; 2013). The following morning the sections were rinsed in SPB and immersed for 1.5 hours in SPB/triton containing biotinylated anti-mouse antibodies made in horse (Vector, Burlingame, CA, used at a dilution of 1:200), again rinsed, and then placed for 1–2 hours in SPB/triton containing avidin-D/biotinylated horseradish peroxidase complex (ABC, Vector, used at a dilution of 1:200). After further rinses, the sections were reacted in one of two ways. For preparations in which the intent was to reveal only PV (n=5) or NOS (n=5) immunoreactivity, DAB chromogen was generated with the aid of a coupled glucose oxidase reaction (Itoh et al., 1979). Briefly, sections were placed in a solution of 0.05 M SPB containing DAB (0.05%), β-D-glucose (0.2%), NH4Cl (0.04%), and 0.0004% glucose oxidase (Sigma) for 20–40 minutes after which they were rinsed in distilled water and mounted in serial order on glass slides. Then the DAB reaction product was intensified by placing the slides sequentially through 0.005% aqueous OsO4 and aqueous thiocarbohydrazide (0.1 mg/100ml H2O) followed by a second immersion in the osmium solution, with brief rinses between. Sections reacted with PV antibodies and intended to undergo further processing to reveal immunoreactivity against calbindin-D 28 kD (CB, n=3), another good marker of basal forebrain organization (Zahm, 1999), were instead reacted in a solution containing 15 mg of DAB dissolved in 42 ml of distilled water to which was added 8 ml of 5% aqueous nickel ammonium sulfate, 50 ml of 0.05 M Tris-HCl buffer (pH 8.0) and 20 μl of 3% H2O2. After ten minutes, these sections were rinsed and then placed overnight in SPB/triton containing mouse anti-CB antibodies. The following morning they were rinsed in SPB/triton and immersed in SPB containing unconjugated biotin (Sigma, at a concentration of 0.01%) for 1 hour in order to block unreacted biotin sites on avidin already present in the sections. After further rinsing in SPB, the sections were again immersed for 1–2 hours at room temperature in SPB containing biotinylated anti-mouse IgGs made in horse (Vector, used at 1:200) and, after further rinsing, in SPB containing ABC reagents (Vector, used as described above). The sections were again rinsed three times in SPB prior to being reacted with DAB using the coupled glucose oxidase method as described above, after which they were rinsed and mounted in serial order on glass slides. Mounted sections were coverslipped with Permount (Fisher, St. Louis, MO).
Primary antibodies and specificity controls
Omission of primary antibodies produced tissue lacking specific immunoreactivity. Where the antigen was available to us, we did preabsorbtion controls (see below under anti-NOS). For anti-CB and anti-PV, we relied on information provided by the vendors regarding primary antibody specificity (see below). Patterns of immunostaining generated by these antibodies in our hands are consistent with those published in the literature (e.g., for anti CB and anti-PV - Celio and Heizmann, 1981, Gerfen et al., 1985, Cowan et al., 1990; for anti-NOS - Rodrigo et al., 1994). Additional information on the characterization of the antibodies and specificity controls is given below.
anti-CB
This is a monoclonal antibody raised against purified bovine kidney calbindin-D 28 kD and isolated from mouse ascites fluid (Sigma Chemical Company, St. Louis, MO, cat. # C9848) The vendor states that [1] the antibody does not react with other members of the EF-hand family such as calbindin-D 9K, calretinin, myosin light chain, parvalbumin, S-100a, S-100b, S100A2 (S100L) and S100A6 (calcyclin); [2] species cross-reactivity was observed with human, bovine, goat, sheep, porcine, rabbit, dog, cat, guinea-pig, rat and mouse; [3] a weaker reactivity was observed with chicken CB.
anti-NOS
This is a polyclonal antibody raised in rabbit against amino acids 251–270 of nitric oxide synthase (GDNDRVFNDLWGKDNVPVILC) conjugated to keyole limpet cyanin (Sigma, cat. # N7155). NOS immunoreactivity was abolished in our hands by preabsorption with the cognate peptide (10 μg/ml).
anti-PV
This is a monoclonal antibody raised against purified carp muscle parvalbumin and isolated from mouse ascites fluid (Sigma, clone PA-235, cat. # P3088). The vendor states that [1] the antibody is immunospecific for parvalbumin as determined by indirect immunoperoxidase staining and immunoblotting; [2] the antibody reacts specifically with parvalbumin of cultured nerve cells and tissue originating from human, monkey, rat, mouse, chicken and fish; [3] it specifically stains the 45Ca-binding spot of parvalbumin (M.W. 12,000, pI of 4.9) by immunobinding.
Maps and photomicrographs
Basal forebrain territories were photographed (Fig. 1A–D) and mapped (Fig. 1E and F) according to patterns of immunohistochemical staining in representative frontal sections. Mapping was done under brightfield optics (Olympus BX51) with the aid of the Neurolucida dedicated hardware-software platform (MFB Bioscience, Williston, VT). Digital micrographs were captured using a Nikon Optiphot microscope and Q Imaging Fast 1394 digital camera and adjusted for brightness and contrast with Adobe Photoshop (CS2) software. The plates were constructed using Adobe Illustrator (CS2) software.
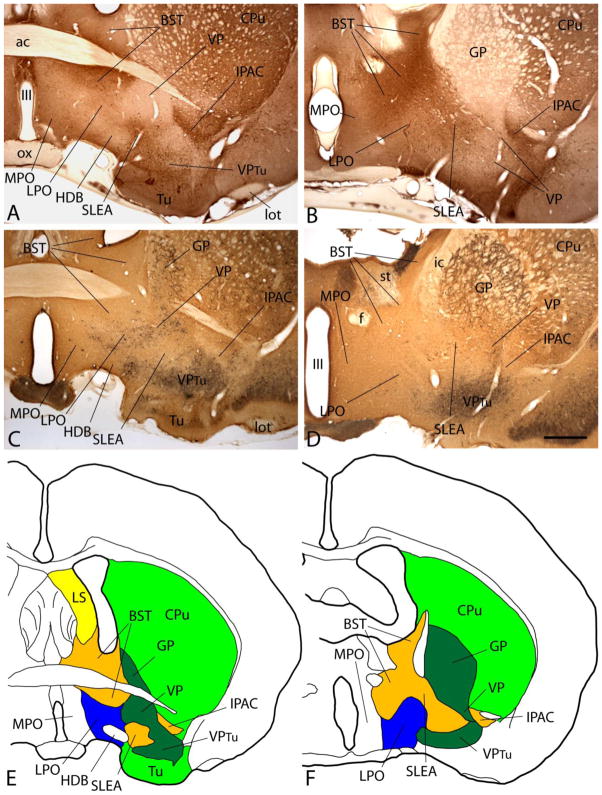
Figure 1.
A–D: Photomicrographs illustrating sections through the basal forebrain processed to exhibit nitric oxide synthase (NOS, A and B) or parvalbumin and calbindin-D 28kD (PV and CB, C and D) immunoreactivity (-ir). B and D are cut at a more caudal level than A and B. Note that NOS-ir is strong in extended amygdala stuctures such as the bed nucleus of stria terminalis (BST) and interstitial nucleus of the posterior limb of the anterior commissure (IPAC) and weak in the ventral pallidum (VP). Lateral preoptic area (LPO) exhibits an intermediate density of NOS-ir. PV-ir neurons (black dots) in C and D occupy the VP and, to lesser extent, LPO. E and F: The immunohistochemical patterns in A and B and C and D were used as a basis to draw the maps reflecting the respective levels of the forebrain in E and F. Scale bar: 1 mm for all photomicrographs. Additional abbreviations: ac - anterior commissure; CPu - caudate-putamen; f - fornix; GP - globus pallidus; HDB - horizontal limb of the diagonal band; ic - internal capsule; lot - lateral olfactory tract; LS - lateral septum; MPO - medial preoptic area; ox - optic chiasm; SLEA - sublenticular extended amygdala; st - stria terminalis; Tu - olfactory tubercle; VPtu - ventral pallidum in the deep layer of the olfactory tubercle; III - third ventricle.
Placement of guide cannulae
Rats were deeply anesthetized as described above and placed in a Kopf stereotaxic instrument. An incision was made to expose the skull in which a small burr hole was drilled in line with the intended injection site. Guide cannulae, consisting of 13 mm lengths of 22 gauge stainless steel tubing (PlasticsOne, Roanoke, VA) were implanted in the openings. Unilateral guide cannulae aimed at the preoptic area (n=48) or other basal forebrain structures, including the bed nucleus of stria terminalis (BST, n=6), sublenticular extended amygdala (SLEA, n=7), caudate-putamen (CPu, n=5), sublenticular substantia innominata (SLSI, n=5) and globus pallidus (GP, n=5), were implanted at an angle of 20° in the mediolateral plane so as to avoid the lateral ventricle. Unilateral guide cannulae aimed at the ventral pallidum (n=13) were implanted mainly vertically. Another group of rats was implanted with bilateral guide cannulae aimed vertically at the ventral pallidum on both sides of the midline (n=30). A final group of rats was implanted with a single vertical guide cannula targeting the left lateral ventricle (n=3). All guide cannulae were set to a depth 2 mm short of the center of the intended target. Cannulae were secured with dental cement anchored to stainless steel screws turned into the skull. Stainless steel wire obturators were inserted into the guide cannulae to maintain patency. The incisions were closed with cyanoacrylate glue and the rats were kept warm until they awakened. A solution containing lidocaine was infused into the incisions before they were closed. The rats were given an i.p. injection of Rimadyl (carprophen, 10 mg/kg) before they awoke and on each of the next three days.
