Abstract
Purpose
The aim of the present study is to assess the effects of respiratory motion on the image quality of two-dimensional (2D), free-breathing, black-blood coronary wall (magnetic resonance) MR imaging.
Materials and methods
This study was compliance with the HIPPA. With the approval of the institution review board, 230 asymptomatic participants, including 164 male patients (72.9 ± 4.4 years) and 66 female patients (72.4 ± 5.1 years), were recruited. Written informed consent was obtained. A 2D navigator(NAV)-gated, black-blood coronary wall MR imaging sequence was run on the left main artery, the left anterior descending artery and the right coronary artery. The drift of the location of the NAV and scan efficiency were compared between good (scored 2 or 3) and poor images (scored 1). Age, body weight, body weight index (BMI), heart rate, length of the rest period of cardiac motion, diaphragm excursion and breathing frequency were compared using a t-test between the "successful" (having 2 or 3 good images) and "unsuccessful" cases (having 1 or 0 good images). A logistic regression model was applied to identify the contributors to good image quality.
Results
The drift of the NAV location and the scan efficiency were higher in the 411 good images compared with the 279 poor images. Minimal drift of the NAV location and low body weight were identified as independent predictors of good images after using a logistic regression model to adjust for multiple physiological and technical factors.
Conclusions
The stability of respiratory motion significantly influences the image quality of 2D, free-breathing, black-blood coronary wall MR imaging.
Keywords: Coronary wall MR imaging, Respiratory motion, Effects
Introduction
Coronary artery disease (CAD) is a leading cause of death in older populations[1]. The features of vascular remodeling, a major manifestation of coronary plaques, are expected to serve as biomarkers for evaluating the progression of CAD[2, 3]. Over the past decade, coronary wall magnetic resonance (MR) imaging has been introduced for observing coronary remodeling in vivo, especially for quantitatively assessing subclinical atherosclerosis[4–6]. However, such a noninvasive imaging method is limited by the continual motion of the coronary tree in the three-dimensional (3D) source space from various physiological activities, including heartbeats and respiratory motions. To minimize the effect of motion on image quality, segmented imaging data from the coronary wall are generally collected within a highly selected "acquisition window" for minimal motion during multiple heartbeat and respiration cycles. In free-breathing coronary wall MR imaging, a "cardiac motion-free" duration for imaging is chosen via electrocardiogram (ECG) triggering; a "respiratory freezing period," usually in the end expiration, is generally identified by dynamically tracking the position of the diaphragm using two-dimensional (2D), motion-adapted navigator (NAV) echo[7, 8]. Only the signal received during the overlapping section between the quiescence of cardiac palpitation and respiration is accepted for filling the k-space.
Unfortunately, current motion correction/suppression strategies are still unable to completely eliminate the adverse effects of motion on cardiovascular MR scans. Rapid cardiac motion, associated with faster heart rates and shorter rest periods in cardiac cycles, has already been proven to be a prominent determinant of poor image quality in coronary wall MR imaging[9]. However, there are no existing studies demonstrating how the characterization of respiration behavior affects the performance of coronary wall MR imaging in asymptomatic subjects with dominant risk factors for CAD, such as older age, diabetes mellitus (DM) and hypertension (HTN)[10]. This knowledge gap might stem from the fact that respiratory movement, comprising both volunteer motion and autonomic motion, is difficult to describe and predict, even under normal physical conditions.
The aim of the present study was to quantitatively assess the effects of respiratory motion on the performance of 2D, free-breathing, black-blood coronary wall MR imaging in elderly patients with low heart rates.
Methods
Patient study
With the approval of the institutional review board (IRB), 230 asymptomatic participants of the Chicago Healthy Aging Low Risk MRI Angiography (CHARISMA) study, including 164 male subjects (mean age 72.9 ± 4.4 years old, range 66 – 85 years, body weight 84.7 ± 13.3 kg, heart rate 59.8 ± 5.9 beats/minute, range 44 – 70 beats/minute, breath frequency 16.1 ± 2.1 breaths/minute) and 66 female subjects (mean age 72.4 ± 5.1 years old, range 65 – 84 years, body weight 71.9 ± 13.9 kg, heart rate 61.5 ± 5.6 beats/minute, range 48 – 70 beats/minute, breath frequency 16.4 ± 2.3 breaths/minute), were enrolled. The inclusion criterion for the present cohort was asymptomatic aging (66 – 85 years old) without a documented history of cardiovascular diseases. The exclusion criteria were contraindications for MR scanning, heart rate > 70 beats/minute and renal dysfunction. Written informed consent was obtained from all of the participants.
