Abstract
Delta opioid receptors (DORs) have been considered as a potential target to relieve pain as well as treat depression and anxiety disorders, and are known to modulate other physiological responses, including ethanol and food consumption. A small number of DOR selective drugs are in clinical trials, but no DOR selective drugs have been approved by the Federal Drug Administration and some candidates have failed in phase II clinical trials, highlighting current difficulties producing effective delta opioid based therapies. Recent studies have provided new insights into the pharmacology of the DOR, which is often complex and at times paradoxical. This review will discuss the existing literature focusing on four aspects: 1) Two DOR subtypes have been postulated based on differences in pharmacological effects of existing DOR-selective ligands 2) DORs are expressed ubiquitously throughout the body and central nervous system and are, thus, positioned to play a role in a multitude of diseases. 3) DOR expression is often dynamic, with many reports of increased expression during exposure to chronic stimuli, such as stress, inflammation, neuropathy, morphine, or changes in endogenous opioid tone. 4) A large structural variety in DOR ligands implies potential different mechanisms of activating the receptor. These combined features of DOR pharmacology illustrate the potential benefit of designing tailored or biased DOR ligands.
Keywords: opioid receptor, GPCR, trafficking, localization, drug, alcohol, depression, anxiety, allodynia
1. Introduction
Opioids are some of the earliest drugs used medicinally, with utility dating back thousands of years to the use of opium. Opioid based mixtures continued to be used for centuries without a proper understanding of the mechanism of action. It was not until the beginning of the 19th century that some of the active analgesic alkaloids, like morphine and codeine, were isolated from opium. This was followed a century later, in the midst of the Second World War, with the synthesis of the first synthetic opiates (Brownstein 1993). By this time, receptor theory had become reasonably well established (Maehle et al. 2002) and in 1973 binding studies with radiolabeled opioids performed by Pert, Snyder, Terenius and Simon suggested the existence of multiple different opioid receptors with distinct distribution patterns (Kuhar et al. 1973; Pert and Snyder 1973; Simon et al. 1973; Terenius 1973) and see Simon et al for review (Simon and Hiller 1978)). This hypothesis was further supported by the isolation of endogenous opioids (Cox et al. 1976; Goldstein 1976; Goldstein et al. 1979; Hughes et al. 1975). Using morphine and ketacyclazocine Martin et al proposed the existence of kappa (KOR) and mu (MOR) opioid receptors (Martin et al. 1976) and, a year later, the delta opioid receptor (DOR) was proposed based on the preference of leu-enkephalin to bind to receptors in the mouse vas deferens (Lord et al. 1977). These studies were soon followed by the cloning of the genes encoding the endogenous opioids pro-opiomelanocortin (Nakanishi et al. 1979), preproenkephalin (Noda et al. 1982) and prodynorphin genes (Civelli et al. 1985), and subsequently the cloning of the genes for the receptors, DOR (Evans et al. 1992; Kieffer et al. 1992), MOR (Chen et al. 1993; Thompson et al. 1993; Wang et al. 1993) and KOR (Li et al. 1993; Meng et al. 1993; Minami et al. 1993).
DOR subtypes
Prior to the cloning of the DOR gene, several studies reported ambiguous DOR pharmacology (Negri et al. 1991b; Vaughn et al. 1990; Xu et al. 1991). Some of these studies proposed the existence of an “uncomplexed” DOR and a DOR that was “complexed” with the MOR. These DOR subtypes were labeled DORcx, the DOR in complex with MOR, and DORncx, the non-complexed DOR (Rothman et al. 1992a; Rothman et al. 1992b; Rothman et al. 1991). New DOR selective ligands were also identified and synthesized and several groups classified them into two classes, whereby agonists of one class would not produce cross-tolerance to an agonist of the other class (Mattia et al. 1991). The existence of more than one class of DOR was further supported by observations that antagonist(s) from one class could not block the effects of an agonist from the other class (Mika et al. 2001; Sofuoglu et al. 1991; Sofuoglu et al. 1993; Thorat and Hammond 1997).
These findings led investigators to propose the existence of two distinct DOR subtypes, DOR1 and DOR2. Based mostly on pharmacological studies of cross tolerance and selective antagonism, some DOR ligands were classified as DOR1-selective (DPDPE, TAN67, DALCE and BNTX) and other as DOR2-selective (Deltorphin II, DSLET, Naltriben and 5'NTII). However, the underlying molecular mechanisms responsible for this subtype classification is poorly understood (Dietis et al. 2011), especially once it was clear there was only a single DOR gene and unlike for the MOR (Pasternak 2004), splice variants were much less apparent (Gaveriaux-Ruff et al. 1997). Also it has proven difficult to recapitulate DOR subtype specific pharmacology in vitro in cells expressing only DOR (Parkhill and Bidlack 2002). This suggests that the subtypes only appear post-translational at the protein-level, rather than at the gene-level. This would agree, for example, with the observation that protein-protein interactions between the DOR and MOR contribute to subtype pharmacology. Another option is that simultaneous blockade or stimulation of DORs and MORs that are expressed in the same brain circuit, but not necessarily in the same neuron, could produce a pharmacological effect distinct from ligands that only occupy DORs, and not MORs. For the latter hypothesis to be true one would expect to see a clear difference in DOR/MOR selectivity ratio between the DOR subtypes. However, it does not appear that DOR1 selective ligands display higher MOR affinity compared to DOR2 selective ligands (Table 1), which would argue against the “circuitry” hypothesis.
Table 1.
Binding affinities for the delta and mu opioid receptor for a selection of DOR1, DOR2, and DOR-selective ligands.
| Ligand | DOR | MOR | Selectivity | [3H]DOR | [3H]MOR | Ref‡ | |
|---|---|---|---|---|---|---|---|
| Delta1 | TAN-67 | 0.647 | 775 | 1197 | [3H]NTI | [3H]CTOP | [1] |
| DPDPE | 14 | >1000 | >71 | [3H]NTI | [3H]DAMGO | [2] | |
| BNTX | 0.66 | 18 | 27.3 | [3H]NTI | [3H]DAMGO | [2] | |
| DALCE | 4.1 | 55 | 13.4 | [3H]DPDPE | [3H]DAMGO | [3] | |
| Delta2 | SNC80 | 0.181 | 3900 | 21547 | [3H]DPDPE | [3H]DAMGO | [4] |
| Naltriben | 0.013 | 12 | 923 | [3H]NTI | [3H]DAMGO | [2] | |
| DeltorphinII | 3.3 | >1000 | >303 | [3H]NTI | [3H]DAMGO | [2] | |
| DSLET | 4.8 | 39 | 8.1 | [3H]NTI | [3H]DAMGO | [2] | |
| 5'NTII | 5.9 | ND | NA | [3H]DPDPE | NA | [5] | |
| Delta | ADL5859 | 0.84 | >10000 | >11904 | [3H]DPN | [3H]DPN | [6] |
| TIPPpsi | 0.308 | 3230 | 10487 | [3H]DSLET | [3H]DAMGO | [7] | |
| ARM390 | 0.87 | 3800 | 4367 | [125I]Delt II | [125I]FK33824 | [8] | |
| NTI | 0.02 | 64 | 3200 | [3H]NTI | [3H]DAMGO | [2] | |
| AZD2327 | 0.49 | 770 | 1571 | [125I]Delt II | [125I]FK33824 | [9] | |
| JNJ | 2 | 1191 | 595 | [3H]DPDPE | [3H]DAMGO | [10] | |
| SB235863 | 4.81 | 906 | 188 | [3H]DADLE | [3H]DAMGO | [11] | |
| ICI174,864 | 193 | 24700 | 128 | [3H]DADLE | [3H]DAMGO | [7] | |
| SOri9409 | 2.2 | 51 | 23 | [3H]DADLE | [3H]DAMGO | [12] | |
| DADLE | 0.74 | 16 | 21 | [3H]NTI | [3H]DAMGO | [2] | |
| Leu-Enkephalin | 4 | 3.4 | 0.9 | [3H]NTI | [3H]DAMGO | [2] | |
| Biphalin | 2.6 | 1.4 | 0.5 | [3H]DPDPE | [3H]CTOP | [13] | |
| Met-Enkephalin | 1.7 | 0.65 | 0.4 | [3H]NTI | [3H]DAMGO | [2] |
(ND = not determined, NA = not applicable)
Reference: [1] (Knapp et al. 1995), [2] (Raynor et al. 1994), [3] (Bowen et al. 1987), [4] (Knapp et al. 1996), [5] (Portoghese et al. 1992), [6] (Le Bourdonnec et al. 2008), [7] (Schiller 2004), [8] (Wei et al. 2000), [9] (Hudzik et al. 2011), [10] (Codd et al. 2009), [11] (Petrillo et al. 2003), [12] (Xu et al. 2001), [13] (Misicka et al. 1997)
DOR selective ligands
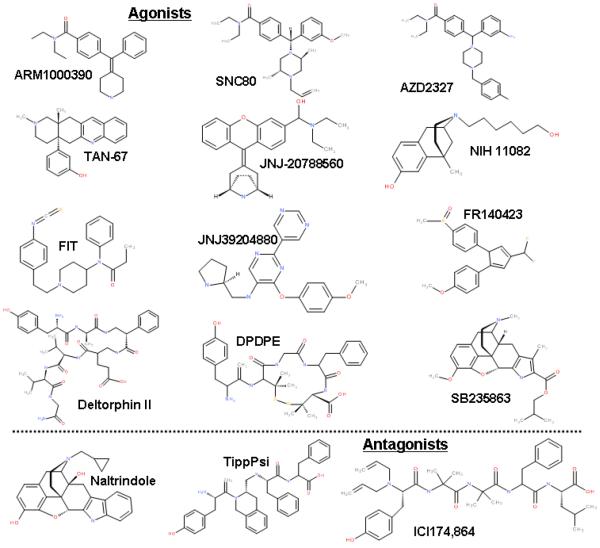
There is a large structural variety in compounds that have moderate to high affinity for the DOR (Figure 1). Endogenous opioids have been isolated from humans (e.g. Met- and Leu-enkephalin) as well as from other species such as frogs (e.g. deltorphin I and II) (Erspamer et al. 1989) snake venom (Picolo and Cury 2004) and spinach (Yang et al. 2003). Some of these peptides have served as template or have been derivatized to create more selective, potent and/or stable peptidic ligands that are less prone to enzymatic degradation, such as [D-Ala2, D-Leu5]-Enkephalin (DADLE), D-Ser2-Leu-Enkephalin-Thr6 (DSLET), [d-Pen2,d-Pen5]-Enkephalin (DPDPE) and the tetrapeptide JOM-13 (David et al. 1982; Kosterlitz et al. 1980; Mosberg et al. 1983; Mosberg and Kroona 1992). A major concern with these compounds from a therapeutic point of view is their poor ability to cross the blood brain barrier, although some reports have suggested the existence of pumps that can transport DPDPE and deltorphin II across the blood brain barrier (Fiori et al. 1997; Williams et al. 1996). For that reason, numerous small non-peptidic, DOR-selective alkaloids have been developed. Some of these compounds are morphinan derivatives (BNTX, NTI, SoRI-9409), whereas others use different alkaloid scaffolds such as diarylmethylpiperazines (SNC80, ARM1000390, AZD2327) (Calderon 2011), isoquinoline (TAN-67, KNT-127), spiro (ADL5747) and diarylmethylenepiperidine (JNJ-39204880). More recently, bi-functional and bivalent ligands (Ananthan 2006; Fujita et al. 2004; Harvey et al. 2012) have been designed that have affinity for DOR as well as for MOR including KSK-103 (Miyamoto et al. 1993a; Purington et al. 2011), MDAN (Daniels et al. 2005), L2 and L4 (Harvey et al. 2012). The bivalent ligands have been designed in part to test the hypothesis that DORs heterodimerize with MORs to form an opioid receptor target with unique functionality (Abdelhamid et al. 1991; Gendron et al. 2007b; Gomes et al. 2004; van Rijn et al. 2012b; van Rijn and Whistler 2009). The bi-functional ligands would fit the hypothesis that ligands acting on DOR and MOR can produce a unique functional response that is different from selective DOR or MOR ligands.