Behavioral testing
Three days after cannulae implantations and at least 20 minutes prior to testing, the rats were brought to the behavioral studies room, where locomotion was measured in standard activity monitoring chambers, each comprising a square, white floor space (43.2 × 43.2 cm) bounded on the sides by clear plexiglass walls 30.5 cm in height (Med Associates, St. Albans, VT). Fixtures containing horizontal rows of 16 point-source infrared illuminators and detectors spaced at 2.54 cm were bracketed to the walls on opposite sides of the chambers at heights of 2.54 and 12.7 cm from the floor. The lower of these comprised two sets of illuminators and detectors mounted on adjacent walls at a right angle to each other so as to form a grid of 16 × 16 intersecting beams for measurement of horizontal (forward) locomotion, whereas the upper consisted of a single illuminator/detector pair, which detected vertical locomotion (rearing). Beam breaks were evaluated with the aid of Activity Monitor software (Med Associates). The monitoring chambers were housed inside dimly illuminated sound attenuating chambers with exhaust fans running (Med Associates). Prior to being placed in the activity monitoring chambers, the rats were removed from the home cages, the obturators were removed and injector cannulae connected by a small length of polyethylene tubing (PE 20 0.38/1.09, Stoelting, Wood Dale, IL, cat. # 51155) to the Luer needle of a 1.0 μl Hamilton syringe were inserted into the guide cannulae. To mitigate the resistance of brain tissue at the injector tip to the flow of infusate and thus facilitate its uniform spread into the targeted area, a mock injector extending 2 mm past the ends of the guide cannulae was inserted briefly and then removed after which the actual injection cannula was inserted and advanced 1.5 mm past the ends of the guide cannulae. On all subsequent trials only the injector cannula was inserted and always advanced 1.5 mm past the guide cannula. The infusate was propelled by a syringe pump (Harvard Apparatus, Holliston, MA, model ‘11’ plus) calibrated to eject solution at a rate of 0.25 μl/min.
The infusions were done mainly using groups of six rats of which each was first infused with saline for 1 minute at a rate of 0.25 μl/minute. One minute after the infusions were completed, the rats were placed in the locomotor apparatus and beam breaks were monitored for a period of 20 minutes, after which the rats were returned to their home cages. After about a half hour rest, the rats were again infused, this time with 0.25 μl of saline containing 1(S),9(R)-(−)-bicuculline methbromide (Sigma, St. Louis, MO) prepared at a dilution of 1 mg bicuculline / 3 ml saline, which resulted in the delivery of 67 ng (0.18 nmol) of bicuculline. After 1 minute, the rats were placed in the activity chambers and monitored for 20 minutes as before. The concentration of bicuculline infused in the present study produced the most robust locomotor activation in our previous investigation (Reynolds et al., 2006).
Fold difference analysis and mapping of locomotion
In order to localize sites in the forebrain from which locomotor activation was most effectively elicited by bicuculline infusions, bicuculline-elicited responses were expressed in relation to those elicited by immediately preceding saline infusions (see preceding section) as fold differences [beam breaks/20 minutes following bicuculline infusion]/[beam breaks/20 minutes following saline infusion]. Fold differences were rank ordered as follows: 1 = 0–1.49 fold; 2 = 1.5–2.49 fold; 3 = 2.5–3.49 fold; 4 = > 3.5 fold; and the ranks were coded in symbols and mapped (see Results). Where more than one infusion was tested at a given site (see Table 1), the maximum locomotor response to bicucullline at that site was used to prepare the map.
Table 1.
Fold changes in horizontal locomotion associated with bicuculline as compared to saline infusions
| LPO-MPO | |||
|---|---|---|---|
| 10111 - 3.3 | 11257 - 6.19, 0.34 | 12014 - 0.82, 3.17, 0.85 | 12058 - 1.53, 3.35, 0.42 |
| 10112 - 7.7 | 11258 - 5.13, 4.87 | 12017 - 1.32, 2.02, 1.24 | 12059 - 3.16, 1.55, 0.05 |
| 10117 - 1.41 | 11259 - 0.91 | 12018 - 1.03, 1.86, 1.91 | 12060 - 0.61, 4.35, 0.4 |
| 10120 - 3.31 | 11272 - 0.87, 6.6 | 12021 - 4.01, 6.3, 2.46, 3.49 | 12061 - 4.41, 3.65, 0.33 |
| 10121 - 0.95, 0.82, 0.92 | 11273 - 2.79, 1.73, 0.44 | 12022 - 2.8, 1.08, 3.93, 3.87 | 12062 - 4.95, 4.4, 1.09 |
| 10124 - 1.11, 0.81, 1.59 | 12001 - 0.48, 1.11 | 12023 - 4.73, 3.25, 2.45, 1.3 | 12063 - 9.11, 6.14, 3.48 |
| 10130 - 2.42 | 12002 - 0.63, 6.33 | 12024 - 10.56, 8.21, 5.75, 4.01 | 12064 - 7.72, 2.11, 6.58 |
| 10135 - 5.13 | 12009 - 1.19, 3.34 | 12028 - 3.04, 6.18 | 12065 - 4.26, 1.99, 3.95 |
| 11250 - 9.6, 2.4 | 12010 - 1.53, 3.78 | 12031 - 3.87, 9.7, 3.39, 6.4 | 12066 - 4.54, 3.73, 3.25 |
| 11251 - 4.4, 0.99 | 12011 - 7.04, 7.4 | 12032 - 1.86, 3.25, 1.23, 7.22 | 12067 - 7.97, 2.65, 0.74 |
| 11256 - 1.91, 3.1 | 12012 - 5.05, 3.37 | 12057 - 1.16, 3.52, 0.31 | 12098 - 8.94 |
| VP | BST | SLEA | |
|---|---|---|---|
| 10110 - 0.74 | 13036 - 1.71, 1.48, 1.07 | 10113 - 0.9, 0.95 | 10114 - 0.51 |
| 10116 - 1.53 | 13037 - 2.08, 2.29, 1.8 | 10131 - 0.33, 1.01 | 10115 - 0.67 |
| 10132 - 2.82 | 13038 - 0.049, 0.087 | 12006 - 1.14 | 10133 - 0.48 |
| 12096 - 0.59 | 13042 - 1.2, 1.43 | 12099 - 0.46 | 13047 - 1.6, 1.37 |
| 12097 - 1.44 | 13043 - 0.43, 0.98 | 12101 - 1.09 | 13049 - 1.48, 1.34 |
| 12100 - 1.07 | 13048 - 1.98, 1.6 | 13041 - 0.76, 1.02 | 13050 - 1.38, 2.62 |
| 13029 - 1.04, 2.56, 2.47 | 13052 - 1.65, 2.55 |
| CPu | Other | |
|---|---|---|
| 12005 - 0.95, 0.85, 1.09 | 11289-VDB - 2.3, 0.79 | 12037-SLSI - 0.67, 1.19 |
| 13021 - 0.72, 0.64 | 11290-VDB - 0.95, 0.54 | 13023-GP - 1.14, 1.33 |
| 13024 - 0.72, 0.64 | 12027-VDB - 5.27, 4.08 | 13030-GP - 1.88, 2.9, 3.24 |
| 13025 - 0.63, 1.31 | 12033-SLSI - 1.07, 1.41, 1.48, 0.67 | 13035-GP - 1.74, 1.79, 1.94 |
| 13039 - 0.62, 1.65 | 12034-SLSI - 0.3, 3, 0.15, 0.82 | 13040-GP - 0.95, 1.37 |
| 12035-SLSI - 2.36, 2.36 | 13051-GP - 1.33, 2.1 | |
| 12036-SLSI - 2.5, 0.89 |
Case numbers are given to the left in each column. Each case number is followed by one or several fold values, each reflecting a separate comparison of saline-and bicuculline-elicited of locomotion. Fold difference is [beam breaks/20 minutes following bicuculline infusion]/[beam breaks/20 minutes following saline infusion] (see Methods). Increases of 3.0 or greater are bolded and underscored for emphasis. Structures in which infusions were made can be referenced in Figures 1E and F and 2.
Abbreviations: BST - bed nucleus of stria terminalis; CPu - caudate-putamen; GP - globus pallidus; LPO-MPO - lateral and medial preoptic area; SLEA -sublenticular extended amygdala; SLSI - sublenticular substantia innominata; VDB - vertical limb of the diagonal band; VP ventral pallidum
Localization of infusions
The rats were deeply anesthetized and perfused precisely as described above. The perfused brains were removed and placed in the same fixative for at least four hours and then overnight at 4° C with agitation in distilled H2O containing 30% sucrose. Five adjacent series of 50 μm thick sections were cut on a cryostat of which the first was thaw mounted on subbed glass slides and the rest were collected and stored in separate glass vials at −20° C in cryoprotectant consisting of 0.1 M SPB containing 30% sucrose and 30% ethylene glycol. The mounted sections were defatted in Coplin jars in 1:1 alcohol/chloroform for at least four hours, immersed in 0.1% cresyl violet solution for 5–10 minutes, rinsed quickly in distilled water, differentiated in 95% ethyl alcohol for 2–30 minutes, dehydrated in 100% alcohol 2×5 min, cleared in xylene 2×5 minutes and coverslipped with Permount. The ends of the infusion cannula tracts were identified by light microscopy with the 2x objective and plotted visually onto maps prepared by reference to the immunohistochemical preparations (see Figs. 1 and 2).