Participants with existing DM and HTN were identified based on their medical records. The information on the participants is shown in table 1.
Table 1.
Participant characteristics
| Parameters | Male (N = 164) | Female (N = 66) | Significance (p) |
|---|---|---|---|
| Age (years) | 72.9 ± 4.4 | 72.4 ± 5.1 | 0.416 |
| Body weight (Kg) | 84.7 ± 13.3 | 71.9 ± 13.9 | < 0.001 |
| BMI | 27.4 ± 4.4 | 26.9 ± 4.7 | 0.421 |
| Heart rate (beats/minutes) | 59.8 ± 5.9 | 61.5 ± 5.6 | 0.037 |
| respiratory frequency (breaths/minute) | 16.1 ± 2.1 | 16.4 ± 2.3 | 0.879 |
| Prevalence of DM (Percentage) | 34(21) | 15(23) | 0.738 |
| Prevalence of HTN (Percentage) | 93(57) | 35(53) | 0.661 |
Imaging protocols
All coronary scans were performed with a 1.5 Tesla MR scanner (ESPREE, SIEMENS, GERMANY) by two certified clinical MR technologists. For each participant, a 3-plane, fast-localization sequence was run first for anatomic orientation for the whole scan. The 2-chamber, 4-chamber and short axis views were localized for coronary imaging. Then, 4-chamber cardiac cine images were acquired with a segmented 2D steady-state free precession (SSFP) sequence. The whole-heart coronary MR angiography was acquired with a NAV-gated, ECG-triggered, fat-saturated, T2-prepared, segmented 3D SSFP sequence. The NAV signal for respiratory gating was acquired with a spin-echo readout. Both the 90- and 180-degree pulses were selective and were planned with a graphical user interface. A 10-mm slice thickness was used for both pulses in this study. A 90-degree pulse for excitation was planned in the sagittal view, and a 180-degree pulse for refocusing was planned in the sagittal view, tilted (30 degrees) toward the coronal plane (sagittal > coronal). The cross section of both pulses formed an echo in the head-foot direction. A ± 2.5-mm acceptance window was used. Imaging data for which the liver-lung boundary fell within this acceptance window were accepted. Imaging data acquired outside of this acceptance window were rejected, and the same k-space lines were repeated in subsequent heartbeats until the NAV echo fell within the acceptance window. To address the drift of respiratory motion during the imaging period, a motion-adaptive gating algorithm was utilized. This algorithm constantly tracked the motion of the diaphragm (search window: ± 32 mm) over the previous 20 heartbeats, and the position of the acceptance window was dynamically updated. The cross section of excitation and the refocusing of pulses for the NAV navigator gating were placed at the dome of the liver-lung interface. This protocol was established based on the localization images acquired in both the axial and coronal views. To observe and measure the motion of the diaphragm, coronal images going through the diaphragm dome (right) were repeatedly acquired, using a single-shot SSFP sequence. The other imaging parameters included the following: field of view (FOV) = 320 × 320 mm2; TR/TE = 3.7/1.7 ms; flip angle = 90 degrees; k-space lines acquired per heartbeat = 17 – 35; readout bandwidth = 870 Hz/pixel; and parallel acquisition factor = 2. The in-plane spatial resolution was 1.1 × 1.1 mm2. The slice thickness was 0.7 mm (interpolated from 1.4 mm). During image acquisition, beta blocker was not applied to lower heart rates. The 3D multiplanar reformations (MPR) were performed on the MR angiography images to localize the left main artery (LM), proximal left anterior descending artery (LAD), and right coronary artery (RCA). Cross-sectional black-blood images of the proximal portions of the coronary arteries were acquired using a NAV-gated (described above), ECG-triggered, double inversion recovery (DIR)-prepared, 2D turbo spin echo (TSE) sequence at 5 mm from the origins of the RCA, the LM and the LAD (perpendicular to the long axis of the vessel) for quantitative analysis[11]. The other imaging parameters included the following: TR = 800 ms; TE = 33 ms; 11–13 k-space lines per cardiac cycle; bandwidth = 305 Hz/pixel; FOV = 420 × 420 mm2; matrix = 416 × 416; and slice thickness = 4.0 mm. In all of the cases, the duration of data acquisition was individually optimized to begin after the onset of the coronary rest period (mid-diastole) and not to exceed the rest period. A spectral-selective adiabatic inversion recovery (SPAIR) pulse was used to suppress fat signals surrounding the coronary walls.