Figure 1.
Overview of a variety of selective delta-opioid receptor agonists and antagonists.
DOR subtypes
As mentioned in the introduction, complex pharmacological observations that could not be reconciled by a single DOR have led to the proposed existence of multiple DOR subtypes (DOR1 and DOR2). Yet, despite that numerous new DOR selective compounds have been synthesized, none of these DOR compounds have been classified as DOR1 or DOR2 beyond those mentioned in the introduction. Additionally, little research is currently performed that is specifically designed to understand the basis of the two subtypes. The potential of DOR agonists to act on different targets has become more interesting due to findings by us and others that subtype-selective DOR ligands can have opposing behavioral effects. More specifically, the DOR2 selective antagonist NTB reduces ethanol intake (Krishnan-Sarin et al. 1995; van Rijn and Whistler 2009), yet a DOR1 agonist TAN-67 also decreases ethanol intake (van Rijn and Whistler 2009), and a DOR agonist SNC80, increases both ethanol intake (Nielsen et al. 2012; van Rijn et al. 2010) and ethanol lever pressing (Platt and Bano 2011). The differential effects on ethanol intake of these compounds were further supported by findings that these two DOR subtype selective agonists also modulate ethanol preference in opposite directions. Specifically, TAN-67 increases ethanol place preference (Matsuzawa et al. 1998; van Rijn et al. 2012a), whereas SNC80 decreases ethanol place preference (van Rijn et al. 2012a). The place preference data would suggest that a DOR1 agonist reduces ethanol intake by increasing the reinforcing effect of ethanol, whereas SNC80 increases ethanol intake by reducing ethanol reinforcement learning. This is in sharp contrast with the opioid antagonist naltrexone, which decreases ethanol place preference as well as ethanol intake (Le et al. 1993; Middaugh and Bandy 2000; Phillips et al. 1997; van Rijn and Whistler 2009), suggesting that TAN-67, SNC80 and naltrexone affect ethanol related behavior in three different ways.
We and others have provided evidence suggesting certain DOR subtype selective ligands require the presence of both DOR and MOR to be effective (Gendron et al. 2007b; van Rijn et al. 2012b) for example in relation to ethanol intake (van Rijn and Whistler 2009). Studies have shown that DOR can directly interact with MOR to form a DOR-MOR heteromer (George et al. 2000; Gomes et al. 2000). This DOR-MOR heteromer has the ability to activate signal transduction properties different from DOR monomers/homomers (Hasbi et al. 2007; Rozenfeld and Devi 2007). Thus heteromerization of DORs with MORs can be responsible for the differential effects observed with DOR subtype-selective ligands.
DOR subtype selectivity appears to play an important role in the ability of DOR ligands to induce or suppress coughing. The DOR1 antagonist BNTX is an antitussive (Kamei et al. 1994b), whereas the DOR1 agonist DPDPE inhibited the antitussive effects of MOR agonists morphine and DAMGO (Kamei et al. 1991). Conversely, the DOR2 agonist deltorphin II enhanced the antitussive responses of DAMGO (Kamei et al. 1993b). The selective DOR agonist SB 227122 reduced citric acid induced coughing in guinea pigs (Kotzer et al. 2000), suggesting that SB 227122 may activate DOR2. The effect of SB 227122 was blocked by the DOR antagonist SB 244525, which by itself had no effect on coughing (Kotzer et al. 2000). It is currently not known if SB 244525 has DOR subtype selectivity. And while the non-subtype-selective DOR antagonist NTI functioned as antitussive (Kamei et al. 1993a), this may rather be caused by its effects on KOR at high enough concentrations. To avoid any off target effects, antagonists with improved selectivity for DOR over KOR and MOR have been developed and these drugs: TRK-850 [(5R,9R,13S,14S)-17-cyclopropylmethyl-6,7-didehydro-4,5-epoxy-5′,6′-dihydro-3-methoxy-4′H-pyrrolo[3,2,1-ij]quinolino[2′,1′:6,7]morphinan-14-ol methanesulfonate] (Sakami et al. 2008b), and the more metabolically stable TRK-851 (Sakami et al. 2008a) show strong antitussive effects. Being antagonists, side effects such as respiratory depression and dependence may be less of an issue with these drugs. A recent paper showed the presence of an antitussive alkaloid, kopsinine, with DOR affinity in Kopsia Hainanensis (Tan et al. 2010). Kopsinine, however, most likely acted as an agonist, and therefore may have a worse side effect profile than a non-selective DOR or DOR1-selective antagonist. Currently, no studies have investigated if the DOR1-selective antitussive effects require the presence of MORs, which could hint at the target being a DOR-MOR heteromer.
There also appears to be subtype selectivity in the ability of DOR ligands to reduce physical dependence on morphine, as the DOR1 antagonists DALCE (Miyamoto et al. 1994) and BNTX (Suzuki et al. 1997) do not reduce precipitated withdrawal symptoms as effectively as DOR2 antagonists (Miyamoto et al. 1993a). Inhibition of DOR with DOR-(Chefer and Shippenberg 2009; Suzuki et al. 1994), DOR1- (Suzuki et al. 1994) and DOR2-selective(Billa et al. 2010; Suzuki et al. 1994) antagonists has also been shown to block morphine place preference acquisition. The DOR1 agonist TAN-67 enhances morphine conditioned place preference (Suzuki et al. 1996), yet a DOR2 antagonists prevents sensitized morphine place preference (Shippenberg et al. 2009) and can precipitate morphine withdrawal (Miyamoto et al. 1993b), suggesting that both DOR subtypes modulate aspects of the reinforcing effects and physical dependence inducing properties of morphine—and that they may do so in opposite directions. This complexity is also observed in studies that show that endogenous opioids that produce antinociception (Kim et al. 2003; Vanderah et al. 1992) when released after cold water swim stress can be blocked by a DOR2 antagonist (5'NTII) but not a DOR1 antagonist (DALCE) (Vanderah et al. 1992).
Differences in expression levels and localization of DOR subtypes within certain brain circuits complicate assessing the subtype selectivity of DOR selective ligands. Whereas the DOR agonist SNC80 produces anxiolytic-like effects in both naïve and ethanol withdrawn mice, the DOR1 agonist TAN-67 reduces anxiety-like behavior only in mice that have consumed ethanol (van Rijn et al. 2010), suggesting an emergence of DOR1s in ethanol withdrawn mice. Similarly the DOR2 antagonist NTB, but not the DOR1 antagonist BNTX, increases anxiety-like behavior (Saitoh et al. 2005). Yet, microinjection of the DOR1 agonist DPDPE in the central nucleus of the amygdala decreases anxiety (Randall-Thompson et al. 2010) in naïve mice. Similarly anxiolytic-like effects for the DOR1 agonist KNT-127 have been reported in rats (Saitoh et al. 2012). This suggests that location and expression level of DOR1 and DOR2s may affect anxiety-like behavior to different extents, such that it becomes difficult to determine whether the effects of a systemically injected DOR-subtype selective compound is mediated by only that subtype. It is important to note that several DOR ligands including both DOR1 and DOR2 subtype-selective agonists (Pakarinen et al. 1995; Ukai et al. 1997) disrupt learning (Le Merrer et al. 2013), although less than scopolamine (Jutkiewicz et al. 2003), a muscarinic antagonist known to impair learning. Thus it is conceivable that a systemically injected DOR agonist at a dose where it affects learning, may influence task performance when measuring for example responses in place preference (Le Merrer et al. 2012), elevated plus maze or forced swim paradigms. This is particularly important to consider when an agonist response changes between local and systemic administration.
Selectively targeting a single DOR subtype could potentially reduce side effects, or at least display a different side effect profile. For example, Saitoh et al demonstrated that the DOR1 agonist KNT-127 has a lower potency to induce convulsion than SNC80 (Saitoh et al. 2011). The DOR1 agonists TAN-67 and DPDPE did not induce seizures themselves (Tortella et al. 1984; Yajima et al. 2000), whereas the DOR2 agonist deltorphin II does (Capasso and Cavallo 2005; Di Giannuario et al. 2001). DOR1- but not DOR2-selective ligands modulate the ability of the antiepileptic drug zonisamide to alleviate Parkinson's symptoms (Yamamura et al. 2009). A small number of studies have investigated the role of DOR subtypes on immune responses; a DOR1 agonist can activate granulocytes and immunocytes (Stefano et al. 1992), whereas only a DOR2, but not a DOR1 agonist can suppress splenic immune responses (Rahim et al. 2001). However, it appears that DOR subtype selectivity plays less of an important role for some other potential DOR-mediated side effects. Many DOR1- and DOR2-subtype selective agonists (DPDPE (Charron et al. 2008; Lasukova et al. 2009; Yang et al. 2011), SNC-121(Patel et al. 2006), Eribis peptide 94(Karlsson et al. 2011), fentanyl isothiocyanate[FIT] (Gross et al. 2005), BW373U86 (Peart et al. 2011), TAN-67 (Guo et al. 2005), ARD-353 (Watson et al. 2006), deltorphin II (Maslov et al. 2010a) have cardioprotective abilities. While a study by Mslov et al suggested that only the DOR2 selective deltorphin II, but not DPDPE, reduced infarct size (Maslov et al. 2010b), these differences may relate to experimental procedures (e.g. duration of occlusion, reperfusion). Thus it appears that there are indications such as ethanol use, seizure and cough that react differently to DOR1- than to DOR2-subtype-selective ligands (Table 2), as well as physiological responses that do not show DOR-subtype selectivity. While DOR selective therapies have not yet been approved by the Federal Drug Administration, several DOR agonists are in clinical trials for treatment of neuropathic/inflammatory pain, as well as anxiety/depression and as antitussive.
Table 2.