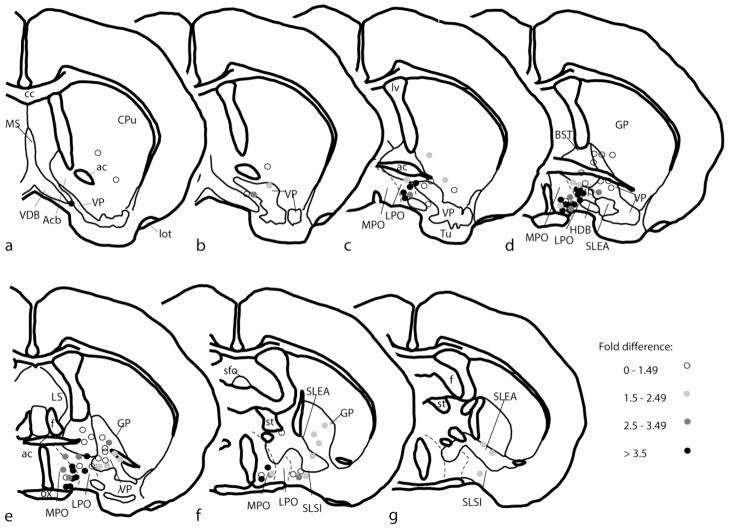
Figure 2.
A–G. Diagrams of a series of frontal sections ordered from rostral to caudal that were drawn on the basis of immunohistochemical observations as illustrated in Fig. 1. Round symbols indicate the positions of the tips of ejection cannulae from which bicuculline and saline were infused into the brain to generate data illustrated in Fig. 3 and Tables 2 and 3. The symbols are coded such that increasing density of gray tone indicates increasingly robust locomotor responses to infusion of bicuculline. Fold difference refers to the amount of distance traversed in an activity chamber according to the following relationship: [beam breaks/20 minutes following bicuculline infusion]/[beam breaks/20 minutes following saline infusion]. Structures and abbreviations are as provided in Fig. 1 legend.
Results
Anatomy
Fig. 1A and B reveals that NOS immunoreactivity (-ir) is moderately to heavily expressed in extended amygdala structures, including the bed nucleus of stria terminalis (BST), interstitial nucleus of the posterior limb of the anterior commissure (IPAC) and sublenticular extended amygdala (SLEA). In contrast, NOS-ir (brown in Fig. 1A and B) is nearly absent in the ventral pallidum (VP) and has an intermediate level of expression in the lateral preoptic area (LPO). Material processed to exhibit PV-ir (black in Fig. 1C and D) reveals boundaries between the LPO, extended amygdala structures and ventral pallidum similar to those shown by NOS-ir. PV-ir neurons are numerous in striatopallidal structures, particularly VP (Fig. 1C), and, to a lesser degree, LPO. Fig. 1C and D also show that CB-ir (brown) is less heavily expressed in extended amygdala structures, as compared to striatopallidal. In view of the coherence of these several patterns of immunoreactivity, which is consistent with much literature cited in the Introduction, Materials and Methods and Discussion, they were used in the present study to aid in the generation of diagrams showing boundaries between basal forebrain structures, representative examples of which are shown in Figures 1E and F. Such diagrams were utilized to localize bicuculline infusion sites (Fig. 2).
Locomotor activation and localization of effective infusion sites
Unilateral infusions of bicuculline into the basal forebrain often were followed by some degree of activation of locomotion and rearing, but also often did not affect locomotion or, occasionally, diminished it, as compared to a preceding saline infusion. The variability of the effects of these infusions was such that infusions near each other frequently produced disparate effects on locomotion (Fig. 2), as did infusions into the same site in different sessions (Table 1). Rats exhibiting bicuculline-elicited locomotor activation had normal posture and perfectly coordinated movements even when, occasionally, they were so active as to escape the 12″ (30.5 cm) high plexiglass walls of the recording chamber. They never behaved as if perturbed or anxious or exhibited obvious fearfulness, as might be reflected in burrowing beneath the cage bedding or engaging in compulsive burying behavior, or aggressiveness, as might be reflected in piloerection and aggressive biting. Nor did they give the impression of ‘exploring’, insofar as the locomotion of rats responding to an initial bicuculline infusion was no different than that of rats well-habituated to the activity monitor that had received activating infusions in multiple test sessions.
The most robust locomotor activations elicited by unilateral bicuculline infusions occupied a cluster centered over the rostral parts of the LPO and medial preoptic area (MPO), which, to denote this, hereafter will be referred to in this paper as LPO/MPO, despite their known structural and functional differences. Mann-Whitney rank sum tests revealed that the ranks (Fig. 2) and actual fold difference values (Table 1) for infusions into the LPO/MPO (n = 48) were significantly greater (P=<0.001 for ranks and fold difference values) than corresponding values acquired by combining infusion sites from all of the other evaluated forebrain structures (n = 48). Moreover, analysis using the Kruskal-Wallis one-way ANOVA on ranks with post hoc comparisons by Dunn’s test revealed that the ranks and actual fold difference values associated associated with bicuculline infusions into LPO/MPO were significantly greater (P = <0.001) than those associated with infusions into VP (n=13), extended amygdala (n = 13) and pooled remaining structures (e.g., CPu, GP, SLSI et al., n = 22). Due to the fact that more infusions were assessed in some cases than others, a possibility exists that sampling bias or cumulative effects of multiple infusions of bicuculline influence the comparison. However, the same statistical tests using only first infusions from each case (first of values listed in Tables 1 and 2) also revealed significantly different fold difference values for LPO vs. all other tested structures (P=<0.001) and LPO vs. VP (p<0.003).
Table 2.
Bilateral infusions of bicuculline: locomotion and abnormal movements
| Case | Infusion sites | Abnormal movements | Fold differences in sequential trials | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Right side | Left side | pivoting | 1 | 2 | 3 | 4 | 5 | 6 | ||||
| gnawing | ||||||||||||
| grasping | ||||||||||||
| galloping | ||||||||||||
| A. | ||||||||||||
| 11017 | lVP | lVP, VPtu | 0.11 | |||||||||
| 11018 | vmCPu | vmCPu | 0.69 | |||||||||
| 11038 | vBST, VP | vmCPu, dVP | X | X | 0.39 | 0.36 | ||||||
| 11039 | VP | vmCPu, dVP | 1.53 | 1.30 | ||||||||
| 11053 | VP | VP | X | X | 5.95 | 2.90 | ||||||
| 11063 | Acb, VP, CPu | VP, VPtu | X | X | 1.42 | 0.51 | 0.38 | |||||
| 11068 | VPtu | mTu (Str&VP) | 2.39 | 1.78 | 1.37 | |||||||
| 11070 | lVP,VPtu | VP, VPtu | 0.94 | 1.85 | 2.05 | |||||||
| 11231 | CPu, lVP | VP | 4.37 | 1.57 | ||||||||
| 12044 | VP | VP, VPtu | X | X | 0.93 | 1.60 | 1.30 | |||||
| 12045 | VP | VP | X | X | 1.02 | 0.87 | 0.67 | |||||
| 12047 | VP, CPu | VP | X | X | 1.43 | 1.51 | 1.07 | |||||
| 12048 | VP, VPtu | vCPu, VP | X | X | 0.95 | 2.58 | 2.21 | |||||
| 12049 | vmCPu, VP | vmCPu, VP | X | X | 2.41 | 2.95 | 3.96 | |||||
| 13094 | vmCPu, VP | vmCPu, VP | X | X | X | 0.83 | 2.35 | 1.80 | 3.48 | 1.39 | 0.10 | |
| 13096 | vGP, VP | vGP, VP | X | X | 1.04 | 0.68 | 2.55 | 2.46 | 0.06 | |||
| 13097 | VP | VP | X | X | 0.92 | 0.88 | 1.58 | 0.40 | 0.17 | 0.14 | ||
| 13098 | VP | VP | X | X | 0.62 | 0.83 | 2.09 | 2.04 | 0.06 | 0.23 | ||
| 13106 | VP | VP | X | 1.53 | 0.88 | |||||||
| 13107 | VP | VP | X | X | 1.77 | 1.76 | ||||||
| 13109 | VP | VP | X | X | 0.68 | 0.77 | ||||||
| 13110 | VP | VP | X | X | 0.45 | 0.66 | ||||||
| B. | ||||||||||||
| 11052 | VP, LPO, vBST | VP, LPO | X | X | 2.12 | 2.99 | ||||||
| 11062 | VP, LPO | clVP | 4.82 | 10.7 | 2.82 | 4.59 | ||||||
| 11066 | clVP, HDB | cmVP, LPO | X | X | 0.98 | 4.29 | 4.50 | |||||
| 11067 | vCPu, cAcb | VP, cmCPu, BST | X | X | 0.63 | 0.28 | 1.77 | 1.34 | ||||
| 11069 | lVP, VPtu | mTu (Str), LPO | X | X | 3.47 | 6.73 | 4.34 | |||||
| 11230 | VP, LPO | CPu | X | X | 1.17 | 6.18 | ||||||
| 11232 | VP, vCPu, BST | VP, vCPu, BST | 3.47 | 3.77 | ||||||||
| 12046 | VP, LPO | VP | X | X | 1.04 | 2.84 | 4.94 | |||||
A. Cases (not included in Table 1) in which infusion sites involved the ventral pallidum (VP) and other indicated structures, but not the lateral preoptic area (LPO), on both sides of the midline. Abnormal movements are as described in the the text. Fold difference is beam breaks/20 minutes following bicuculline infusion]/[ beam breaks/20 minutes following saline infusion] (see Methods). Fold difference values more than 3.0 are bolded and undescored for emphasis.