Image evaluation and data processing
The coronary wall images were transferred to a dedicated imaging workstation (Dell, Studio XPS 435T, installed with a Linux system, Ubuntu 11.0) for semi-automatic analysis. All of the images were graded independently by two authors (reader #1 had 5 years of experience in cardiovascular imaging and reader #2 had 8 years of experience in radiology), using a three-point system based on the following criteria[5, 12]: 1) vessel (lumen) not visible or not eligible for analysis; 2) good image quality, eligible for analysis, vessel (lumen) may have minor signal loss or image artifacts; and 3) excellent image quality, vessel (lumen) is observed continuously with minor signal loss. If the two reviewers could not reach a consensus on a certain image, a third reviewer (reader #3 had 8 years of experience in radiology) determined the final score. Reader #1 ranked all of the images again 2 months after the first review, using the same criteria, to test for intra-observer variation. Coronary images with a score of "2" or "3" were considered to have "good” image quality. A "successful" case was defined as 2 or 3 coronary segments being of good image quality, while an "unsuccessful" case had only 1 or 0 good images.
The breathing behavior of the participants during the scanning was measured after the MR scans by reader #2. From the NAV data recorded during the scans, the drift of the position of the NAV during the coronary wall scan (absolute value between the lowest level and the highest level) and the scan efficiency (defined as the percentage of accepted ECG triggers among the available ECG triggers) were acquired. Breath frequency (a breath was counted as a round trip of diaphragm movement at the cephalocaudal axis of the body) and diaphragm excursion (from the lowest level to the highest level) were measured from serial coronal images that dynamically showed the location of the right dome of the diaphragm.
Statistical methods
There were two levels of quantitative analysis in this study. On a per-segment basis, the drift of the NAV location and the scan efficiency were compared between coronary segments with good image quality and those with poor image quality using t tests. A logistic regression model was applied to investigate the contributions of various factors to image quality, including sex, age, body weight, body weight index (BMI, defined as [weight in kg / (height in meters × height in meters)]), respiratory frequency, diaphragm excursion, drift of the NAV location, drift directions during individual scanning, scan efficiency, heart rate and length of the rest period in cardiac cycles (mid-diastole). Pearson’s correlation coefficient (r) was used to investigate the relationships among various factors. Cohen's kappa coefficient was applied to measure intra-observer agreement (for reader #1) and inter-observer agreement (between reader #1 and #2) of image grading. On a per-patient basis, sex composition and the prevalence of DM and HTN between "successful” cases and "unsuccessful” cases were compared using Chi-squared tests. Age, body weight, BMI, heart rate, length of the rest period of cardiac motion in a cardiac cycle, diaphragm excursion and breathing frequency were compared with t-tests between the two subject groups.
The measurements were expressed in the format of mean ± one standard deviation (SD). All statistical processing was performed with SPSS software (SPSS Inc., version 13.0, Chicago, IL). Statistical significance was set at a two-tailed p-value < 0.05.
Results
Overall, 411 out of 690 coronary segments (including 139 LMs, 141 LADs and 131 RCAs) were considered to be good images, resulting in a 59.6% overall success rate for coronary wall MR imaging in 230 participants. There were no significant differences in the success rates among various coronary branches (p > 0.05).