Schematic overview of the physiological effects of delta opioid receptor subtype-elective agonists and antagonists. (NE = no effect).
| Physiological effect | Agonist | Antagonist | References‡ |
|---|---|---|---|
| Anxiety-like behavior | δ1↓, δ2↓ | δ1 NE, δ2↑ | [1–5] |
| Ethanol consumption | δ1↓, δ2↑ | δ 2 ↓ | [3, 6–8] |
| Ethanol place preference | δ1↑, δ2↓ | δ ↓ | [9, 10] |
| Cough | δ1↑, δ2↓ | δ 1 ↓ | [11–13] |
| Seizure threshold | δ1 NE/↑, δ2↓ | - | [14–19] |
| Locomotion | δ 2 ↑ | - | [20, 21] |
| HIV infection | δ 2 ↓ | - | [22] |
| Morphine dependence | - | δ1 NE, δ2↓ | [23–26] |
| Intestinal motility | δ1↓, δ2↓ | - | [27–32] |
| Depression-like behavior | δ1↓, δ2↓ | - | [16, 33] |
| Ischemic injury | δ1↓, δ2↓ | - | [34–39] |
| Hyperalgesia | δ1↓, δ2↓ | - | [40–46] |
| Memory | δ1↓, δ2↓ | - | [47, 48] |
[1] (van Rijn et al. 2010), [2] (Saitoh et al. 2005) [3], (Margolis et al. 2008), [4] (Sugiyama et al. 2012), [5] (Randall-Thompson et al. 2010), [6] (Krishnan-Sarin et al. 1995) [7] (van Rijn and Whistler 2009), [8] (Matsuzawa et al. 1998), [9] (Bie et al. 2009a), [10] (van Rijn et al. 2012a), [11] (Kamei et al. 1994b), [12] (Kamei et al. 1993b), [13] (Kamei et al. 1991), [14] (Tortella et al. 1984), [15] (Tortella et al. 1988), [16] (Saitoh et al. 2011), [17] (Yajima et al. 2000), [18] (Di Giannuario et al. 2001), [19] (Capasso and Cavallo 2005), [20] (Mizoguchi et al. 1996), [21] (Fraser et al. 2000b), [22](Sharp et al. 1998), [23] (Miyamoto et al. 1994), [24] (Miyamoto et al. 1993a), [25] (Miyamoto et al. 1993b), [26] (Suzuki et al. 1997), [27] (Broccardo and Improta 1992b), [28] (Broccardo and Improta 1992a), [29] (Krevsky et al. 1991), [30] (Fox-Threlkeld et al. 1994), [31] (Pairet and Ruckebusch 1984), [32] (Shook et al. 1989), [33] (Torregrossa et al. 2006), [34] (Charron et al. 2008), [35] (Maslov et al. 2010b), [36] (Maslov et al. 2010a), [37] (Guo et al. 2005), [38] (Yang et al. 2011), [39] (Lasukova et al. 2009), [40] (Stewart and Hammond 1994), [41] (Mika et al. 2001), [42] (Taiwo and Levine 1991), [43] (Fraser et al. 2000a), [44] (Hurley and Hammond 2000), [45] (Cahill et al. 2003), [46] (Holdridge and Cahill 2007), [47] (Ukai et al. 1997), [48] (Castellano and Pavone 1985)
DOR expression and potential role in (patho)physiology
Early studies using in situ hybridization and autoradiography revealed that the delta opioid receptor and its cognate endogenous opioids are widely expressed throughout the human body and central nervous system (Dilts and Kalivas 1990; Goodman et al. 1980; Harlan et al. 1987; Mansour et al. 1994; Mansour et al. 1987; Shivers et al. 1986). The production of a transgenic mouse line expressing a DOR fused to a green fluorescent protein (DOR-eGFP) has provided further confirmation for the ubiquitous nature of DOR expression (Erbs et al. 2012; Faget et al. 2012; Poole et al. 2011; Scherrer et al. 2006) and seems to match localization patterns of DORs produced by immunohistochemistry (Cahill et al. 2001a). This is significant as an often recurring issue for DOR expression studies is antibody specificity (Chabot-Dore et al. 2008; Scherrer et al. 2009). It is important to note that the mRNA and protein expression as well as cellular localization and trafficking pattern of DOR-eGFP deviates from the wild-type receptor (Scherrer et al. 2006; Wang et al. 2008). This would limit the DOR-eGFP mice use to regional localization studies, and may make it harder to draw conclusions with regard to cellular localization and redistribution after chronic stimulation if not additionally confirmed in wild-type mice.
Like MORs, DORs are expressed in pain circuits, both in supraspinal (Le Merrer et al. 2009) and spinal regions (Onofrio and Yaksh 1983), although, there may be some differences in the types of nociception the DOR is involved in (Scherrer et al. 2009; van Rijn et al. 2012b), compared to MOR, DORs are not as abundantly expressed in midbrain regions such as the periaqueductal grey (Pradhan et al. 2011). Often DOR mediated antinociception is observed in models of chronic pain, such as chronic inflammation or neuroconstriction (Cahill et al. 2003; Holdridge and Cahill 2007; Kabli and Cahill 2007; Zhang et al. 2006b). However, also in naïve mice DOR agonists can produce potent mechanical antinociception (Ochi et al. 1999; Scherrer et al. 2009; van Rijn et al. 2012b), an effect that has been attributed to DOR expression in peripheral NaV1.8-positive primary nociceptive neurons (Gaveriaux-Ruff et al. 2011).
The DOR is expressed in regions commonly associated with depression and mood disorders. These include the hippocampus, nucleus accumbens and frontal cortex which are associated with motor and motivational control (Tejedor-Real et al. 1998). DORs are also expressed in brain regions implicated in stress: the amygdala, locus coeruleus (Alvira-Botero and Garzon 2006; Scherrer et al. 2006) and regions of the hypothalamus where they are co-localized with stress hormones (Williams and Milner 2011) and can regulate corticotropin releasing hormone release (Williams and Milner 2011). The antitussive effects of opioids, including DOR selective opioids stems from actions in the CNS but also in part, from the localization of opioid receptors on sensory nerve fibers in the vagus nerve in the lung (Belvisi and Hele 2009).
Current research has revealed potential roles for DORs in neurodegenerative diseases. For example decreased expression of DORs was observed in putamen and amygdaloid regions of Alzheimer patients (Barg et al. 1993; Hiller et al. 1987; Mathieu-Kia et al. 2001; Rinne et al. 1993). Endogenous opioids acting on DORs can inhibit long term potentiation, a form of cellular memory, in the dentate gyrus of the hippocampus (Xie and Lewis 1995), a brain region well known for its role in memory formation (Neves et al. 2008). Thus DOR selective opioids may serve a role in preventing or treating Alzheimer's disease. Parkinson's disease is another neurodegenerative disease, caused by a loss of dopaminergic neurons in the substantia nigra, leading to a lack of muscle movement control due to the inability for the neurons to properly relay signals (Davie 2008). DOR selective ligands may be of therapeutic benefit for Parkinson's Disease considering that DORs are expressed in parts of the basal ganglia (Mansour et al. 1987; Scherrer et al. 2006) and several DOR agonists are reported to induce hyperlocomotion when administered systemically (Perrine et al. 2006), intracerebroventricularly, or into the nucleus accumbens (Churchill et al. 1995; Havemann et al. 1983; Ingman et al. 2003; Katsuura and Taha 2010; Kelley et al. 1996; Michael-Titus et al. 1989; Michael-Titus et al. 1990; Mickley et al. 1990; Mizoguchi et al. 1996; Murray and Cowan 1990; Negri et al. 1991a; Ukai et al. 1989).
Expression of DORs on cardiac myocytes (Ventura et al. 1989) has made these receptors interesting targets to study ischemia and stroke. In particular DOR agonists may protect against ischemic injury (For review see (Johnson and Turner 2010; Schultz and Gross 2001)). Recent studies have suggested a role for DORs in human immunodeficiency virus (HIV) infection. It has been shown that DORs are expressed on T-cells (Carr et al. 1988), which play a key role in HIV infection, and that the DOR agonists SNC80 and deltorphin inhibit the infection rate of HIV into T-cell derived jurkat cells (Sharp et al. 1998). The observation that DOR expression is upregulated in human peripheral blood lymphocytes after stimulation with phytohaemagglutinin (Sharp et al. 2001), which proliferates these cells to activated T-like cells (O'Donovan et al. 1995) increases it functional relevance as potential (co-)therapy of HIV infection.
The expression of DORs in certain brain areas may explain some side effects commonly observed with opioid receptor targeted therapeutics in lab animals and humans. For example DORs have been identified in several brain regions involved in sleep, including the ventral division of the reticular oral pontine nucleus (Alvira-Botero and Garzon 2006) as well as in the nucleus of the solitary tract of the brain stem (Reinoso-Barbero and de Andres 1995). The expression of these DORs could account for some of the somnolence side effects commonly observed during opioid therapies (Cherny et al. 2001; Swegle and Logemann 2006). DORs are also reported to be expressed in respiratory centers of the brain stem (Alheid and McCrimmon 2008; Sales et al. 1985; Stasinopoulos et al. 2000) (dorsal and ventral respiratory group of the medulla oblongata and pontine respiratory group in the rostral pons), and several DOR agonists (SNC80 (Szeto et al. 1999), DSLET (Morin-Surun et al. 1984), DPDPE (Johnson et al. 2008; Szeto et al. 1999), deltorphin II (Szeto et al. 1999) and DADLE (Johnson et al. 2008; Pazos and Florez 1983; 1984)) have reported to modulate respiration.
Using DOR-eGFP knock-in mice, DORs have been identified on submucosal and myenteric neurons (Poole et al. 2011), and it is known that DOR selective compounds can modulate GI motility and transit (Broccardo and Improta 1992b; Broccardo et al. 1998; Chamouard et al. 1994; Negri et al. 1999), potentially leading to constipation or diarrhea. Besides affecting GI motility, opioid receptors including DORs have also been implicated in renal function (Holt et al. 2005; Sezen et al. 1998). It is thought that these effects can be attributed to stimulation of peripheral opioid receptors on kidney cells by enkephalins released from the parasympathetic ganglia (de Groat and Kawatani 1989) as well as effects mediated by DORs in the paraventricular and hypothalamic supraoptic nuclei (Tsushima et al. 1993).
DOR dynamic expression
An intriguing aspect of DOR pharmacology is that while the receptor is ubiquitously expressed, many studies have reported instances of dynamic changes in DOR expression and localization, in particular translocation of DORs from large dense core vesicles to the cell surface (Zhang et al. 2006b). During certain chronic conditions the functional activity of DORs is increased as evidenced by findings that DOR-selective agonists become more potent and efficacious under conditions of inflammatory pain, chronic opioid use, chronic ethanol consumption and repeated stress (Cahill et al. 2007; Cahill et al. 2003; Cahill et al. 2001b; Commons 2003; Kabli and Cahill 2007; van Rijn et al. 2012b; Zhang et al. 2006b) (Table 3). Induction of inflammation and neuropathy causes a cellular redistribution of DORs (Cahill et al. 2003; Kabli and Cahill 2007). Chronic morphine has been shown to increase DOR expression in several areas of the central nervous system (Bie et al. 2010; Cahill et al. 2001b; Hack et al. 2005; Lucido et al. 2005; Ma et al. 2006), including the central nucleus of the amygdala (Chieng and Christie 2009), where the DORs could play a role in opioid withdrawal anxiety.
Table 3.