B. Cases in which the infusion site on at least one side of the midline involved the LPO or transition area between the VP and LPO.
Note the greater density of bolded entries in section B.
Additional abbreviations: Acb - accumbens; BST - bed nucleus of stria terminalis; c - caudal; CPu - caudate-putamen; GP - globus pallidus; HDB - horzontal limb of the diagonal band; m - medial; l - lateral; Str - striatal, as in the striatal part of the olfactory tubercle; VPtu - ventral pallidum occupying the deep layer of the olfactory tubercle
Unilateral infusions into the LPO/MPO and ventral pallidum
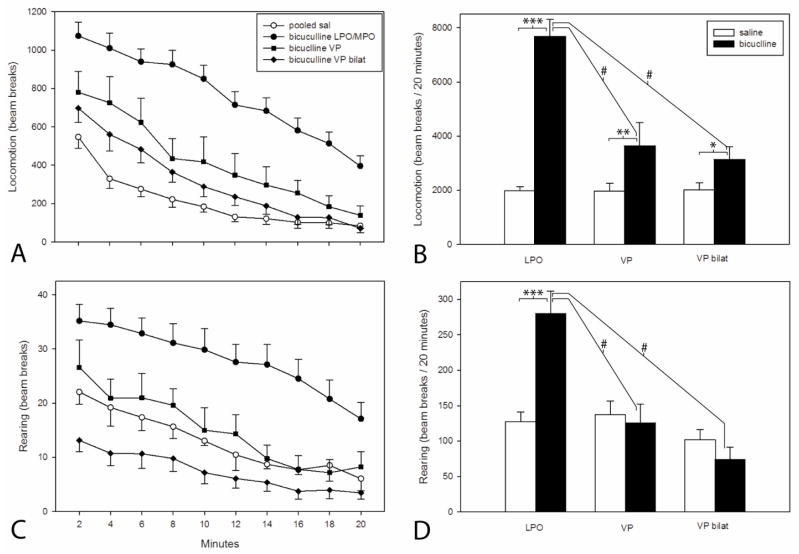
To strengthen the analysis, a comparison of total beam breaks elicited during the 20 minute test period by bicuculline and saline (Fig. 3) using paired t tests revealed that significantly greater locomotion was elicited by bicuculline than saline infusions into the LPO/MPO (n= 48, P=<0.001) and VP (n=13, P = <0.025). Additionally, a Kruskal-Wallis one-way ANOVA on ranks with post hoc comparisons by Dunn’s test revealed that significantly greater locomotion was elicited by bicuculline in the LPO/MPO than VP (P = <0.001). Interestingly, rearing was significantly increased in association with unilateral bicuculline infusion in the LPO/MPO (P = <0.001), but not VP (Fig. 3B, see also 3D). Unilateral infusions of bicuculline into other forebrain structures, including the bed nucleus of stria terminalis, sublenticular extended amygdala and caudate-putamen did not increase locomotion relative to saline (Table 1).
Figure 3.
A and C: Plots illustrating ambulatory locomotor activation following infusion of bicuculline hydrobromide (67 ng/0.25 μl) into the lateral preoptic area (LPO), ventral pallidum unilaterally (VP) and bilaterally (VP bilat) and an equivalent volume of saline into the same structures. In the absence of differences between groups, the data for saline infusions into LPO, VP and VP bilat were pooled. After the infusions, the cannulae were left in place for one minute and then the rats were placed immediately into the activity monitors. Bins are 2 min in duration. B and D: Bar graphs reflecting total locomotion reflected in the respective adjacent line/scatter graphs (A and C). Pairs of bars were tested with paired t-tests and the bicuculline infusions were tested with a Kruskal-Wallis one-way ANOVA on ranks with post hoc comparisons by Dunn’s test. Paired t-test: *** P=<0.001, ** P=0.025, * P=0.017. Kruskal Wallis test with Dunn’s post hoc comparison: # P=<0.001.
Bilateral infusions into ventral pallidum
Bilateral infusions of bicuculline into the ventral pallidum produced moderate activation of horizontal locomotion (Fig. 3A and B) similar to that observed following unilateral VP infusions and, interestingly, a non-significant trend toward a reduction in rearing (Fig. 3D, see also Fig. 3C). Furthermore, rats that received bilateral infusions of bicuculline into the VP exhibited abundant abnormal movements consisting of repetitive bouts of compulsive rotatory locomotion, comprising one to several full rotations in the form of tight pivots around one of the hindlimbs (Table 2). When observed, pivots in one direction might be followed momentarily by a series of pivots in the same or opposite direction. Rats that exhibited pivoting movements also engaged in persistent, non-aggressive, gentle gnawing of accessible objects, such as, e.g., the handler’s lab coat or finger or, during pivots, the subject’s own tail. In two cases, rats with bilateral VP infusions of bicuculline compulsively grasped with the digits of both forepaws at buttons on the investigator’s lab coat. In a single case, the rat adopted a gait abnormality in which the forepaws and hindpaws were not used alternately, but rather were engaged symmetrically, resulting in a hopping or ‘galloping’ forward progression. Abnormal movements were observed in 73% of cases in which infusion sites on both sides of the brain involved the VP (Table 2), but only one case of a total of 96 in which unilateral infusions were done and in only 2 cases with bilateral infusions of which only one substantially involved the VP. Series of bilateral infusions of bicuculline into the bed nucleus of stria terminalis (n=6) and caudate-putamen (n=6) done in a separate study (data not shown) produced no abnormal movements. Importantly, if one of the intended bilateral VP sites was misplaced such that it partially occupied the VP, but also occupied or bordered the LPO/MPO, robust locomotor activation was likely to be observed and accompanied by abnormal movements (Table 2, section B).
Bicuculline infusions into the lateral ventricle
When tested with a one-way ANOVA following infusions into the lateral ventrical (n=3), total horizontal and vertical ambulatory counts for bicuculline (1509±235 and 49±10, respectively), although not significantly different from saline (1930±394 and 127±37), trended downward, which is opposite of the result observed following infusions of bicuculline into the LPO/MPO.
Discussion
Anatomy
At the time when basal forebrain-elicited locomotor activation was first observed (see references in the Introduction), the concept of a ‘ventral striatopallidum’ was relatively new (Heimer et al., 1972; Heimer and Wilson, 1975; Switzer et al., 1982), as was a companion conceptualization, the extended amygdala (de Olmos and Ingram, 1972; Alheid and Heimer, 1988). These novel ‘functional-anatomical’ entities, founded on the basis of sound cytoarchitectural, histochemical, immunohistochemical and connectional criteria in rodents and primates (Heimer and Alheid, 1991; Heimer et al., 1991; 1997; 1999; Alheid et al., 1995; 1998; 1999; Sakamoto et al., 1999), essentially usurped territory classically assigned to the preoptic area and ‘subpallidal’ region. The immunohistochemical observations described herein were not intended to revisit basal forebrain organization comprehensively, but rather were to be used to generate a map to localize our infusion sites. In this regard, the markers we selected produced conspicuous labeling differences distinguishing VP, LPO/MPO and extended amygdala, consistent with the extensive literature cited above. It is important to point out, however, that hard lines separating brain structures in maps are poor reflections of the actual situation in the basal forebrain, wherein structures more often than not blend into each other along imperceptible boundaries referred to as transition zones (Alheid et al., 1994; Zahm et al., 2011; 2013). This is particularly true of the caudomedial part of the VP which merges with the LPO along precisely such a transitional boundary, where an exceedingly dense GABAergic striatopallidal innervation terminating on typical pallidal-type neurons gives way to a less dense innervation of neurons characteristic of the LPO (Zahm, 1989). Also in this region, typical VP outputs to, e.g., the subthalamic nucleus and substantia nigra reticulata, diminish in favor of efferents aligned more exclusively with the trajectory of the medial forebrain bundle (Zahm, 1989). Consistent with the caudalward decline in numbers of GABAergic inputs from VP toward LPO, Mogenson and Yang (1991) reported that the spontaneous firing rate of neurons is greater moving from VP to the subpallidal region, which in part includes the LPO. In view of these differences between VP and LPO, we anticipated differences in how infusions of bicuculline into each would influence locomotor activation.
We were surprised to encounter locomotor activations following bicuculline infusions into the MPO, which, as a modulator of neuroendocrine function with important roles in sexual and parental behavior and various forms of aggression (Newman, 1999), is distinct from the LPO. We speculate that MPO-elicted locomotor activation may reflect a transitional character of the boundary between LPO and MPO.