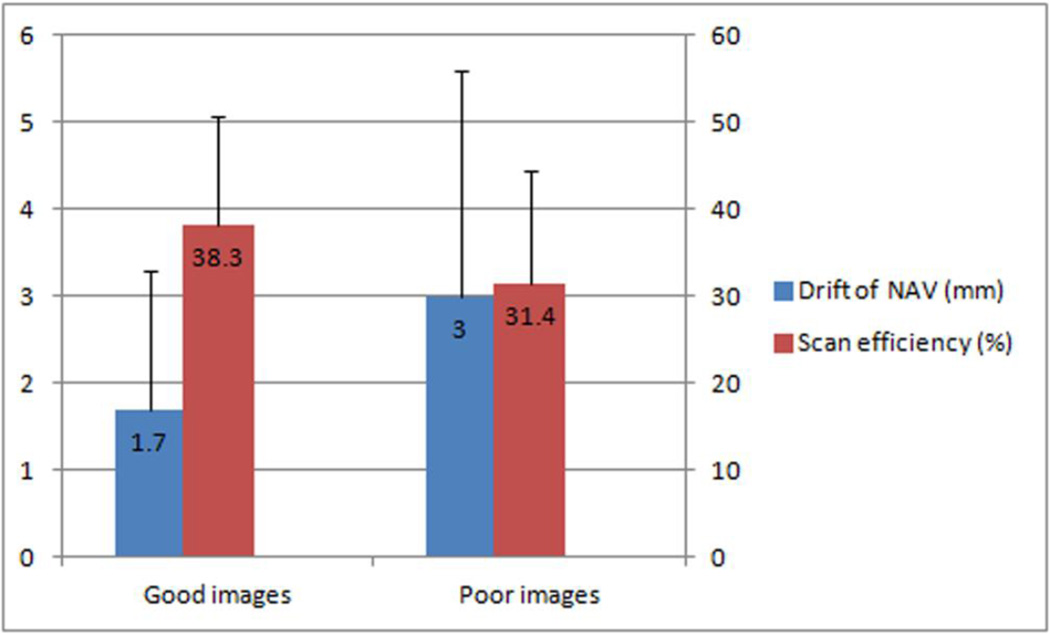
On a per-segment basis, there were significant differences in the drift of the NAV location (1.7 ± 1.6 mm vs. 3.0 ± 2.5 mm, p < 0.001) and the scan efficiency (38.3% ± 12.5% vs. 31.4% ± 13.1%, p < 0.001) between the 411 coronary segments with good image quality and the 279 segments with poor image quality (see figure 1). There was a tight correlation between the drift of the NAV location and the scan efficiency (r = 0.680, p < 0.001). Using a logistic regression model, minimal drift of the NAV location (EXP(B) = 0.840, 95%CI 0.755 – 0.935, p = 0.001) and low body weight (EXP(B) = 0.962, 95%CI 0.942 – 0.982, p < 0.001) were identified as independent predictors of good image quality (See table 2). Good intra-observer (Kappa = 0.867, p < 0.001) and inter-observer agreement (Kappa = 0.793, p < 0.001) for image grading were found in all coronary segments.
Figure 1.
Minimal drift of the NAV location and high scan efficiency were affiliated with good images.
Table 2.
Determinants for good image quality of coronary wall MR imaging
| Covariates | Exp (B) | EXP (B) 95% CI | p value | |
|---|---|---|---|---|
| lower | Upper | |||
| Age (years) | 1.035 | 0.997 | 1.075 | 0.070 |
| Gender (male vs. Female) | 1.023 | 0.988 | 1.048 | 0.156 |
| Body weight (Kg) | 0.962 | 0.942 | 0.982 | < 0.001 |
| BMI | 0.993 | 0.939 | 1.051 | 0.801 |
| Heart Rate (beats/minutes) | 0.974 | 0.941 | 1.008 | 0.127 |
| Rest period (msec) | 1.004 | 0.999 | 1.008 | 0.104 |
| Respiratory discursion (mm) | 1.002 | 0.977 | 1.028 | 0.859 |
| Frequency of breath (breath / minute) | 1.043 | 0.962 | 1.131 | 0.305 |
| Drift of NAV (mm) | 0.840 | 0.775 | 0.935 | 0.001 |
| Scan efficiency (%) | 1.023 | 1.000 | 1.045 | 0.016 |
On a per-patient basis, there were significant differences in the length of the rest period in a single cardiac cycle (189 ± 45 ms vs. 175 ± 47 ms, p = 0.023) and in body weight (77.6 ± 14.1 kg vs. 87.2 ± 13.8 kg, p < 0.001) and BMI (26.4 ± 4.6 vs. 28.8 ± 3.9, p < 0.001) between the 149 "successful” cases (55 cases with good image quality for all 3 coronary branches,107 male) and the 81 "unsuccessful” cases" (57 male). There were no significant differences between the two subject groups in gender composition (72% male vs. 70% male, p = 0.817), prevalence of DM (21% vs. 23%, p = 0.738), prevalence of HTN (57% vs. 53%, p = 0.661), age (73.1 ± 4.7 years old vs. 72.2 ± 4.4 years old, p = 0.130), heart rate (60.2 ± 5.5 beats/minute vs. 60.6 ± 6.6 beats/minute, p = 0.719), respiratory frequency (16.2 ± 2.2 breaths/minute vs. 16.3 ± 2.1 breaths/minute, p = 0.907) or diaphragm excursion (14.8 ± 6.8 mm vs. 14.6 ± 6.7 mm, p = 0.867) (see table 3).