Overview of conditions for which changes in DOR expression or function or potency of DOR ligands have been reported.
| Increased DOR expression, potency and/or function | References‡ |
|---|---|
| Chronic opioid exposure | [1–13] |
| (Chronic) inflammation | [14–22] |
| Alcohol (withdrawal) | [23–29] |
| Stress | [30–33] |
| Cancer | [34–36] |
| Obesity/diabetes | [37–39] |
| Hypoxia (preconditioning) | [40,41] |
| Neuropathy | [42] |
| Fibromyalgia | [43] |
| Seizure | [44] |
| Failing heart | [45] |
[1] (Cahill et al. 2001b), [2] (Morinville et al. 2003), [3] (Ma et al. 2006), [4] (Bie et al. 2009b), [5] (Chieng and Christie 2009), [6] (Lucido et al. 2005), [7] (Morinville et al. 2004a), [8] (Hack et al. 2005), [9] (Lesscher et al. 2003), [10] (Hyytia et al. 1999), [11] (Khotib et al. 2004), [12] (Bie et al. 2010), [13] (Zhang and Pan 2010), [14] (Cahill et al. 2003), [15] (Codd et al. 2009), [16] (Morinville et al. 2004b), [17] (Gendron et al. 2007b), [18] (Gendron et al. 2007a), [19] (Pol et al. 1994), [20] (Shook et al. 1989), [21] (Wade et al.), [22] (Hurley and Hammond 2000), [23] (van Rijn et al. 2010), [24] (van Rijn et al. 2012b), [25] (Margolis et al. 2008), [26] (Bie et al. 2009a), [27] (Nielsen et al. 2012), [28] (Charness et al. 1986), [29] (Mendez et al. 2004), [30] (Margolis et al. 2011), [31] (Commons 2003), [32] (Hebb et al. 2005), [33] (Pohorecky et al. 1999), [34] (Krajnik et al. 2010), [35] (Madar et al. 2007), [36] (Schreiber et al. 1998), [37] (Evans et al. 2001), [38] (Kamei et al. 1997), [39] (Kamei et al. 1994a), [40] (Zhang et al. 2006a), [41] (Ma et al. 2005), [42] (Kabli and Cahill 2007), [43] (Salemi et al. 2007), [44] (Madar et al. 1997), [45] (Bolte et al. 2009)
Several studies have recently investigated the role of DORs on ethanol intake. Both in vitro and in vivo studies suggest that ethanol exposure modulates DOR expression levels. For example, DORs are upregulated in neuroblastoma cell culture exposed to ethanol (Charness et al. 1986). Increased [3H]DPDPE binding was observed after ethanol exposure in several brain regions including the prefrontal cortex, the nucleus accumbens, different areas of the striatum and the substantia nigra pars reticulate (Mendez et al. 2004). Moreover, DOR function is enhanced in the central nucleus of the amygdala (Bie et al. 2009a), ventral tegmental area (Margolis et al. 2008), striatum (Nielsen et al. 2012) and spinal cord (van Rijn et al. 2012b) after long term ethanol consumption. Yet, functional DORs may already be present in naïve animals based on GTPγS activation studies in several brain regions including, cortex (Nielsen et al. 2012) hippocampus, cerebellum and colliculus (Saland et al. 2004) as well as cAMP inhibition studies in striatum (Shen et al. 1997) and electrophysiology performed in the central nucleus of the amygdala (Kang-Park et al. 2007). Margolis and co-workers found that rats that consume large amounts of ethanol are in general more anxious and express fewer functional DORs in the ventral tegmental area (Margolis et al. 2008). Interestingly, ethanol exposure increases GTPγS activation by the DOR1 agonist TAN-67, but not the DOR2 agonist SNC80 in rat spinal cord (Nielsen et al. 2012). Similarly, it has been reported that obese-diabetic (ob/ob) mice have increased binding of the DOR1 agonist DPDPE in their extensor digitorum longus, and that DPDPE, but not the DOR2 agonist deltorphin II, increases glucose transport in these mice (Evans et al. 2001).
Stress appears to play a strong role in DOR expression. During stress the body does not only release stress hormones such as corticotropin releasing hormone, but it also releases endogenous opioids/enkephalins (Amir et al. 1980; Kalivas and Abhold 1987), suggesting that DORs may be part of a negative feedback system. Indeed, in already stressed mice, DPDPE drastically reduces the response to future stress (Hebb et al. 2005). In rats, foot-shock stress specifically enhances postsynaptic DOR activity in dopaminergic neurons of the ventral tegmental area (Margolis et al. 2011). Stress induced by exposure to cold water in a forced swim paradigm has been reported to cause a cellular redistribution of DORs (Commons 2003). Similarly, stress induced by housing condition or social rank also affects DOR activity (Pohorecky et al. 1999). Downregulation of DORs has been observed in rats that have been sleep deprived by placing them on a small platform surrounded by water. This downregulation may occur due to endocytosis and degradation of DORs after activation by endogenous enkephalins, released by the stressful event. However, it is also possible that this is an adaptation designed to induce alertness and wakefulness (Fadda et al. 1991). Indeed, naltrindole increases wakefulness (Moss et al. 1993), suggesting that activation of DORs by endogenous opioids or exogenous opioids (Reinoso-Barbero and de Andres 1995) induce sleep.
There have been several reports that suggest increases of DOR activity and expression in intestinal tissue by inflammatory stress (Wade et al.). Shook et al found that while DPDPE did not inhibit transit in naïve mice it reduced castor oil induced diarrhea(Shook et al. 1989), which could suggest that castor oil enhances DOR activity. Similarly, diarrhea caused by croton oil induced intestinal inflammation potentiated DOR agonist inhibition of GI transit (Pol et al. 1994). Collectively, these studies indicate that the type and duration of stress affect the expression level of DORs and thus may influence the efficacy of DOR-selective ligands.
While most studies on DOR expression level were performed in rodent and other animal models, some data in humans suggest similar plasticity in DOR expression levels. Human studies have found increases in DOR selective (Madar et al. 1997) opioid PET radioligand binding after an epileptic seizure. Antibodies (Krajnik et al. 2010), RT-PCR (Schreiber et al. 1998) and PET (Madar et al. 2007) studies of human lung carcinoma cells revealed higher expression of DORs compared to normal healthy lung tissue.
3. Future prospects for the development of DOR selective drug treatments
DOR subtypes
Our hypothesis of DOR subtypes, from the overarching literature is that the observation of DOR subtype-selective effect can not simply be explained by invoking the existence of two DOR targets. Instead, we believe that the types of response a DOR-selective ligand will produce strongly depend on the site of action and the presence of other receptors, MOR in particular. In some cases an effect that may have been attributed to a DOR subtype may actually be binding of that ligand to MORs or another receptor as it turns out that the drug is still active in DOR KO mice (He and Lee 1998; Matthes et al. 1998; Scherrer et al. 2004; van Rijn et al. 2012b; Zhu et al. 1999). In some cases the effect may be mediated by a combined effect of the DOR subtype-selective ligand to a target that requires both DOR and MOR (Gendron et al. 2007b; van Rijn et al. 2012b; van Rijn and Whistler 2009). Sometimes, both DOR1 and DOR2 subtype-selective drugs act on the same target (Wild et al. 1993). In other cases this may be attributed to biased agonism or differences in receptor trafficking (Pradhan et al. 2010). It is important to realize that for example a “DOR2 selective” ligand such as deltorphin II can act on MOR in spinal cord neurons mediating thermal pain, but on DOR-MORs in neurons mediating mechanical pain (van Rijn et al. 2012b), and on DORs in yet another region (Hosohata et al. 2000). Similarly, in our ethanol studies we propose that SNC80 acts like a DOR2 agonist as its effect is the opposite of a DOR2 antagonist (van Rijn and Whistler 2009). This is in line with some other studies that found SNC80 effects only to be influenced by NTB, but not BNTX (Baker and Meert 2002; Rawls et al. 2005; Saitoh et al. 2005). Yet other studies have found SNC80 being blocked by both BNTX and NTB (Pacheco et al. 2005), or only BNTX, thus suggesting DOR1 agonism (Zhu et al. 2009). This complexity, when better understood, can provide an incredible opportunity to tailor drugs to produce the proper response for a specific indication. The aforementioned large structural diversity of DOR selective drugs that have been designed so far holds promise for the development of tailored DOR drugs for selective therapies.
Peripheral vs Central effects
Since some of the effects of DOR selective ligands arise from activation of DORs located in the periphery, such as cardiac, mucosal and lung tissue, rather than the central nervous system, this should facilitate the development of peripherally restricted (e.g. peptides, hydrophilic ligands or quartanary nitrogen containing ligands) DOR selective ligands. These drugs could potentially be used as anti-tussive, to reduce constipation or to protect against ischemic injury, with reduced risk of central nervous system side effects.
Transient DOR expression
As summarized in this review, cellular redistribution of DORs and increases in the efficacy of DOR selective drugs have been observed after chronic inflammation, neuropathy, opioid exposure, ethanol exposure, stress, ischemia and potentially cancer and obesity (Table 3). It is important to investigate the exact triggers and mechanisms for the cellular redistribution of DORs and how selectively this occurs. Recent studies have provided detailed information on the redistribution of DORs from large dense core vesicles and the proposed mechanisms (Bao et al. 2003; Bie et al. 2010; Guan et al. 2005; Zhang et al. 2006b). Yet several questions remain to be answered satisfactory: How long does the cellular redistribution of DORs last? How region-specific is this cellular translocation and how much does this depend on the event that triggers the redistribution? What is the role of the plasma enkephalin tone during the redistribution? In other words, does stress change cellular compartmentalization of DORs throughout cells in the body, whereas a local event such as castor oil exposure, only redistribute DORs in cells along the gastro-intesintal tract? If DORs are translocated only in a specific region this would allow for selective treatment of the disorders and a reduced liability of side effects. On the other hand if DORs are translocated in cells throughout the body, rather than in only a subpopulation, it may for example be possible to first eliminate peripheral DORs by administration of a peripherally restricted DOR agonist with a strong internalization profile, which should eliminate those DORs, as DORs are targeted for degradation (Whistler et al. 2002). Subsequently a DOR agonist with a low internalization profile, such as ARM1000390 (Pradhan et al. 2009) could be administered to treat a central nervous system disorder without major side effects in the periphery. Conditional DOR and preproenkephalin knockout mice could be useful in addressing temporal and mechanistic aspects of DOR translocation. Another tool that may assist in answering some of these questions is the availability of fluorescent DOR ligands (Arttamangkul et al. 2000; Balboni et al. 2004; Chen et al. 2005; Korlipara et al. 1997; Kshirsagar et al. 1998; Morinville et al. 2004a). A better understanding of the DOR pharmacology (i.e. subtype and expression level of DORs) within a certain circuit may also lead to design and synthesis of better/more (subtype-) selective fluorescent DOR probes and PET ligands (Clayson et al. 2001; Elmore et al. 2011) to investigate DOR function specifically within this circuit.
Sex and age differences
The realization of the versatility of DORs as potential drug target should spur on more investigations of the effects of DOR selective effects in females, as currently the majority of research has been performed in males (Craft 2003; Dahan et al. 2008). It is known that gender differences exist for the pharmacological effects of KOR selective drugs (Rasakham and Liu-Chen 2011). Recent studies suggest some sex differences, that result in changes in potency and efficacy may also exist for DOR selective ligands (Dahan et al. 2008; Saloman et al. 2011; Wilson et al. 2002). Additionally, DOR expression level changes during development (Zhu et al. 1998) and in areas such as the dorsal striatum DOR expression may decline with age (Nielsen et al. 2012). Therefore it will be important to understand the contribution of age and gender to an already complex DOR pharmacology.