Locomotion
The present results indicate that unilateral infusions of bicuculline into the LPO/MPO increase horizontal locomotion and rearing with far greater probability and elicit locomotor activations of substantially greater magnitude than unilateral infusions of bicuculline into any other basal forebrain structure, including the VP. Fold difference values for horizontal locomotion following infusions into the LPO/MPO reliably surpassed 3.5 and, frequently, were more than 5 (Table 1). In contrast, unilateral and bilateral infusions of bicuculline into the VP typically produced fold differences in locomotion relative to saline of less than 2 (Tables 1 and 2) and these were unassociated with increased rearing. In addition, bilateral infusions of bicuculline into the VP produced abormal movements. Direct comparison of LPO/MPO-elicited and VP-elicited total locomotion/session was consistent with the fold difference evaluations in that LPO/MPO infusions produced significantly greater amounts of horizontal locomotion and rearing during the 20 minute test period than did VP infusions (Fig. 3). The present results agree with a previous study done in our laboratory (Reynolds et al., 2006) and a series of earlier papers in which locomotor activation elicited by GABAA antagonists and glutamate agonists was said to be localized to the “lateral preoptic area/substantia innominata”, but in illustrations was mapped to the preoptic area exclusively (Shreve and Uretsky, 1988; 1989; 1991; Willins et al., 1992). Finally, the present finding of minimal to moderate locomotor activation following unilateral and bilateral infusions of bicuculline into the VP are reflected by recent reports involving infusions of GABAA antagonists into the VP that left locomotor activation unmentioned (Kemppainen et al., 2012) or tested for and failed to detect it (June et al., 2003).
Methodological considerations
Bicuculline and its methyl derivatives competitively block GABA at the GABAA receptor, in contrast to picrotoxin, which utilizes a mixed/non-competitive mechanism involving the associated chloride channel (Zukin et al., 1974; Simmonds, 1982; Krishek et al., 1996). Accordingly, bicuculline methochloride was determined to be a more potent and selective antagonist of GABA than picrotoxin (Dray, 1975). In a direct comparison of efficacies following forebrain infusions, picrotoxin produced more robust locomotor activations, but a less coherent dose response curve, than bicuculline methbromide (Austin and Kalivas, 1990). In view of these considerations, we selected to use bicuculline methbromide in this and our earlier study (Reynolds et al., 2006), despite knowing that mainly picrotoxin had been used in earlier, similar studies (Mogenson and Nielsen, 1983; Mogenson et al., 1985; Shreve and Uretsky, 1988; 1991; Austin and Kalivas, 1990; 1991)
The present results, obtained using microinfusions, reflect methodological problems typical of the technique. Thus, although we have no measure of the volumes of tissue effectively disinhibited by our infusions, the modest volume of vehicle (0.25 μl) and amount of bicuculline (67 ng) we used were slightly less than used in other studies where the compounds were shown in illustrations to have occupied approximately spherical volumes between 0.5 and 1.0 mm in diameter (e.g., Johnson et al., 1996; Chapman and Zahm, 1996). While this encourages confidence that our compound activated a volume of tissue somewhat less than shown in those studies, it seems possible that the geometry of our infusions may have been shaped by preferential diffusion along longitudinally-running fascicles of myelinated and unmyelinated axons that pervade basal forebrain. Thus, uncertainty attends the size and shape of the volume of tissue disinhibited by our infusions. This and the omnipresent potential for clogging of injection cannlae by brain tissue may in part underlie the substantial variability of our behavioral results. Furthermore, we cannot be certain if indeterminate subpopulations of neurons were preferentially activated by bicuculline. In this regard, e.g., we presume that the functional effects of a bicuculline infusion are due more to its capacity to release neurons with high intrinsic firing rates from GABA-mediated tonic suppression than by actions on tonically quiescent neurons.
We attempted to avoid disrupting the lateral ventricles by setting our guide cannulae at an angle of 20° from vertical in the mediolateral plane and, for most infusions, succeeded. However, the most medial of our cannula placements, which, by happenstance, were among those targeting LPO/MPO, frequently did disrupt the lateral ventricle, such that locomotor activation observed following such infusions could have reflected leakage of bicuculline into the ventricular system. To control for this, we infused bicuculline directly into the lateral ventricle in three rats and observed no significant effect on locomotion as compared to saline.
Our results complement those of Mogenson and Nielson (1983), who infused the GABAA antagonist, picrotoxin, in a sequence of increasing doses at various sites throughout the ‘subpallidal’ region, which in their description encompasses the sublenticular substantia innominata, lateral preoptic area, bed nucleus of stria terminalis and ventral globus pallidus. They ultimately took no position on where in basal forebrain is most sensitive to such infusions, but their Figure 1 indicates that infusions in and near the lateral preoptic area required the least amount of picrotoxin to elicit robust activations of locomotion. We infused bicuculline at a single concentration found in our previous study to maximize locomotion upon infusion into the LPO/MPO (Reynolds et al., 2006) and then monitored the magnitude of locomotor responses generated at various immunohistochemically identified sites throughout the subpallidal region. Because we observed that locomotor activations were not limited exclusively to infusions of bicuculline into LPO/MPO, but occasionally were seen after infusions at some distance from it, it is necessary to entertain the possibility that the substrate for bicuculline-elicited locomotor activation extends into parts of the basal forebrain at some distance from the LPO/MPO, possibly as a distributed neuronal network comprising elements that are less sensitive or consistently activateable at increasing distance from the LPO/MPO. Occasional strong locomotor activation in response to bicuculline infusion at sites distant from the LPO/MPO could be due to stimulation of sensitive nodes in such a presumptive network. It is perhaps more likely, however, that the decreasing probability of activating locomotion following infusions at distant sites simply reflects greater distances that bicuculline would need to diffuse in order to activate the LPO/MPO. Robust activations following infusions in very distal sites could reflect fortuitously facilitated diffusion, e.g., along glial interfaces or fiber tracts. It should also be apparent that these alternative mechanisms are not mutually exclusive and that either would benefit from deposition of more voluminous or concentrated GABAA antagonist infusions at sites increasingly distant from the LPO/MPO, consistent with our results and Mogenson’s and Nielsen’s (1983) map.
Functional considerations
The data indicate that unilateral activation by disinhibition of some subset of basal forebrain neurons centered mainly in the LPO/MPO provides a potent facilitatory modulation of locomotion and rearing, presumably exerted via descending projections from LPO/MPO to a neuronal network located more caudally in the brainstem that controls the actions of brainstem and spinal cord motor effectors (Holstege, 1991, 1992). This relatively distal impact on locomotor circuitry would account for how a profound disruption of normal patterns of neuronal activity in a particular structure, LPO/MPO, powerfully affects the quantity but not the quality or physical integrity of locomotion (Holstege et al., 2004). Stated differently, the data suggest that LPO/MPO has no role in synthesizing motor patterns, but rather regulates level of activation in a different network that does synthesize locomotion. Identical considerations may apply to the modest increase in locomotion observed following unilateral bicuculline infusions into the VP, although, as noted above, this could instead reflect diffusion of bicuculline from the VP to the LPO/MPO. Alternatively in this regard, bicuculline-elicited locomotor activations were less prevalent following infusions in the rostral part of the VP as compared to its caudal part (Fig. 2), where numerous corticopetal cholinergic neurons are present (Zaborszky et al., 1991). The action of bicuculline on these cholinergic neurons might lead to the locomotor activations elicited by infusions at caudal VP sites.
Similar to unilateral infusions, bilateral infusions of bicuculline into the VP in our hands produced small increases of locomotion and, in contrast to the unilateral infusions, profoundly altered the quality of movements, such that abundant compulsive pivoting and gnawing, occasionally accompanied by forepaw grasping and, on one occasion, a gait abnormality we refer to as ‘galloping’, were observed. Mechanisms underlying such abnormalities are at present unknown, but their relatively minimal association with generalized locomotor activation and the extent to which they resemble distorted normal movements suggests that descending projections from the VP exert less control over brainstem networks that modulate the abundance of locomotion and more, presumably direct, control over those responsible for synthesizing motor patterns. In view of this, one may be inclined to speculate that descending projections from the VP are concerned with intended movement, or, stated differently, intentional actions (see also Root et al., 2013). Insofar as most of these involve movements to one side or the other, it is intuitively agreeable that descending projections from the VP are mainly unilateral (Groenewegen et al., 1993), but problematic as to why it takes bilateral infusions of bicuculline to elicit a VP effect on motor function. In contrast, LPO/MPO has bilateral distribution of downstream projections (Geisler and Zahm, 2006b), consistent with its role in the modulation of bilaterally potent general locomotion.
Mental correlates of LPO/MPO-elicited locomotion?