Table 3.
Comparisons between "successful" cases and "unsuccessful" cases
| Case groups | Successful (N = 149) |
Unsuccessful (N = 81) |
Significance (P) |
|---|---|---|---|
| Male (percentage) | 106 (70) | 58 (74) | 0.817 |
| Age (years) | 73.1 ± 4.7 | 72.2 ± 4.4 | 0.130 |
| Body weight (Kg) | 77.6 ± 14.0 | 87.2 ± 13.8 | < 0.001 |
| BMI | 26.4 ± 4.6 | 28.8 ± 3.9 | < 0.001 |
| Heart Rate (beats/minutes) | 60.2 ± 5.5 | 60.5 ± 6.6 | 0.719 |
| Rest period (msec) | 185 ± 49 | 175 ± 47 | 0.023 |
| Respiratory frequency (breaths/minute) | 16.2 ± 2.2 | 16.3 ± 2.1 | 0.907 |
| Diaphragm Excursion (mm) | 14.8 ± 6.8 | 14.6 ± 6.6 | 0.867 |
| DM (percentage) | 33 (22) | 16 (20) | 0.672 |
| HTN (percentage) | 88 (59) | 40 (49) | 0.158 |
Coronary wall MR images of different image qualities are shown in Figures 2 – 4 to demonstrate the contributors to optimal image quality.
Figure 2.
A 76-year-old female with a 5-year history of HTN. Her heart rate was 58 beats/minute. The rest period of cardiac motion was 220 msec. Her body weight was 51.2 kg, and her BMI was 22.5. Her breathing frequency was 18 breaths/minute. The excursion of the diaphragm was 16 mm.
a. For the LM, the initial location of the NAV was 127 mm. The green box is the acceptance window of the NAV (± 2.5 mm) and the red box is the search window for the diaphragm (± 32 mm). *
b. The location of the NAV was 127 mm at the end of the scan. The drift of the NAV was 0 mm during the scan. The scan efficiency was 49%.
c. The image quality of the LM was ranked as 3.
Figure 4.
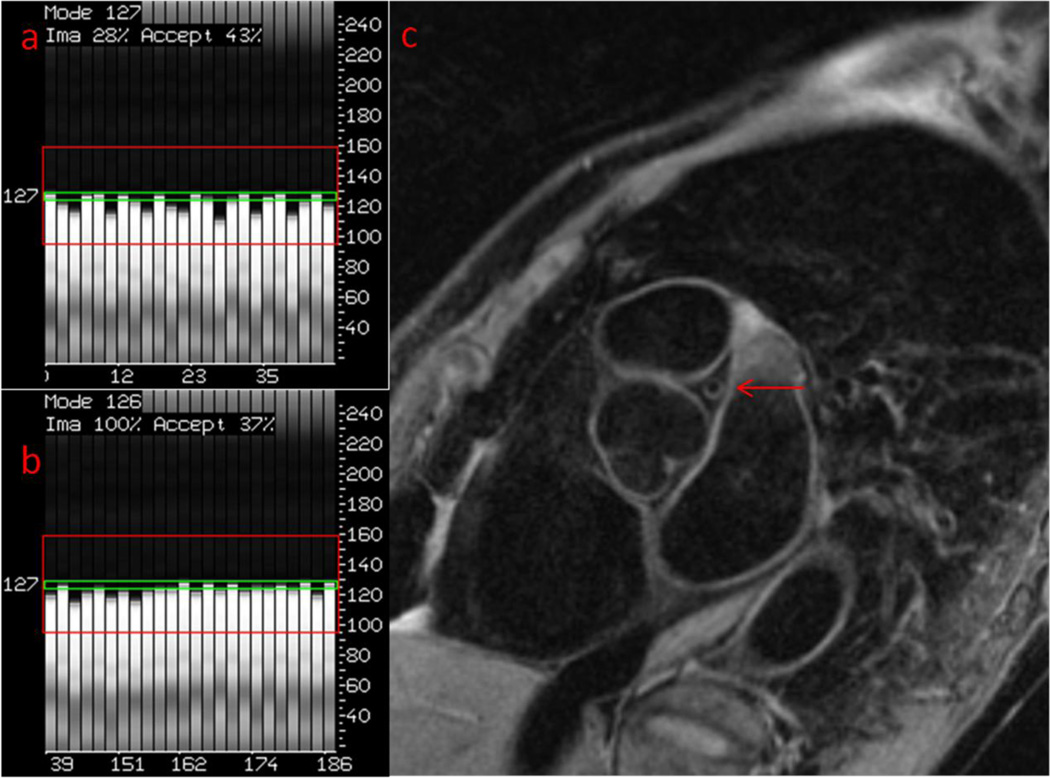
A 72-year-old, healthy female. Her heart rate was 52 beats/minute. The rest period of cardiac motion was 280 msec. Her body weight was 57.4 kg, and her BMI was 23.0. Her breathing frequency was 18 breaths/minute. The excursion of the diaphragm was 18 mm.