Ongoing and future clinical trials
A small number of DOR selective drugs have recently entered clinical trials. AZD2327 is currently in phase II clinical trials for treatment of anxious major depressive disorder, both as a potential anti-depressant and anxiolytic. ALD5859 and ALD5747 have completed phase II clinical trials for the treatment of ostheoarthritic knee pain, post-herpetic neuralgia and acute dental pain after third molar extraction, but failed to show significant effects to warrant further clinical investigations. TRK-851 may be close to entering clinical trials for the treatment of persistent cough (Nagase and Fujii 2011; Sakami et al. 2008a).
In summary, the wide spread distribution of DORs throughout the body allows it to be a factor in the etiology of many diseases. The existence of conditional and global DOR and preproenkephalin knockout mice as well as a large library of selective DOR ligands has been of great help to dissect the role of DORs in different diseases. The recent development of optogenetic tools may be used to study the effect of neuronal signal transduction of DOR expressing neurons, which combined with retrograde labeling could establish more detailed information on the networks in which DORs are playing a role. The large effort in synthesizing structurally diverse DOR selective drugs gives DORs a great potential for translational research. It will be interesting to follow the current DOR selective drugs through their clinical trials and the hope is that they will pave the way for initiating more clinical trials with other DOR selective drugs for multiple difference indications.
Acknowledgements
We thank Drs Li He and Laura Milan-Lobo for their valuable comments and suggestions. This work was funded by the Foundation for Alcohol Research-ABMRF (RMvR), the National Institute for Alcoholism and Alcohol Abuse grants K99AA020539 (RMvR), R01AA020401 (JLW), P50AA017072-03, National Institute on Drug Abuse grants R01 DA015232 and DA019958 (JLW). Funds were also provided by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco to JLW.
Footnotes
Author contrbution: RMvR (wrote paper), JNDF, (wrote paper), JLW (wrote paper)
References
- Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respir Physiol Neurobiol. 2008;164:3–11. doi: 10.1016/j.resp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvira-Botero MX, Garzon M. Cellular and subcellular distributions of delta opioid receptor activation sites in the ventral oral pontine tegmentum of the cat. Brain Res. 2006;1123:101–11. doi: 10.1016/j.brainres.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Amir S, Brown ZW, Amit Z. The role of endorphins in stress: evidence and speculations. Neurosci Biobehav Rev. 1980;4:77–86. doi: 10.1016/0149-7634(80)90027-5. [DOI] [PubMed] [Google Scholar]
- Ananthan S. Opioid ligands with mixed mu/delta opioid receptor interactions: an emerging approach to novel analgesics. Aaps J. 2006;8:E118–25. doi: 10.1208/aapsj080114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Alvarez-Maubecin V, Thomas G, Williams JT, Grandy DK. Binding and internalization of fluorescent opioid peptide conjugates in living cells. Mol Pharmacol. 2000;58:1570–80. doi: 10.1124/mol.58.6.1570. [DOI] [PubMed] [Google Scholar]
- AK. Meert TF. Functional effects of systemically administered agonists and antagonists of mu, delta, and kappa opioid receptor subtypes on body temperature in mice. J Pharmacol Exp Ther. 2002;302:1253–64. doi: 10.1124/jpet.102.037655. [DOI] [PubMed] [Google Scholar]
- Balboni G, Salvadori S, Dal Piaz A, Bortolotti F, Argazzi R, Negri L, Lattanzi R, Bryant SD, Jinsmaa Y, Lazarus LH. Highly selective fluorescent analogue of the potent delta-opioid receptor antagonist Dmt-Tic. J Med Chem. 2004;47:6541–6. doi: 10.1021/jm040128h. [DOI] [PubMed] [Google Scholar]
- Bao L, Jin S-X, Zhang C, Wang L-H, Xu Z-Z, Zhang F-X, Wang L-C, Ning F-S, Cai H-J, Guan J-S. Activation of Delta Opioid Receptors Induces Receptor Insertion and Neuropeptide Secretion. Neuron. 2003;37:121–133. doi: 10.1016/s0896-6273(02)01103-0. [DOI] [PubMed] [Google Scholar]
- Barg J, Belcheva M, Rowinski J, Ho A, Burke WJ, Chung HD, Schmidt CA, Coscia CJ. Opioid receptor density changes in Alzheimer amygdala and putamen. Brain Res. 1993;632:209–15. doi: 10.1016/0006-8993(93)91155-l. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Hele DJ. Cough sensors. III. Opioid and cannabinoid receptors on vagal sensory nerves. Handb Exp Pharmacol. 2009:63–76. doi: 10.1007/978-3-540-79842-2_4. [DOI] [PubMed] [Google Scholar]
- Bie B, Zhang Z, Cai YQ, Zhu W, Zhang Y, Dai J, Lowenstein CJ, Weinman EJ, Pan ZZ. Nerve growth factor-regulated emergence of functional delta-opioid receptors. J Neurosci. 2010;30:5617–28. doi: 10.1523/JNEUROSCI.5296-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bie B, Zhu W, Pan ZZ. Ethanol-induced delta-opioid receptor modulation of glutamate synaptic transmission and conditioned place preference in central amygdala. Neuroscience. 2009a;160:348–58. doi: 10.1016/j.neuroscience.2009.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bie B, Zhu W, Pan ZZ. Rewarding morphine-Induced Synaptic Function of Delta-Opioid Receptors on Central Glutamate Synapses. J Pharmacol Exp Ther. 2009b doi: 10.1124/jpet.108.148908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billa SK, Xia Y, Moron JA. Disruption of morphine-conditioned place preference by a delta2-opioid receptor antagonist: study of mu-opioid and delta-opioid receptor expression at the synapse. Eur J Neurosci. 2010;32:625–31. doi: 10.1111/j.1460-9568.2010.07314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte C, Newman G, Schultz Jel J. Kappa and delta opioid receptor signaling is augmented in the failing heart. J Mol Cell Cardiol. 2009;47:493–503. doi: 10.1016/j.yjmcc.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen WD, Hellewell SB, Kelemen M, Huey R, Stewart D. Affinity labeling of delta-opiate receptors using [D-Ala2,Leu5,Cys6]enkephalin. Covalent attachment via thiol-disulfide exchange. J Biol Chem. 1987;262:13434–9. [PubMed] [Google Scholar]
- Broccardo M, Improta G. Antidiarrheal and colonic antipropulsive effects of spinal and supraspinal administration of the natural delta opioid receptor agonist, [D-Ala2]deltorphin II, in the rat. Eur J Pharmacol. 1992a;218:69–73. doi: 10.1016/0014-2999(92)90148-w. [DOI] [PubMed] [Google Scholar]
- Broccardo M, Improta G. Antidiarrheal effect of deltorphin II, a highly selective delta opioid receptor agonist, in the rat. Pharmacol Res. 1992b;25(Suppl 1):5–6. doi: 10.1016/1043-6618(92)90513-b. [DOI] [PubMed] [Google Scholar]
- Broccardo M, Improta G, Tabacco A. Central effect of SNC 80, a selective and systemically active delta-opioid receptor agonist, on gastrointestinal propulsion in the mouse. Eur J Pharmacol. 1998;342:247–51. doi: 10.1016/s0014-2999(97)01470-2. [DOI] [PubMed] [Google Scholar]
- Brownstein MJ. A brief history of opiates, opioid peptides, and opioid receptors. Proc Natl Acad Sci U S A. 1993;90:5391–3. doi: 10.1073/pnas.90.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, Morinville A. Trafficking of delta-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol Sci. 2007;28:23–31. doi: 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O'Donnell D, Beaudet A. Immunohistochemical distribution of delta opioid receptors in the rat central nervous system: evidence for somatodendritic labeling and antigen-specific cellular compartmentalization. J Comp Neurol. 2001a;440:65–84. doi: 10.1002/cne.1370. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Hoffert C, O'Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001b;21:7598–607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon SN. Nonpeptidic delta (delta) opioid agonists and antagonists of the diarylmethylpiperazine class: what have we learned? Top Curr Chem. 2011;299:121–40. doi: 10.1007/128_2010_83. [DOI] [PubMed] [Google Scholar]
- Capasso A, Cavallo F. L-NAME prevents EEG and behavioral alterations induced by Morphine and Deltorphin II in the rabbit Biomedical Research. 2005;16:56–58. [Google Scholar]
- Carr DJ, Kim CH, deCosta B, Jacobson AE, Rice KC, Blalock JE. Evidence for a delta-class opioid receptor on cells of the immune system. Cell Immunol. 1988;116:44–51. doi: 10.1016/0008-8749(88)90208-0. [DOI] [PubMed] [Google Scholar]
- Castellano C, Pavone F. Dose- and strain-dependent effects of dermorphin and [D-Ala2-D-Leu5]enkephalin on passive avoidance behavior in mice. Behav Neurosci. 1985;99:1120–7. doi: 10.1037//0735-7044.99.6.1120. [DOI] [PubMed] [Google Scholar]
- Chabot-Dore AJ, Zaretsky S, Wilcox GL, Stone LS. Delta opioid receptor immunohistochemistry and western blot analysis in mouse spinal cords: a comparitive study between antibodies from multiple sources Society for Neuroscience. Washington D.C.: 2008. [Google Scholar]
- Chamouard P, Rohr S, Meyer C, Baumann R, Angel F. Delta-opioid receptor agonists inhibit neuromuscular transmission in human colon. Eur J Pharmacol. 1994;262:33–9. doi: 10.1016/0014-2999(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Charness ME, Querimit LA, Diamond I. Ethanol increases the expression of functional delta-opioid receptors in neuroblastoma x glioma NG 108-15 hybrid cells. J Biol Chem. 1986;261:3164–9. [PubMed] [Google Scholar]
- Charron C, Messier C, Plamondon H. Neuroprotection and functional recovery conferred by administration of kappa- and delta 1-opioid agonists in a rat model of global ischemia. Physiol Behav. 2008;93:502–11. doi: 10.1016/j.physbeh.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Shippenberg TS. Augmentation of morphine-induced sensitization but reduction in morphine tolerance and reward in delta-opioid receptor knockout mice. Neuropsychopharmacology. 2009;34:887–98. doi: 10.1038/npp.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chung NN, Lemieux C, Zelent B, Vanderkooi JM, Gryczynski I, Wilkes BC, Schiller PW. [Aladan3]TIPP: a fluorescent delta-opioid antagonist with high delta-receptor binding affinity and delta selectivity. Biopolymers. 2005;80:325–31. doi: 10.1002/bip.20200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Mestek A, Liu J, Hurley JA, Yu L. Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol. 1993;44:8–12. [PubMed] [Google Scholar]