Locomotor activation was viewed by some earlier investigators as the behavioral expression of a sense of impending consummation from which intended motor acts may be launched (Roberts and Carey, 1965; Mary Christopher and Butter, 1968; Hotsenpiller et al., 2001). In view of such ruminations, it is tempting to speculate further that the specificity and sensitivity with which locomotor activation is elicited from the LPO/MPO, combined with the convergence there of outputs from reward- and threat-sensitive functional-anatomical macrosystems (Zahm et al., 2013), makes the LPO/MPO a likely substrate to blend competing and cooperating influences descending via the macrosystems from the cerebral cortex, consistent with a proximal contribution of LPO/MPO to motivational processes. However, it is also known that activation of LPO/MPO stimulates VTA dopamine (DA) neurons (Reynolds et al., 2006), resulting in ‘DA release in the accumbens’ (Blaha et al., 1990; Laitinen et al., 1990; Rivest et al., 1991; Sotty et al., 1998; 2000), which, according to Berridge and Robinson (1998) embodies the psychic state of ‘wanting’ and is invariably accompanied, e.g., by increased locomotor activity (Kelly et al., 1975; Kalivas et al., 1983; Kalivas and Duffy, 1990; Steinberg et al., 1994), energized responses to second-order conditioned stimuli (Cador et al., 1991; Taylor and Robbins, 1984; 1986), establishment of conditioned place preference (Glimcher et al., 1984; Heidbreder et al., 1992), potentiation of brain stimulation reward (Rompre et al., 1992; Rompre and Gratton, 1992; Rompre, 1995), self-administration (Glimcher et al., 1997) and both behavioral and neurochemical sensitization (Kalivas et al., 1982; Kalivas & Taylor, 1985; Elliott & Nemeroff, 1986; Kalivas & Duffy, 1990). But then, infusion of GABAA receptor agonists or excitatory amino acid receptor antagonists into the LPO/MPO and VP, to both of which the accumbens densely projects (Zahm et al., 2013), abolishes these kinds of effects (Mogenson and Nielson, 1983; Swerdlow and Koob, 1984; Shreve and Uretsky, 1988; Austin and Kalivas, 1989; Willens et al., 1992). Furthermore, the sense that dopamine release is essential to the mediation of all these functions is called to question by the observation that LPO/MPO-elicited locomotor activation persists in complete blockade of DA receptors (Zahm et al., 2012). Moreover, intracranial electrical self-stimulation sites are abundant in LPO (Bushnik et al., 2000) and electrical self-stimulation of the LPO (and other sites) persists, albeit with attenuated vigor, in the presence of DA depletion and reward-blocking doses of antipsychotic drugs (e.g., Fibiger et al., 1976; 1987; Gallistel et al., 1985, but see Breese et al., 1971). Together, these observations lead one to wonder if the mental state underlying motivation is not attributable, at least in part, to events occuring in the LPO/MPO. Indeed, they beg a question as to what extent accumbens outputs associated with rewarding aspects of basal forebrain function (e.g., Johnston et al., 1996; Tindell et al., 2004; 2005; 2006; Lodge and Grace, 2006, see also Root et al., 2012) are linked to LPO/MPO, VP, both, or, possibly, a transition area intervening between the two (Zahm, 1989). In any event, whether or not the initial attribution by the brain of ‘reward’ status is an exclusively DAergic event, signals subserving or permitting the perception of reward (or its absence), however triggered, are promptly transmitted, via complex connectivity to DAergic neurons in the ventral mesencephalon (Phillipson, 1979; Zahm et al., 2001; Geisler and Zahm, 2005; 2006a; b; Geisler et al., 2007; Jhou et al., 2009; Zahm et al., 2011; Watabe-Uchida et al., 2012; reviewed in Sesack and Grace, 2010; Lavezzi and Zahm, 2012), which, in turn, disseminate the information in other parts of the brain. LPO/MPO may have a quite proximal role in these processes.
Acknowledgments
This work was supported by USPHS NIH grant NS-23805.
Literature Cited
- Alheid GF, Beltramino C, Braun A, Miselis RR, François C, de Olmos JS. Transition areas of the striatopallidal system and extended amygdala in the rat and primate: Observations from histochemistry and experiments with mono- and trans-synaptic tracer. In: Percheron G, McKenzie JS, Féger J, editors. The Basal Ganglia IV, Vol. 41, New Ideas and Data on Structure and Function. New York: Plenum Press; 1994. pp. 95–107. [Google Scholar]
- Alheid GF, Beltramino CA, De Olmos JS, Forbes MS, Swanson DJ, Heimer L. The neuronal organization of the supracapsular part of the stria terminalis in the rat: the dorsal component of the extended amygdala. Neuroscience. 1998;84:967–996. doi: 10.1016/s0306-4522(97)00560-5. [DOI] [PubMed] [Google Scholar]
- Alheid GF, de Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The Rat Nervous System. 2. San Diego: Academic Press; 1995. pp. 495–578. [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Shammah-Lagnado SJ, Beltramino CA. The interstitial nucleus of the posterior limb of the anterior commissure: a novel layer of the central division of extended amygdala. Ann N Y Acad Sci. 1999;877:645–654. doi: 10.1111/j.1749-6632.1999.tb09294.x. [DOI] [PubMed] [Google Scholar]
- Austin MC, Kalivas PW. Blockade of enkephalinergic and GABAergic mediated locomotion in the nucleus accumbens by muscimol in the ventral pallidum. Jpn J Pharmacol. 1989;50:487–490. doi: 10.1254/jjp.50.487. [DOI] [PubMed] [Google Scholar]
- Austin MC, Kalivas PW. Enkephalinergic and GABAergic modulation of motor activity in the ventral pallidum. J Pharmacol Exp Ther. 1990;252:1370–1377. [PubMed] [Google Scholar]
- Austin MC, Kalivas PW. Dopaminergic involvement in locomotion elicited from the ventral pallidum/substantia innominata. Brain Res. 1991;542:123–131. doi: 10.1016/0006-8993(91)91005-l. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Coury A, Fibiger HC, Phillips AG. Effects of neurotensin on dopamine release and metabolism in the rat striatum and nucleus accumbens: cross-validation using in vivo voltammetry and microdialysis. Neuroscience. 1990;34:699–705. doi: 10.1016/0306-4522(90)90176-5. [DOI] [PubMed] [Google Scholar]
- Breese GR, Howard JL, Leahy JP. Effect of 6-hydroxydopamine on electrical self stimulation of the brain. Br J Pharmacol. 1971;43:255–257. doi: 10.1111/j.1476-5381.1971.tb07177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnik T, Bielajew C, Konkle AT. The substrate for brain-stimulation reward in the lateral preoptic area. I. Anatomical mapping of its boundaries. Brain Res. 2000;881:103–111. doi: 10.1016/s0006-8993(00)02564-6. [DOI] [PubMed] [Google Scholar]
- Cador M, Taylor JR, Robbins TW. Potentiation of the effects of reward-related stimuli by dopaminergic-dependent mechanisms in the nucleus accumbens. Psychopharmacology (Berl) 1991;104:377–385. doi: 10.1007/BF02246039. [DOI] [PubMed] [Google Scholar]
- Celio MR, Heizmann CW. Calcium-binding protein parvalbumin as a neuronal marker. Nature. 1981;293:300–302. doi: 10.1038/293300a0. [DOI] [PubMed] [Google Scholar]
- Chapman MA, Zahm DS. Altered Fos-like immunoreactivity in terminal regions of the mesotelencephalic dopamine system is associated with reappearance of tyrosine hydroxylase immunoreactivity at the sites of focal 6-hydroxydopamine lesions in the nucleus accumbens. Brain Res. 1996;736:270–279. doi: 10.1016/0006-8993(96)00714-7. [DOI] [PubMed] [Google Scholar]
- Chen JC, Liang KW, Huang YK, Liang CS, Chiang YC. Significance of glutamate and dopamine neurons in the ventral pallidum in the expression of behavioral sensitization to amphetamine. Life Sci. 2001;68:973–983. doi: 10.1016/s0024-3205(00)00995-4. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ, Emson PC, Heizmann CW. Parvalbumin containing GABAergic interneurons in the rat neostriatum. J Comp Neurol. 1990;302:197–205. doi: 10.1002/cne.903020202. [DOI] [PubMed] [Google Scholar]
- Dray A. Comparison of bicuculline methochloride with bicuculline and picrotoxin as antagonists of amino acid and monamine depression of neurones in the rat brainstem. Neuropharmacol. 1975;14:887–891. doi: 10.1016/0028-3908(75)90117-3. [DOI] [PubMed] [Google Scholar]
- Elliott PJ, Nemeroff CB. Repeated neurotensin administration in the ventral tegmental area: effects on baseline and D-amphetamine-induced locomotor activity. Neurosci Lett. 1986;68:239–244. doi: 10.1016/0304-3940(86)90149-7. [DOI] [PubMed] [Google Scholar]
- Fibiger HC, Carter DA, Phillips AG. Decreased intracranial self-stimulation after neuroleptics or 6-hydroxydopamine: evidence for mediation by motor deficits rather than by reduced reward. Psychopharmacology (Berl) 1976;47:21–27. doi: 10.1007/BF00428696. [DOI] [PubMed] [Google Scholar]