a. For the LM, the initial location of the NAV was 123 mm.
b. The location of the NAV was 120 mm at the end of the scan. The drift of the NAV was 3 mm during the scan. The scan efficiency was 24%.
c. The image quality of the LM was ranked as 1.
* The presentation of the location of the NAV on MR scanners may be vendor-specified.
Discussion
In the present study, we found that minimal drift of the NAV location and low body weight were independent predictors of optimal image quality in 2D, free-breathing, black-blood coronary wall MR imaging. We quantitatively demonstrated the effects of respiratory motion, which is a presumed technical barrier for coronary wall MR imaging, in an older population with low heart rates.
Breathing patterns may vary greatly among individuals. In a physiological study, the six most common rhythms of breathing were described in healthy volunteers. Fairly regular rhythm, which is the most common respiratory type, was defined as a breathing pattern with constant frequency and ventilation. Five irregular breathing patterns, which comprised changing, intermittent periods or tidal volume fluctuations, were also observed in normal subjects. Older adults were more likely to have irregular breathing than youths[13]. Nevertheless, only those breathing activities that directly affect heart motion need to be addressed in cardiac MR imaging. Previous studies proved that the movement of the heart during regular tidal breathing is dominated by motion at the cephalocaudal axis, which is linearly related to the motion of the diaphragm in the same directions[14, 15]. Using MR imaging, diaphragm motion can be dynamically monitored[7]. In studies using small sample sizes of healthy volunteers[7, 8], the large drift of the end-expiration position of the diaphragm has been related to low scan efficiency and to the image quality of free-breathing 3D coronary MR angiography. Lower scan efficiency suggested poor image quality and required a repositioning of the NAV windows[8]. Although 2D free-breathing coronary wall MR imaging uses similar motion-control strategies, its success rate (approximately 30% – 60%) has been reported to be significantly lower than whole-heart MR angiography (86% – 92%)[5, 11, 16–20]. Such a discrepancy suggests that these two imaging methods have different susceptibilities to certain physiological conditions. In our study, it was not necessary to adjust the location of the NAV manually during scanning because the motion-adaptive technique allowed the acceptance window of the NAV to adjust automatically, according to the position of the dome. Therefore, the drifts of the NAV, either up-drift or down-drift, closely reflected the locations of the dome at end expiration. Our results also showed that scan efficiency has a close relationship to the drift of the NAV location. Although scan efficiency was found to be higher in good images than in poor images, it did not serve as an independent determinant of image quality in the present study.
To highlight the effects of respiratory motion, we only included older subjects with slow heart rates in the cohort for analysis because uncontrolled cardiac motion on its own will destroy a coronary wall MR image. In addition, medications for lowering heart rates, such as beta blockers, may potentially affect breathing behaviors[21]. Because coronary wall MR imaging has not been routinely applied in clinical practice, the cut-off point (70 beats/minute) in the present study was selected based on a criterion used in a multi-center clinical study that used multiple-slice computed tomography coronary angiography[22]. However, we still found that the rest periods in "successful" cases were significantly longer than those in "unsuccessful" cases. This result suggested notable effects of cardiac motion on image quality, even under conditions of low heart rates.
In addition to motion-related factors, some other physiological conditions may also significantly affect MR image quality. We found that low body weight, which is unrelated to both heart beat and respiratory movement, is also a strong predictor of image quality in coronary wall MR imaging. Similar to the phenomenon described in an MR imaging study of obese patients, this finding may be explained by a thinner body wall incurring less attenuation of MR signals[23].