- Cherny N, Ripamonti C, Pereira J, Davis C, Fallon M, McQuay H, Mercadante S, Pasternak G, Ventafridda V. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol. 2001;19:2542–54. doi: 10.1200/JCO.2001.19.9.2542. [DOI] [PubMed] [Google Scholar]
- Chieng B, Christie MJ. Chronic morphine treatment induces functional delta-opioid receptors in amygdala neurons that project to periaqueductal grey. Neuropharmacology. 2009;57:430–7. doi: 10.1016/j.neuropharm.2009.06.034. [DOI] [PubMed] [Google Scholar]
- Churchill L, Roques BP, Kalivas PW. Dopamine depletion augments endogenous opioid-induced locomotion in the nucleus accumbens using both mu 1 and delta opioid receptors. Psychopharmacology (Berl) 1995;120:347–55. doi: 10.1007/BF02311183. [DOI] [PubMed] [Google Scholar]
- Civelli O, Douglass J, Goldstein A, Herbert E. Sequence and expression of the rat prodynorphin gene. Proc Natl Acad Sci U S A. 1985;82:4291–5. doi: 10.1073/pnas.82.12.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson J, Jales A, Tyacke RJ, Hudson AL, Nutt DJ, Lewis JW, Husbands SM. Selective delta-opioid receptor ligands: potential PET ligands based on naltrindole. Bioorg Med Chem Lett. 2001;11:939–43. doi: 10.1016/s0960-894x(01)00112-3. [DOI] [PubMed] [Google Scholar]
- Codd EE, Carson JR, Colburn RW, Stone DJ, Van Besien CR, Zhang SP, Wade PR, Gallantine EL, Meert TF, Molino L, Pullan S, Razler CM, Dax SL, Flores CM. JNJ-20788560 [9-(8-azabicyclo[3.2.1]oct-3-ylidene)-9H-xanthene-3-carboxylic acid diethylamide], a selective delta opioid receptor agonist, is a potent and efficacious antihyperalgesic agent that does not produce respiratory depression, pharmacologic tolerance, or physical dependence. J Pharmacol Exp Ther. 2009;329:241–51. doi: 10.1124/jpet.108.146969. [DOI] [PubMed] [Google Scholar]
- Commons KG. Translocation of presynaptic delta opioid receptors in the ventrolateral periaqueductal gray after swim stress. J Comp Neurol. 2003;464:197–207. doi: 10.1002/cne.10788. [DOI] [PubMed] [Google Scholar]
- Cox BM, Goldstein A, Hi CH. Opioid activity of a peptide, beta-lipotropin-(61–91), derived from beta-lipotropin. Proc Natl Acad Sci U S A. 1976;73:1821–3. doi: 10.1073/pnas.73.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM. Sex differences in opioid analgesia: “from mouse to man”. Clin J Pain. 2003;19:175–86. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesth Analg. 2008;107:83–95. doi: 10.1213/ane.0b013e31816a66a4. [DOI] [PubMed] [Google Scholar]
- Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci U S A. 2005;102:19208–13. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M, Moisand C, Meunier JC, Morgat JL, Gacel G, Roques BP. [3H]Tyr-D-Ser-Gly-Phe-Leu-Thr: a specific probe for the delta-opiate receptor subtype in brain membranes. Eur J Pharmacol. 1982;78:385–7. doi: 10.1016/0014-2999(82)90046-2. [DOI] [PubMed] [Google Scholar]
- Davie CA. A review of Parkinson's disease. Br Med Bull. 2008;86:109–27. doi: 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Kawatani M. Enkephalinergic inhibition in parasympathetic ganglia of the urinary bladder of the cat. J Physiol. 1989;413:13–29. doi: 10.1113/jphysiol.1989.sp017639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S, Sagratella S, Loizzo A. Dexamethasone blocking effects on mu- and delta-opioid-induced seizures involves kappa-opioid activity in the rabbit. Neuropsychobiology. 2001;43:213–20. doi: 10.1159/000054892. [DOI] [PubMed] [Google Scholar]
- Dietis N, Rowbotham DJ, Lambert DG. Opioid receptor subtypes: fact or artifact? Br J Anaesth. 2011 doi: 10.1093/bja/aer115. [DOI] [PubMed] [Google Scholar]
- Dilts RP, Kalivas PW. Autoradiographic localization of delta opioid receptors within the mesocorticolimbic dopamine system using radioiodinated [2-D-penicillamine, 5-D-penicillamine]enkephalin (125I-DPDPE) Synapse. 1990;6:121–32. doi: 10.1002/syn.890060203. [DOI] [PubMed] [Google Scholar]
- Elmore CS, Brush K, Schou M, Palmer W, Dorff PN, Powell ME, Hoesch V, Hall JE, Hudzik T, Halldin C, Dantzman CL. Synthesis of a delta opioid agonist in [2H6], [2H4], [11C], and [14C] labeled forms. Journal of Labelled Compounds and Radiopharmaceuticals. 2011;54:847–854. [Google Scholar]
- Erbs E, Faget L, Scherrer G, Kessler P, Hentsch D, Vonesch JL, Matifas A, Kieffer BL, Massotte D. Distribution of delta opioid receptor-expressing neurons in the mouse hippocampus. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erspamer V, Melchiorri P, Falconieri-Erspamer G, Negri L, Corsi R, Severini C, Barra D, Simmaco M, Kreil G. Deltorphins: a family of naturally occurring peptides with high affinity and selectivity for delta opioid binding sites. Proc Natl Acad Sci U S A. 1989;86:5188–92. doi: 10.1073/pnas.86.13.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AA, Tunnicliffe G, Knights P, Bailey CJ, Smith ME. Delta opioid receptors mediate glucose uptake in skeletal muscles of lean and obese-diabetic (ob/ob) mice. Metabolism. 2001;50:1402–8. doi: 10.1053/meta.2001.28158. [DOI] [PubMed] [Google Scholar]
- Evans CJ, Keith DE, Jr., Morrison H, Magendzo K, Edwards RH. Cloning of a delta opioid receptor by functional expression. Science. 1992;258:1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- Fadda P, Tortorella A, Fratta W. Sleep deprivation decreases mu and delta opioid receptor binding in the rat limbic system. Neurosci Lett. 1991;129:315–7. doi: 10.1016/0304-3940(91)90489-g. [DOI] [PubMed] [Google Scholar]
- Faget L, Erbs E, Le Merrer J, Scherrer G, Matifas A, Benturquia N, Noble F, Decossas M, Koch M, Kessler P, Vonesch JL, Schwab Y, Kieffer BL, Massotte D. In vivo visualization of delta opioid receptors upon physiological activation uncovers a distinct internalization profile. J Neurosci. 2012;32:7301–10. doi: 10.1523/JNEUROSCI.0185-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori A, Cardelli P, Negri L, Savi MR, Strom R, Erspamer V. Deltorphin transport across the blood-brain barrier. Proc Natl Acad Sci U S A. 1997;94:9469–74. doi: 10.1073/pnas.94.17.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox-Threlkeld JE, Daniel EE, Christinck F, Hruby VJ, Cipris S, Woskowska Z. Identification of mechanisms and sites of actions of mu and delta opioid receptor activation in the canine intestine. J Pharmacol Exp Ther. 1994;268:689–700. [PubMed] [Google Scholar]
- Fraser GL, Gaudreau GA, Clarke PB, Menard DP, Perkins MN. Antihyperalgesic effects of delta opioid agonists in a rat model of chronic inflammation. Br J Pharmacol. 2000a;129:1668–72. doi: 10.1038/sj.bjp.0703248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GL, Parenteau H, Tu TM, Ducharme J, Perkins MN, Clarke PB. The effects of delta agonists on locomotor activity in habituated and non-habituated rats. Life Sci. 2000b;67:913–22. doi: 10.1016/s0024-3205(00)00690-1. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Tsuda Y, Li T, Motoyama T, Takahashi M, Shimizu Y, Yokoi T, Sasaki Y, Ambo A, Kita A, Jinsmaa Y, Bryant SD, Lazarus LH, Okada Y. Development of potent bifunctional endomorphin-2 analogues with mixed mu-/delta-opioid agonist and delta-opioid antagonist properties. J Med Chem. 2004;47:3591–9. doi: 10.1021/jm030649p. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Nozaki C, Nadal X, Hever XC, Weibel R, Matifas A, Reiss D, Filliol D, Nassar MA, Wood JN, Maldonado R, Kieffer BL. Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain. 2011;152:1238–48. doi: 10.1016/j.pain.2010.12.031. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Peluso J, Befort K, Simonin F, Zilliox C, Kieffer BL. Detection of opioid receptor mRNA by RT-PCR reveals alternative splicing for the delta-and kappa-opioid receptors. Brain Res Mol Brain Res. 1997;48:298–304. doi: 10.1016/s0169-328x(97)00109-5. [DOI] [PubMed] [Google Scholar]
- Gendron L, Esdaile MJ, Mennicken F, Pan H, O'Donnell D, Vincent JP, Devi LA, Cahill CM, Stroh T, Beaudet A. Morphine priming in rats with chronic inflammation reveals a dichotomy between antihyperalgesic and antinociceptive properties of deltorphin. Neuroscience. 2007a;144:263–74. doi: 10.1016/j.neuroscience.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Gendron L, Pintar JE, Chavkin C. Essential role of mu opioid receptor in the regulation of delta opioid receptor-mediated antihyperalgesia. Neuroscience. 2007b;150:807–17. doi: 10.1016/j.neuroscience.2007.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O'Dowd BF. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128–35. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- Goldstein A. Opioid peptides endorphins in pituitary and brain. Science. 1976;193:1081–6. doi: 10.1126/science.959823. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Tachibana S, Lowney LI, Hunkapiller M, Hood L. Dynorphin-(1–13), an extraordinarily potent opioid peptide. Proc Natl Acad Sci U S A. 1979;76:6666–70. doi: 10.1073/pnas.76.12.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A. 2004;101:5135–9. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RR, Snyder SH, Kuhar MJ, Young WS., 3rd Differentiation of delta and mu opiate receptor localizations by light microscopic autoradiography. Proc Natl Acad Sci U S A. 1980;77:6239–43. doi: 10.1073/pnas.77.10.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross ER, Peart JN, Hsu AK, Auchampach JA, Gross GJ. Extending the cardioprotective window using a novel delta-opioid agonist fentanyl isothiocyanate via the PI3-kinase pathway. Am J Physiol Heart Circ Physiol. 2005;288:H2744–9. doi: 10.1152/ajpheart.00918.2004. [DOI] [PubMed] [Google Scholar]