- Fibiger HC, LePiane FG, Jakubovic A, Phillips AG. The role of dopamine in intracranial self-stimulation of the ventral tegmental area. J Neurosci. 1987;7:3888–3896. doi: 10.1523/JNEUROSCI.07-12-03888.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, Gomita Y, Yadin E, Campbell KA. Forebrain origins and terminations of the medial forebrain bundle metabolically activated by rewarding stimulation or by reward-blocking doses of pimozide. J Neurosci. 1985;5:1246–1261. doi: 10.1523/JNEUROSCI.05-05-01246.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat - anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Neurotensinergic afferents of the ventral tegmental area in the rat: [1] re-examination of the origins and [2] responses to acute psychostimulant drug administration. Eur J Neurosci. 2006a;24:116–134. doi: 10.1111/j.1460-9568.2006.04928.x. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. On the retention of neurotensin in the ventral tegmental area (VTA) despite destruction of the main neurotensinergic afferents of the VTA--implications for the organization of forebrain projections to the VTA. Brain Res. 2006b;1087:87–104. doi: 10.1016/j.brainres.2006.02.108. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Baimbridge KG, Miller JJ. The neostriatal mosaic: Compartmental distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proc Natl Acat Sci USA. 1985;82:8780–8784. doi: 10.1073/pnas.82.24.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW, Giovino AA, Hoebel BG. Neurotensin self-injection in the ventral tegmental area. Brain Res. 1987;403:147–150. doi: 10.1016/0006-8993(87)90134-x. [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Margolin DH, Giovino AA, Hoebel BG. Neurotensin: a new ‘reward peptide’. Brain Res. 1984;291:119–124. doi: 10.1016/0006-8993(84)90657-7. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill DB, Lynn M, Justice JB., Jr Dopamine D1/D2 agonists injected into nucleus accumbens and ventral pallidum differentially affect locomotor activity depending on site. Neuroscience. 1999;93:1349–1358. doi: 10.1016/s0306-4522(99)00235-3. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57:113–142. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- Heidbreder C, Gewiss M, De Mot B, Mertens I, De Witte P. Balance of glutamate and dopamine in the nucleus accumbens modulates self-stimulation behavior after injection of cholecystokinin and neurotensin in the rat brain. Peptides. 1992;13:441–449. doi: 10.1016/0196-9781(92)90073-c. [DOI] [PubMed] [Google Scholar]
- Heimer L. The olfactory connections of the diencephalon in the rat. An experimental light- and electron-microscopic study with special emphasis on the problem of terminal degeneration. Brain Behav Evol. 1972;6:484–523. doi: 10.1159/000123728. [DOI] [PubMed] [Google Scholar]
- Heimer L, Alheid GF. Piecing together the puzzle of basal forebrain anatomy. Adv Exp Med Biol. 1991;295:1–42. doi: 10.1007/978-1-4757-0145-6_1. [DOI] [PubMed] [Google Scholar]
- Heimer L, Harlan RE, Alheid GF, Garcia M, de Olmos J. Substantia innominata: A notion which impedes clinical-anatomical correlations in neuropsychiatric disorders. Neuroscience. 1997;76:957–1006. doi: 10.1016/s0306-4522(96)00405-8. [DOI] [PubMed] [Google Scholar]
- Heimer L, de Olmos J, Alheid GF, Pearson J, Sakamoto M, Marksteiner J, Switzer RC., III . The human basal forebrain, part 2. In: Bloom FE, Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy. Vol. 15. Amsterdam: Elsevier; 1999. pp. 57–226. [Google Scholar]
- Heimer L, de Olmos J, Alheid GF, Zaborszky L. “Perestroika” in the basal forebrain: opening the border between neurology and psychiatry. Prog Brain Res. 1991;87:109–165. doi: 10.1016/s0079-6123(08)63050-2. [DOI] [PubMed] [Google Scholar]
- Heimer L, Wilson RD. The subcortical projections of allocortex: Similarities in the neuroal associations of the hippocampus, the piriform cortex and the neocortex. In: Santini M, editor. Golgi Centennial Symposium Proceedings. Raven Press; New York: 1975. pp. 173–193. [Google Scholar]
- Holstege G. Descending motor pathways and the spinal motor system: limbic and non-limbic components. Prog Brain Res. 1991;87:307–421. doi: 10.1016/s0079-6123(08)63057-5. [DOI] [PubMed] [Google Scholar]
- Holstege G. The emotional motor system. Eur J Morphol. 1991;30:67–79. [PubMed] [Google Scholar]
- Holstege G, Mouton LG, Gerrits MN. Emotional motor system. In: Paxinos G, Mai JK, editors. The human nervous system. Amsterdam: Elsevier; 2004. pp. 1306–1324. [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Manvich DF, Kuhar MJ. Cocaine and amphetamine-regulated transcript-containing neurons in the nucleus accumbens project to the ventral pallidum in the rat and may inhibit cocaine-induced locomotion. Neuroscience. 2010;165:179–187. doi: 10.1016/j.neuroscience.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Z, Akiva K, Nomura S, Mizuno N, Hakamura Y, Sugimoto T. Application of a coupled oxidation reaction to electron microscopic demonstraion of horseradish peroxidase: Cobalt-glucose oxidase method. Brain Res. 1979;175:341–347. doi: 10.1016/0006-8993(79)91013-8. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–96. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K, Churchill L, Klitenick MA, Hooks MS, Kalivas PW. Involvement of the ventral tegmental area in locomotion elicited from the nucleus accumbens or ventral pallidum. J Pharmacol Exp Ther. 1996;277:1122–1131. [PubMed] [Google Scholar]
- Johnson PI, Napier TC. Ventral pallidal injections of a mu antagonist block the development of behavioral sensitization to systemic morphine. Synapse. 2000;38:61–70. doi: 10.1002/1098-2396(200010)38:1<61::AID-SYN7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC, Eiler WJ, Grey C, Carroll MR, McCane S, Jones CM, Yin W, Mason D, Cummings R, Garcia M, Ma C, Sarma PV, Cook JM, Skolnick P. The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacology. 2003;28:2124–2137. doi: 10.1038/sj.npp.1300239. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Burgess SK, Nemeroff CB, Prange AJ., Jr Behavioral and neurochemical effects of neurotensin microinjection into the ventral tegmental area of the rat. Neuroscience. 1983;8:495–505. doi: 10.1016/0306-4522(83)90195-1. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse. 1990;5:48–58. doi: 10.1002/syn.890050104. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Klitenick MA, Hagler H, Austin MC. GABAergic and enkephalinergic regulation of locomotion in the ventral pallidum: involvement of the mesolimbic dopamine system. In: Napier TC, Kaliavs PW, Hanin I, editors. The Basal Forebrain. Anatomy to Function. Vol. 295. Plenum Press; NY: 1991. pp. 315–326. Adv Exp Med Biol. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nemeroff CB, Prange AJ., Jr Neuroanatomical site specific modulation of spontaneous motor activity by neurotensin. Eur J Pharmacol. 1982;78:471–474. doi: 10.1016/0014-2999(82)90491-5. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Taylor S. Behavioral and neurochemical effect of daily injection with neurotensin into the ventral tegmental area. Brain Res. 1985;358:70–76. doi: 10.1016/0006-8993(85)90949-7. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Kemppainen H, Raivio N, Kiianmaa K. Role for ventral pallidal GABAergic mechanisms in the regulation of ethanol self-administration. Psychopharmacology (Berl) 2012;223:211–221. doi: 10.1007/s00213-012-2709-x. [DOI] [PubMed] [Google Scholar]
- Krishek BJ, Moss SJ, Smart TG. A functional comparison of the antagonists bicuculline and picrotoxin at recominant GABAA receptors. Neuropharmacol. 1996;35:1289–1298. doi: 10.1016/s0028-3908(96)00089-5. [DOI] [PubMed] [Google Scholar]
- Laitinen K, Crawley JN, Mefford IN, De Witte P. Neurotensin and cholecystokinin microinjected into the ventral tegmental area modulate microdialysate concentrations of dopamine and metabolites in the posterior nucleus accumbens. Brain Res. 1990;523:342–346. doi: 10.1016/0006-8993(90)91511-e. [DOI] [PubMed] [Google Scholar]
- Lavezzi HN, Zahm DS. The mesopontine rostromedial tegmental nucleus: an integrative modulator of the reward system. Basal Ganglia. 2011;1:191–200. doi: 10.1016/j.baga.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Mary Christopher S, Butter CM. Consummatory behaviors and locomotor exploration evoked from self-stimulation sites in rats. J Comp Physiol Psychol. 1968;66:335–339. doi: 10.1037/h0026325. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Brudzynski SM, Wu M, Yang CR, Yim CY. From motivation to Action: A review of dopaminergic regulation of limbic-nucleus accumbens-ventral pallidum-pedunculopontine nucleus circuitries involved in limbic-motor integration. In: Kalivas PW, Barnes CD, editors. Limbic Motor Circuits and Neuropsychiatry. Vol. 295. CRC Press; Boca Raton, FL: 1993. pp. 193–236. Adv Exp Med Biol. [Google Scholar]
- Mogenson GJ, Nielsen MA. Evidence that an accumbens to subpallidal GABAergic projection contributes to locomotor activity. Brain Res Bull. 1983;11:309–314. doi: 10.1016/0361-9230(83)90166-1. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Swanson LW, Wu M. Neural projections from nucleus accumbens to globus pallidus, substantia innominata, and lateral preoptic-lateral hypothalamic area: an anatomical and electrophysiological investigation in the rat. J Neurosci. 1983;3:189–202. doi: 10.1523/JNEUROSCI.03-01-00189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Swanson LW, Wu M. Evidence that projections from substantia innominata to zona incerta and mesencephalic locomotor region contribute to locomotor activity. Brain Res. 1985;334:65–76. doi: 10.1016/0006-8993(85)90568-2. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. In: Napier TC, Kaliavs PW, Hanin I, editors. The Basal Forebrain. Anatomy to Function. Vol. 295. Plenum Press; NY: 1991. pp. 267–290. Adv Exp Med Biol. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Advancing from the Ventral Striatum to the Extended Amydala. Implications for Neuropsychiatry and Drug Abuse. In: McGinty J, editor. Ann NY Acad Sci. Vol. 877. 1999. pp. 242–257. [DOI] [PubMed] [Google Scholar]