Our study had some limitations. First, we did not consider the potential effects of coronary lesions, such as plaque burden, on MR image quality[9]. We believe that plaque burden is a coronary measurement, the accuracy of which relies on good image quality. Therefore, it seems inappropriate to evaluate image quality using a source gauged from such images. Second, we did not compare some common physical characteristics of the images, such as signal-to-noise ratio (SNR) or contrast-to-noise ratio (CNR). We thought it would be inappropriate to analyze these measurements when the coronary wall was blurred on some of the poor-quality images. Third, being limited by our study cohort (an asymptomatic population) and the fixed MR imaging protocols of the CHARISMA study, we were unable to compare the coronary measurements between patients with CAD and healthy controls. Fourth, we noticed that some adjuncts, such as abdominal belts, have been applied to stabilize respiratory motion during coronary MR angiography[17, 24, 25]. However, because of the fixed protocol of this observational study, we could not try possible remedies for adjusting breath modes to improve the image quality of coronary wall MR imaging. For the same reason, we did not evaluate the effects of breathing on other similar coronary wall MR imaging techniques, such as 3D black-blood MR imaging[26, 27]. Fifth, we didn't discriminated "up-drift" or "down-drift" for the location of NAV[8], since the level of NAV may fluctuate to different directions in a single scan.
Conclusions
The stability of respiratory motion significantly influences the image quality of 2D, free-breathing, black-blood coronary wall MR imaging. The drift of the NAV location will be useful for quantitatively evaluating the efficacies of attempts aimed at improving coronary wall MR imaging.
Figure 3.
A 69-year-old male with an 8-year history of DM. His heart rate was 62 beats/minute. The rest period of cardiac motion was 200 msec. His body weight was 68.9 kg, and his BMI was 24.4. His breathing frequency was 15 breaths/minute. The excursion of the diaphragm was 21 mm.
a. For the LM, the initial location of the NAV was 132 mm.
b. The location of the NAV was 133 mm at the end of the scan. The drift of the NAV was 1 mm during the scan. The scan efficiency was 29%.
c. The image quality of the LM was ranked as 2.
Acknowledgments
This study was supported by a grant from the National Institute of Health (R01HL089695) and a grant from the American Heart Association (10CRP3050051)
Footnotes
Financial disclosure:
One co-author, XB, is an employee of SIEMENS health care, Chicago, IL. The data and information of this study are under control by authors who are not SIEMENS employee.
Reference
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics--2012 Update: A Report From the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365:2078–2087. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 3.Fayad ZA, Mani V, Fuster V. The time has come for clinical cardiovascular trials with plaque characterization as an endpoint. Eur Heart J. 2012;33:160–161. doi: 10.1093/eurheartj/ehr243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fayad ZA, Fuster V, Fallon JT, et al. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation. 2000;102:506–510. doi: 10.1161/01.cir.102.5.506. [DOI] [PubMed] [Google Scholar]
- 5.Miao C, Chen S, Macedo R, et al. Positive remodeling of the coronary arteries detected by magnetic resonance imaging in an asymptomatic population: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2009;53:1708–1715. doi: 10.1016/j.jacc.2008.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin K, Lloyd-Jones DM, Liu Y, Bi X, Li D, Carr JC. Potential Quantitative Magnetic Resonance Imaging Biomarkers of Coronary Remodeling in Older Hypertensive Patients. Arterioscler Thromb Vasc Biol. 2012 doi: 10.1161/ATVBAHA.112.245266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor AM, Jhooti P, Firmin DN, Pennell DJ. Automated monitoring of diaphragm end-expiratory position for real-time navigator echo MR coronary angiography. J Magn Reson Imaging. 1999;9:395–401. doi: 10.1002/(sici)1522-2586(199903)9:3<395::aid-jmri6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Taylor AM, Jhooti P, Wiesmann F, Keegan J, Firmin DN, Pennell DJ. MR navigator-echo monitoring of temporal changes in diaphragm position: implications for MR coronary angiography. J Magn Reson Imaging. 1997;7:629–636. doi: 10.1002/jmri.1880070404. [DOI] [PubMed] [Google Scholar]