- Guan J-S, Xu Z-Z, Gao H, He S-Q, Ma G-Q, Sun T, Wang L-H, Zhang Z-N, Lena I, Kitchen I. Interaction with Vesicle Luminal Protachykinin Regulates Surface Expression of δ-Opioid Receptors and Opioid Analgesia. Cell. 2005;122:619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Guo Y, Stein AB, Wu WJ, Zhu X, Tan W, Li Q, Bolli R. Late preconditioning induced by NO donors, adenosine A1 receptor agonists, and delta1-opioid receptor agonists is mediated by iNOS. Am J Physiol Heart Circ Physiol. 2005;289:H2251–7. doi: 10.1152/ajpheart.00341.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack SP, Bagley EE, Chieng BCH, Christie MJ. Induction of {delta}-Opioid Receptor Function in the Midbrain after Chronic Morphine Treatment. J. Neurosci. 2005;25:3192–3198. doi: 10.1523/JNEUROSCI.4585-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan RE, Shivers BD, Romano GJ, Howells RD, Pfaff DW. Localization of preproenkephalin mRNA in the rat brain and spinal cord by in situ hybridization. J Comp Neurol. 1987;258:159–84. doi: 10.1002/cne.902580202. [DOI] [PubMed] [Google Scholar]
- Harvey JH, Long DH, England PM, Whistler JL. Tuned-Affinity Bivalent Ligands for the Characterization of Opioid Receptor Heteromers. ACS Med Chem Lett. 2012;3:640–644. doi: 10.1021/ml300083p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Nguyen T, Fan T, Cheng R, Rashid A, Alijaniaram M, Rasenick MM, O'Dowd BF, George SR. Trafficking of preassembled opioid mu-delta heterooligomer-Gz signaling complexes to the plasma membrane: coregulation by agonists. Biochemistry. 2007;46:12997–3009. doi: 10.1021/bi701436w. [DOI] [PubMed] [Google Scholar]
- Havemann U, Winkler M, Kuschinsky K. The effects of D-ala2, D-Leu5-enkephalin injections into the nucleus accumbens on the motility of rats. Life Sci. 1983;33(Suppl 1):627–30. doi: 10.1016/0024-3205(83)90581-7. [DOI] [PubMed] [Google Scholar]
- He L, Lee NM. Delta opioid receptor enhancement of mu opioid receptor-induced antinociception in spinal cord. J Pharmacol Exp Ther. 1998;285:1181–6. [PubMed] [Google Scholar]
- Hebb AL, Drolet G, Mendella PD, Roach SP, Gauthier MS, Zacharko RM. Intracerebroventricular D-Pen2, D-Pen5-enkephalin administration soon after stressor imposition influences behavioral responsivity to a subsequent stressor encounter in CD-1 mice. Pharmacol Biochem Behav. 2005;82:453–69. doi: 10.1016/j.pbb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Hiller JM, Itzhak Y, Simon EJ. Selective changes in mu, delta and kappa opioid receptor binding in certain limbic regions of the brain in Alzheimer's disease patients. Brain Res. 1987;406:17–23. doi: 10.1016/0006-8993(87)90764-5. [DOI] [PubMed] [Google Scholar]
- Holdridge SV, Cahill CM. Spinal administration of a delta opioid receptor agonist attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Eur J Pain. 2007;11:685–93. doi: 10.1016/j.ejpain.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Holt JD, Watson MJ, Chang JP, O'Neill SJ, Wei K, Pendergast W, Gengo PJ, Chang KJ. DPI-221 [4-((alpha-s)-alpha-((2s,5r)-2,5-dimethyl-4-(3-fluorobenzyl)-1-piperazinyl)benzyl)-N,N-diethylbenzamide]: a novel nonpeptide delta receptor agonist producing increased micturition interval in normal rats. J Pharmacol Exp Ther. 2005;315:601–8. doi: 10.1124/jpet.105.090498. [DOI] [PubMed] [Google Scholar]
- Hosohata Y, Vanderah TW, Burkey TH, Ossipov MH, Kovelowski CJ, Sora I, Uhl GR, Zhang X, Rice KC, Roeske WR, Hruby VJ, Yamamura HI, Lai J, Porreca F. delta-Opioid receptor agonists produce antinociception and [35S]GTPgammaS binding in mu receptor knockout mice. Eur J Pharmacol. 2000;388:241–8. doi: 10.1016/s0014-2999(99)00897-3. [DOI] [PubMed] [Google Scholar]
- Hudzik TJ, Maciag C, Smith MA, Caccese R, Pietras MR, Bui KH, Coupal M, Adam L, Payza K, Griffin A, Smagin G, Song D, Swedberg MD, Brown W. Preclinical pharmacology of AZD2327: a highly selective agonist of the delta-opioid receptor. J Pharmacol Exp Ther. 2011;338:195–204. doi: 10.1124/jpet.111.179432. [DOI] [PubMed] [Google Scholar]
- Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975;258:577–80. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci. 2000;20:1249–59. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytia P, Ingman K, Soini SL, Laitinen JT, Korpi ER. Effects of continuous opioid receptor blockade on alcohol intake and up-regulation of opioid receptor subtype signalling in a genetic model of high alcohol drinking. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:391–401. doi: 10.1007/s002109900070. [DOI] [PubMed] [Google Scholar]
- Ingman K, Salvadori S, Lazarus L, Korpi ER, Honkanen A. Selective delta-opioid receptor antagonist N,N(CH3)2-Dmt-Tic-OH does not reduce ethanol intake in alcohol-preferring AA rats. Addict Biol. 2003;8:173–9. doi: 10.1080/1355621031000117400. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Kinney ME, Wiegel LM. Inhibitory and excitatory effects of micro-, delta-, and kappa-opioid receptor activation on breathing in awake turtles, Trachemys scripta. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1599–612. doi: 10.1152/ajpregu.00020.2008. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Turner SM. Protecting motor networks during perinatal ischemia: the case for delta-opioid receptors. Ann N Y Acad Sci. 2010;1198:260–70. doi: 10.1111/j.1749-6632.2010.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Rice KC, Woods JH, Winsauer PJ. Effects of the delta-opioid receptor agonist SNC80 on learning relative to its antidepressant-like effects in rats. Behav Pharmacol. 2003;14:509–16. doi: 10.1097/00008877-200311000-00003. [DOI] [PubMed] [Google Scholar]
- Kabli N, Cahill CM. Anti-allodynic effects of peripheral delta opioid receptors in neuropathic pain. Pain. 2007;127:84–93. doi: 10.1016/j.pain.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Abhold R. Enkephalin release into the ventral tegmental area in response to stress: modulation of mesocorticolimbic dopamine. Brain Res. 1987;414:339–48. doi: 10.1016/0006-8993(87)90015-1. [DOI] [PubMed] [Google Scholar]
- Kamei J, Iwamoto Y, Misawa M, Nagase H, Kasuya Y. Streptozotocin-induced diabetes selectively enhances antinociception mediated by delta 1- but not delta 2-opioid receptors. Life Sci. 1994a;55:PL121–6. doi: 10.1016/0024-3205(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Kamei J, Iwamoto Y, Suzuki T, Misawa M, Nagase H, Kasuya Y. Antitussive effects of naltrindole, a selective delta-opioid receptor antagonist, in mice and rats. Eur J Pharmacol. 1993a;249:161–5. doi: 10.1016/0014-2999(93)90428-k. [DOI] [PubMed] [Google Scholar]
- Kamei J, Iwamoto Y, Suzuki T, Misawa M, Nagase H, Kasuya Y. Involvement of delta 1-opioid receptor antagonism in the antitussive effect of delta-opioid receptor antagonists. Eur J Pharmacol. 1994b;251:291–4. doi: 10.1016/0014-2999(94)90411-1. [DOI] [PubMed] [Google Scholar]
- Kamei J, Iwamoto Y, Suzuki T, Nagase H, Misawa M, Kasuya Y. Differential modulation of mu-opioid receptor-mediated antitussive activity by delta-opioid receptor agonists in mice. Eur J Pharmacol. 1993b;234:117–20. doi: 10.1016/0014-2999(93)90714-s. [DOI] [PubMed] [Google Scholar]
- Kamei J, Kawai K, Mizusuna A, Saitoh A, Morita K, Narita M, Tseng LF, Nagase H. Supraspinal delta 1-opioid receptor-mediated antinociceptive properties of (−)-TAN-67 in diabetic mice. Eur J Pharmacol. 1997;322:27–30. doi: 10.1016/s0014-2999(97)00085-x. [DOI] [PubMed] [Google Scholar]
- Kamei J, Tanihara H, Kasuya Y. Modulation of mu-mediated antitussive activity in rats by a delta agonist. Eur J Pharmacol. 1991;203:153–6. doi: 10.1016/0014-2999(91)90807-3. [DOI] [PubMed] [Google Scholar]
- Kang-Park MH, Kieffer BL, Roberts AJ, Siggins GR, Moore SD. Presynaptic delta opioid receptors regulate ethanol actions in central amygdala. J Pharmacol Exp Ther. 2007;320:917–25. doi: 10.1124/jpet.106.112722. [DOI] [PubMed] [Google Scholar]
- Karlsson LO, Grip L, Bissessar E, Bobrova I, Gustafsson T, Kavianipour M, Odenstedt J, Wikstrom G, Gonon AT. Opioid receptor agonist Eribis peptide 94 reduces infarct size in different porcine models for myocardial ischaemia and reperfusion. Eur J Pharmacol. 2011;651:146–51. doi: 10.1016/j.ejphar.2010.10.069. [DOI] [PubMed] [Google Scholar]
- Katsuura Y, Taha SA. Modulation of feeding and locomotion through mu and delta opioid receptor signaling in the nucleus accumbens. Neuropeptides. 2010;44:225–32. doi: 10.1016/j.npep.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Bless EP, Swanson CJ. Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J Pharmacol Exp Ther. 1996;278:1499–507. [PubMed] [Google Scholar]
- Khotib J, Narita M, Suzuki M, Yajima Y, Suzuki T. Functional interaction among opioid receptor types: up-regulation of mu- and delta-opioid receptor functions after repeated stimulation of kappa-opioid receptors. Neuropharmacology. 2004;46:531–40. doi: 10.1016/j.neuropharm.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci U S A. 1992;89:12048–52. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Choi SS, Woo RS, Suh HW. Development of antinociceptive tolerance and changes of opioid receptor ligand binding in central nervous system of the mouse forced to single and repeated swimming in the cold water. Brain Res Bull. 2003;61:93–7. doi: 10.1016/s0361-9230(03)00079-0. [DOI] [PubMed] [Google Scholar]
- Knapp RJ, Landsman R, Waite S, Malatynska E, Varga E, Haq W, Hruby VJ, Roeske WR, Nagase H, Yamamura HI. Properties of TAN-67, a nonpeptidic delta-opioid receptor agonist, at cloned human delta- and mu-opioid receptors. Eur J Pharmacol. 1995;291:129–34. doi: 10.1016/0922-4106(95)90134-5. [DOI] [PubMed] [Google Scholar]
- Knapp RJ, Santoro G, De Leon IA, Lee KB, Edsall SA, Waite S, Malatynska E, Varga E, Calderon SN, Rice KC, Rothman RB, Porreca F, Roeske WR, Yamamura HI. Structure-activity relationships for SNC80 and related compounds at cloned human delta and mu opioid receptors. J Pharmacol Exp Ther. 1996;277:1284–91. [PubMed] [Google Scholar]
- Korlipara V, Ells J, Wang J, Tam S, Elde R, Portoghese P. Fluorescent N-benzylnaltrindole analogues as potential delta opioid receptor selective probes. Eur J Med Chem. 1997;32:171–174. [Google Scholar]
- Kosterlitz HW, Lord JA, Paterson SJ, Waterfield AA. Effects of changes in the structure of enkephalins and of narcotic analgesic drugs on their interactions with mu- and delta-receptors. Br J Pharmacol. 1980;68:333–42. doi: 10.1111/j.1476-5381.1980.tb10422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzer CJ, Hay DW, Dondio G, Giardina G, Petrillo P, Underwood DC. The antitussive activity of delta-opioid receptor stimulation in guinea pigs. J Pharmacol Exp Ther. 2000;292:803–9. [PubMed] [Google Scholar]