- de Olmos JS, Ingram WR. The projection field of the stria terminalis in the rat brain. An experimental study. J Comp Neurol. 1972;146:303–334. doi: 10.1002/cne.901460303. [DOI] [PubMed] [Google Scholar]
- Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Geisler S, Berod A, Zahm DS. Neurotensin antagonist acutely and robustly attenuates locomotion that accompanies stimulation of a neurotensin-containing pathway from rostrobasal forebrain to the ventral tegmental area. Eur J Neurosci. 2006;24:188–196. doi: 10.1111/j.1460-9568.2006.04791.x. [DOI] [PubMed] [Google Scholar]
- Rivest R, Jolicoeur FB, Marsden CA. Neurotensin causes a greater increase in the metabolism of dopamine in the accumbens than in the striatum in vivo. Neuropharmacology. 1991;30:25–33. doi: 10.1016/0028-3908(91)90038-d. [DOI] [PubMed] [Google Scholar]
- Roberts WW, Carey RJ. Rewarding effect of performance of gnawing aroused by hypothalamic stimulation in the rat. J Comp Physiol Psychol. 1965;59:317–324. doi: 10.1037/h0022030. [DOI] [PubMed] [Google Scholar]
- Rodrigo J, Springall DR, Uttenthal O, Bentura ML, Abadia-Molina F, Riveros-Moreno V, Martínez-Murillo R, Polak JM, Moncada S. Localization of nitric oxide synthase in the adult rat brain. Philos Trans R Soc Lond B Biol Sci. 1994;345:175–221. doi: 10.1098/rstb.1994.0096. [DOI] [PubMed] [Google Scholar]
- Rompre PP. Psychostimulant-like effect of central microinjection of neurotensin on brain stimulation reward. Peptides. 1995;16:1417–1420. doi: 10.1016/0196-9781(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Rompre PP, Bauco P, Gratton A. Facilitation of brain stimulation reward by mesencephalic injections of neurotensin-(1–13) Eur J Pharmacol. 1992;211:295–303. doi: 10.1016/0014-2999(92)90384-g. [DOI] [PubMed] [Google Scholar]
- Rompre PP, Gratton A. Mesencephalic microinjections of neurotensin-(1–13) and its C-terminal fragment, neurotensin-(8–13), potentiate brain stimulation reward. Brain Res. 1993;616:154–162. doi: 10.1016/0006-8993(93)90204-z. [DOI] [PubMed] [Google Scholar]
- Root DH, Ma S, Barker DJ, Megehee L, Striano BM, Ralston CM, Fabbricatore AT, West MO. Differential roles of ventral pallidum subregions during cocaine self-administration behaviors. J Comp Neurol. 2012 doi: 10.1002/cne.23191. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M, Pearson J, Shinoda K, Alheid GF, de Olmos JS, Heimer L. The human basal forebrain, part 1. An overview. In: Bloom FE, Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy. Vol. 15. Amsterdam: Elsevier; 1999. pp. 1–55. [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacol. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreve PE, Uretsky NJ. Effect of GABAergic transmission in the subpallidal region on the hypermotility response to the administration of excitatory amino acids and picrotoxin into the nucleus accumbens. Neuropharmacology. 1988;27:1271–1277. doi: 10.1016/0028-3908(88)90030-5. [DOI] [PubMed] [Google Scholar]
- Shreve PE, Uretsky NJ. AMPA, kainic acid, and N-methyl-D-aspartic acid stimulate locomotor activity after injection into the substantia innominata/lateral preoptic area. Pharmacol Biochem Behav. 1989;34:101–106. doi: 10.1016/0091-3057(89)90360-2. [DOI] [PubMed] [Google Scholar]
- Shreve PE, Uretsky NJ. GABA and glutamate interact in the substantia innominata/lateral preoptic area to modulate locomotor activity. Pharmacol Biochem Behav. 1991;38:385–388. doi: 10.1016/0091-3057(91)90296-e. [DOI] [PubMed] [Google Scholar]
- Sotty F, Brun P, Leonetti M, Steinberg R, Soubrie P, Renaud B, Suaud-Chagny MF. Comparative effects of neurotensin, neurotensin(8–13) and [D-Tyr(11)]neurotensin applied into the ventral tegmental area on extracellular dopamine in the rat prefrontal cortex and nucleus accumbens. Neuroscience. 2000;98:485–492. doi: 10.1016/s0306-4522(00)90023-x. [DOI] [PubMed] [Google Scholar]
- Simmonds MA. Classification of GABA antagonists with regard to site of action and potency in slices of rat cuneate nucleus. Eur J Pharmacol. 1982:347–358. doi: 10.1016/0014-2999(82)90080-2. [DOI] [PubMed] [Google Scholar]
- Sotty F, Souliere F, Brun P, Chouvet G, Steinberg R, Soubrie P, Renaud B, Suaud-Chagny MF. Differential effects of neurotensin on dopamine release in the caudal and rostral nucleus accumbens: a combined in vivo electrochemical and electrophysiological study. Neuroscience. 1998;85:1173–1182. doi: 10.1016/s0306-4522(97)00691-x. [DOI] [PubMed] [Google Scholar]
- Steinberg R, Brun P, Fournier M, Souilhac J, Rodier D, Mons G, Terranova JP, Le Fur G, Soubrie P. SR 48692, a non-peptide neurotensin receptor antagonist differentially affects neurotensin-induced behaviour and changes in dopaminergic transmission. Neuroscience. 1994;59:921–929. doi: 10.1016/0306-4522(94)90295-x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Koob GF. The neural substrates of apomorphine-stimulated locomotor activity following denervation of the nucleus accumbens. Life Sci. 1984;35:2537–2544. doi: 10.1016/0024-3205(84)90440-5. [DOI] [PubMed] [Google Scholar]
- Switzer RC, 3rd, Hill J, Heimer L. The globus pallidus and its rostroventral extension into the olfactory tubercle of the rat: a cyto- and chemoarchitectural study. Neuroscience. 1982;7:1891–1904. doi: 10.1016/0306-4522(82)90005-7. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology (Berl) 1984;84:405–412. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology (Berl) 1986;90:390–397. doi: 10.1007/BF00179197. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Aldridge JW. Ventral pallidal representation of pavlovian cues and reward: population and rate codes. J Neurosci. 2004;24:1058–1069. doi: 10.1523/JNEUROSCI.1437-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Zhang J, Pecina S, Aldridge JW. Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. Eur J Neurosci. 2005;22:2617–2634. doi: 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Pecina S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J Neurophysiol. 2006;96:2399–2409. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Willins DL, Wallace LJ, Miller DD, Uretsky NJ. alpha-Amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptor antagonists in the nucleus accumbens and ventral pallidum decrease the hypermotility response to psychostimulant drugs. J Pharmacol Exp Ther. 1992;260:1145–1151. [PubMed] [Google Scholar]
- Zaborszky L, Cullinan WE, Braun A. Afferents to basal forebrain cholinergic neurons: an update. In: Napier TC, Kalivas PW, Hanin I, editors. The Basal Forebrain. Anatomy to Function. Plenum Press; New York: 1991. pp. 43–100. [Google Scholar]
- Zahm DS. The ventral striatopallidal parts of the basal ganglia in the rat--II. Compartmentation of ventral pallidal efferents. Neuroscience. 1989;30:33–50. doi: 10.1016/0306-4522(89)90351-5. [DOI] [PubMed] [Google Scholar]
- Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Grosu S, Irving JC, Williams EA. Discrimination of striatopallidum and extended amygdala in the rat: a role for parvalbumin immunoreactive neurons? Brain Res. 2003;978:141–154. doi: 10.1016/s0006-8993(03)02801-4. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Grosu S, Williams EA, Qin S, Berod A. Neurons of origin of the neurotensinergic plexus enmeshing the ventral tegmental area in rat: retrograde labeling and in situ hybridization combined. Neuroscience. 2001;104:841–851. doi: 10.1016/s0306-4522(01)00118-x. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Cheng AY, Lee TJ, Ghobadi CW, Schwartz ZM, Geisler S, Parsely KP, Gruber C, Veh RW. Inputs to the midbrain dopaminergic complex in the rat, with emphasis on extended amygdala-recipient sectors. J Comp Neurol. 2011;519:3159–3188. doi: 10.1002/cne.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Parsley KP, Schwartz ZM, Cheng AY. On lateral septum-like characteristics of outputs from the accumbal hedonic ‘hotspot’ of Pecina and Berridge with commentary on the transitional nature of basal forebrain ‘boundaries’. J Comp Neurol. 2013;521:50–68. doi: 10.1002/cne.23157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Schwartz ZM, Lavezzi HN, Parsley KP. Locomotion elicited by infusion of bicuculline into the lateral preoptic area persists during blockade of dopamine receptors. Soc Neurosci Abstr. 2012:183.13. [Google Scholar]
- Zukin SR, Young AB, Snyder SH. Gamma-aminobutyric acid binding to receptor sites in the rat central nervous system. Proc Natl Acad Sci USA. 1974;71:4802. doi: 10.1073/pnas.71.12.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]