- 9.Malayeri AA, Macedo R, Li D, et al. Coronary vessel wall evaluation by magnetic resonance imaging in the multi-ethnic study of atherosclerosis: determinants of image quality. J Comput Assist Tomogr. 2009;33:1–7. doi: 10.1097/RCT.0b013e3181648606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 11.Lin K, Bi X, Taimen K, et al. Coronary wall MR imaging in patients with rapid heart rates: a feasibility study of black-blood steady-state free precession (SSFP) Int J Cardiovasc Imaging. 2012;28:567–575. doi: 10.1007/s10554-011-9852-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin K, Lloyd-Jones DM, Liu Y, Bi X, Li D, Carr JC. Noninvasive evaluation of coronary distensibility in older adults: a feasibility study with MR angiography. Radiology. 2011;261:771–778. doi: 10.1148/radiol.11110573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tobin MJ, Chadha TS, Jenouri G, Birch SJ, Gazeroglu HB, Sackner MA. Breathing patterns. 1. Normal subjects. Chest. 1983;84:202–205. doi: 10.1378/chest.84.2.202. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Riederer SJ, Ehman RL. Respiratory motion of the heart: kinematics and the implications for the spatial resolution in coronary imaging. Magn Reson Med. 1995;33:713–719. doi: 10.1002/mrm.1910330517. [DOI] [PubMed] [Google Scholar]
- 15.Bogren HG, Lantz BM, Miller RR, Mason DT. Effect of respiration on cardiac motion determined by cineangiography. Implications concerning three-dimensional heart reconstruction using computer tomography. Acta Radiol Diagn (Stockh) 1977;18:609–620. doi: 10.1177/028418517701800601. [DOI] [PubMed] [Google Scholar]
- 16.Jahnke C, Paetsch I, Nehrke K, et al. Rapid and complete coronary arterial tree visualization with magnetic resonance imaging: feasibility and diagnostic performance. Eur Heart J. 2005;26:2313–2319. doi: 10.1093/eurheartj/ehi391. [DOI] [PubMed] [Google Scholar]
- 17.Kato S, Kitagawa K, Ishida N, et al. Assessment of coronary artery disease using magnetic resonance coronary angiography: a national multicenter trial. J Am Coll Cardiol. 2010;56:983–991. doi: 10.1016/j.jacc.2010.01.071. [DOI] [PubMed] [Google Scholar]
- 18.Sakuma H, Ichikawa Y, Chino S, Hirano T, Makino K, Takeda K. Detection of coronary artery stenosis with whole-heart coronary magnetic resonance angiography. J Am Coll Cardiol. 2006;48:1946–1950. doi: 10.1016/j.jacc.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 19.Sakuma H, Ichikawa Y, Suzawa N, et al. Assessment of coronary arteries with total study time of less than 30 minutes by using whole-heart coronary MR angiography. Radiology. 2005;237:316–321. doi: 10.1148/radiol.2371040830. [DOI] [PubMed] [Google Scholar]
- 20.Lin K, Lloyd-Jones DM, Liu Y, Bi X, Li D, Carr JC. Potential quantitative magnetic resonance imaging biomarkers of coronary remodeling in older hypertensive patients. Arterioscler Thromb Vasc Biol. 2012;32:1742–1747. doi: 10.1161/ATVBAHA.112.245266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Short PM, Williamson PA, Lipworth BJ. Effects of hydrocortisone on acute beta-adrenoceptor blocker and histamine induced bronchoconstriction. Br J Clin Pharmacol. 2012;73:717–726. doi: 10.1111/j.1365-2125.2011.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–2336. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 23.de Bucourt M, Streitparth F, Wonneberger U, Rump J, Teichgraber U. Obese patients in an open MRI at 1.0 Tesla: image quality, diagnostic impact and feasibility. Eur Radiol. 2011;21:1004–1015. doi: 10.1007/s00330-010-2005-2. [DOI] [PubMed] [Google Scholar]
- 24.Ishida M, Schuster A, Takase S, et al. Impact of an abdominal belt on breathing patterns and scan efficiency in whole-heart coronary magnetic resonance angiography: comparison between the UK and Japan. J Cardiovasc Magn Reson. 2011;13:71. doi: 10.1186/1532-429X-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McConnell MV, Khasgiwala VC, Savord BJ, et al. Comparison of respiratory suppression methods and navigator locations for MR coronary angiography. AJR Am J Roentgenol. 1997;168:1369–1375. doi: 10.2214/ajr.168.5.9129447. [DOI] [PubMed] [Google Scholar]
- 26.Kim WY, Astrup AS, Stuber M, et al. Subclinical coronary and aortic atherosclerosis detected by magnetic resonance imaging in type 1 diabetes with and without diabetic nephropathy. Circulation. 2007;115:228–235. doi: 10.1161/CIRCULATIONAHA.106.633339. [DOI] [PubMed] [Google Scholar]
- 27.Stuber M, Botnar RM, Danias PG, et al. Double-oblique free-breathing high resolution three-dimensional coronary magnetic resonance angiography. J Am Coll Cardiol. 1999;34:524–531. doi: 10.1016/s0735-1097(99)00223-5. [DOI] [PubMed] [Google Scholar]