- Krajnik M, Schafer M, Sobanski P, Kowalewski J, Bloch-Boguslawska E, Zylicz Z, Mousa SA. Enkephalin, its precursor, processing enzymes, and receptor as part of a local opioid network throughout the respiratory system of lung cancer patients. Hum Pathol. 2010;41:632–42. doi: 10.1016/j.humpath.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Krevsky B, Cowan A, Maurer AH, Butt W, Fisher RS. Effects of selective opioid agonists on feline colonic transit. Life Sci. 1991;48:1597–602. doi: 10.1016/0024-3205(91)90285-j. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Portoghese PS, Li TK, Froehlich JC. The delta 2-opioid receptor antagonist naltriben selectively attenuates alcohol intake in rats bred for alcohol preference. Pharmacol Biochem Behav. 1995;52:153–9. doi: 10.1016/0091-3057(95)00080-g. [DOI] [PubMed] [Google Scholar]
- Kshirsagar T, Nakano AH, Law PY, Elde R, Portoghese PS. NTI4F: a non-peptide fluorescent probe selective for functional delta opioid receptors. Neurosci Lett. 1998;249:83–6. doi: 10.1016/s0304-3940(98)00379-6. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Pert CB, Snyder SH. Regional distribution of opiate receptor binding in monkey and human brain. Nature. 1973;245:447–50. doi: 10.1038/245447a0. [DOI] [PubMed] [Google Scholar]
- Lasukova TV, Maslov LN, Nizkodubova SW, Gorbunov AS, Zibulnikov SY. Role of Intracellular Calcium and Cyclic Nucleotides in Realization of Cardioprotective Effects of delta(1)- and kappa(1)-Opioid Receptor Agonists. Bull Exp Biol Med. 2009;148:877–80. doi: 10.1007/s10517-010-0840-4. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Quan B, Chow S. The effects of selective blockade of delta and mu opiate receptors on ethanol consumption by C57BL/6 mice in a restricted access paradigm. Brain Res. 1993;630:330–2. doi: 10.1016/0006-8993(93)90672-a. [DOI] [PubMed] [Google Scholar]
- Le Bourdonnec B, Windh RT, Ajello CW, Leister LK, Gu M, Chu GH, Tuthill PA, Barker WM, Koblish M, Wiant DD, Graczyk TM, Belanger S, Cassel JA, Feschenko MS, Brogdon BL, Smith SA, Christ DD, Derelanko MJ, Kutz S, Little PJ, DeHaven RN, DeHaven-Hudkins DL, Dolle RE. Potent, orally bioavailable delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-4-(5-hydroxyspiro[chromene-2,4'-piperidine]-4-yl)benzamide (ADL5859) J Med Chem. 2008;51:5893–6. doi: 10.1021/jm8008986. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Faget L, Matifas A, Kieffer BL. Cues predicting drug or food reward restore morphine-induced place conditioning in mice lacking delta opioid receptors. Psychopharmacology (Berl) 2012;223:99–106. doi: 10.1007/s00213-012-2693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Rezai X, Scherrer G, Becker JA, Kieffer BL. Impaired Hippocampus-Dependent and Facilitated Striatum-Dependent Behaviors in Mice Lacking the Delta Opioid Receptor. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, Bailey A, Burbach JP, Van Ree JM, Kitchen I, Gerrits MA. Receptor-selective changes in mu-, delta- and kappa-opioid receptors after chronic naltrexone treatment in mice. Eur J Neurosci. 2003;17:1006–12. doi: 10.1046/j.1460-9568.2003.02502.x. [DOI] [PubMed] [Google Scholar]
- Li S, Zhu J, Chen C, Chen YW, Deriel JK, Ashby B, Liu-Chen LY. Molecular cloning and expression of a rat kappa opioid receptor. Biochem J. 1993;295(Pt 3):629–33. doi: 10.1042/bj2950629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JA, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267:495–9. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Lucido AL, Morinville A, Gendron L, Stroh T, Beaudet A. Prolonged morphine treatment selectively increases membrane recruitment of delta-opioid receptors in mouse basal ganglia. J Mol Neurosci. 2005;25:207–14. doi: 10.1385/JMN:25:3:207. [DOI] [PubMed] [Google Scholar]
- Ma J, Zhang Y, Kalyuzhny AE, Pan ZZ. Emergence of functional delta-opioid receptors induced by long-term treatment with morphine. Mol Pharmacol. 2006;69:1137–45. doi: 10.1124/mol.105.019109. [DOI] [PubMed] [Google Scholar]
- Ma MC, Qian H, Ghassemi F, Zhao P, Xia Y. Oxygen-sensitive {delta}-opioid receptor-regulated survival and death signals: novel insights into neuronal preconditioning and protection. J Biol Chem. 2005;280:16208–18. doi: 10.1074/jbc.M408055200. [DOI] [PubMed] [Google Scholar]
- Madar I, Bencherif B, Lever J, Heitmiller RF, Yang SC, Brock M, Brahmer J, Ravert H, Dannals R, Frost JJ. Imaging delta- and mu-opioid receptors by PET in lung carcinoma patients. J Nucl Med. 2007;48:207–13. [PubMed] [Google Scholar]
- Madar I, Lesser RP, Krauss G, Zubieta JK, Lever JR, Kinter CM, Ravert HT, Musachio JL, Mathews WB, Dannals RF, Frost JJ. Imaging of delta- and mu-opioid receptors in temporal lobe epilepsy by positron emission tomography. Ann Neurol. 1997;41:358–67. doi: 10.1002/ana.410410311. [DOI] [PubMed] [Google Scholar]
- Maehle AH, Prull CR, Halliwell RF. The emergence of the drug receptor theory. Nat Rev Drug Discov. 2002;1:637–41. doi: 10.1038/nrd875. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–38. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–64. [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Fields HL, Hjelmstad GO, Mitchell JM. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J Neurosci. 2008;28:12672–81. doi: 10.1523/JNEUROSCI.4569-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Hjelmstad GO, Fields HL. A novel opioid receptor-mediated enhancement of GABAA receptor function induced by stress in ventral tegmental area neurons. J Physiol. 2011;589:4229–42. doi: 10.1113/jphysiol.2011.209023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–32. [PubMed] [Google Scholar]
- Maslov LN, Barzakh EI, Krylatov AV, Chernysheva GA, Krieg T, Solenkova NV, Lishmanov AY, Cybulnikov SY, Zhang Y. Opioid peptide deltorphin II simulates the cardioprotective effect of ischemic preconditioning: role of delta-opioid receptors, protein kinase C, and K(ATP) channels. Bull Exp Biol Med. 2010a;149:591–3. doi: 10.1007/s10517-010-1000-6. [DOI] [PubMed] [Google Scholar]
- Maslov LN, Lishmanov YB, Oeltgen PR, Barzakh EI, Krylatov AV, Naryzhnaya NV, Pei JM, Brown SA. Comparative analysis of the cardioprotective properties of opioid receptor agonists in a rat model of myocardial infarction. Acad Emerg Med. 2010b;17:1239–46. doi: 10.1111/j.1553-2712.2010.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu-Kia AM, Fan LQ, Kreek MJ, Simon EJ, Hiller JM. Mu-, delta- and kappa-opioid receptor populations are differentially altered in distinct areas of postmortem brains of Alzheimer's disease patients. Brain Res. 2001;893:121–34. doi: 10.1016/s0006-8993(00)03302-3. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M, Nagase H. Involvement of mu- and delta-opioid receptors in the ethanol-associated place preference in rats exposed to foot shock stress. Brain Res. 1998;803:169–77. doi: 10.1016/s0006-8993(98)00679-9. [DOI] [PubMed] [Google Scholar]
- Matthes HWD, Smadja C, Valverde O, Vonesch JL, Foutz AS, Boudinot E, Denavit-Saubie M, Severini C, Negri L, Roques BP, Maldonado R, Kieffer BL. Activity of the delta -Opioid Receptor Is Partially Reduced, Whereas Activity of the kappa -Receptor Is Maintained in Mice Lacking the μ-Receptor. J. Neurosci. 1998;18:7285–7295. doi: 10.1523/JNEUROSCI.18-18-07285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattia A, Vanderah T, Mosberg HI, Porreca F. Lack of antinociceptive cross-tolerance between [D-Pen2, D-Pen5]enkephalin and [D-Ala2]deltorphin II in mice: evidence for delta receptor subtypes. J Pharmacol Exp Ther. 1991;258:583–7. [PubMed] [Google Scholar]
- Mendez M, Morales-Mulia M, Leriche M. [3H]DPDPE binding to delta opioid receptors in the rat mesocorticolimbic and nigrostriatal pathways is transiently increased by acute ethanol administration. Brain Res. 2004;1028:180–90. doi: 10.1016/j.brainres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Meng F, Xie GX, Thompson RC, Mansour A, Goldstein A, Watson SJ, Akil H. Cloning and pharmacological characterization of a rat kappa opioid receptor. Proc Natl Acad Sci U S A. 1993;90:9954–8. doi: 10.1073/pnas.90.21.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael-Titus A, Dourmap N, Costentin J. MU and delta opioid receptors control differently the horizontal and vertical components of locomotor activity in mice. Neuropeptides. 1989;13:235–42. doi: 10.1016/0143-4179(89)90076-0. [DOI] [PubMed] [Google Scholar]
- Michael-Titus A, Dourmap N, Costentin J, Schwartz JC. Role of delta opioid receptors in the effects of inhibitors of enkephalin-degrading peptidases on the horizontal and vertical components of locomotion in mice. Neuropeptides. 1990;15:89–100. doi: 10.1016/0143-4179(90)90044-y. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Mulvihill MA, Postler MA. Brain mu and delta opioid receptors mediate different locomotor hyperactivity responses of the C57BL/6J mouse. Psychopharmacology (Berl) 1990;101:332–7. doi: 10.1007/BF02244050. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Bandy AL. Naltrexone effects on ethanol consumption and response to ethanol conditioned cues in C57BL/6 mice. Psychopharmacology (Berl) 2000;151:321–7. doi: 10.1007/s002130000479. [DOI] [PubMed] [Google Scholar]
- Mika J, Przewlocki R, Przewlocka B. The role of delta-opioid receptor subtypes in neuropathic pain. Eur J Pharmacol. 2001;415:31–7. doi: 10.1016/s0014-2999(01)00814-7. [DOI] [PubMed] [Google Scholar]
- Minami M, Toya T, Katao Y, Maekawa K, Nakamura S, Onogi T, Kaneko S, Satoh M. Cloning and expression of a cDNA for the rat kappa-opioid receptor. FEBS Lett. 1993;329:291–5. doi: 10.1016/0014-5793(93)80240-u. [DOI] [PubMed] [Google Scholar]
- Misicka A, Lipkowski AW, Horvath R, Davis P, Porreca F, Yamamura HI, Hruby VJ. Structure-activity relationship of biphalin. The synthesis and biological activities of new analogues with modifications in positions 3 and 4. Life Sci. 1997;60:1263–9. doi: 10.1016/s0024-3205(97)00069-6. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Bowen WD, Portoghese PS, Takemori AE. Lack of involvement of delta-1 opioid receptors in the development of physical dependence on morphine in mice. J Pharmacol Exp Ther. 1994;270:37–9. [PubMed] [Google Scholar]
- Miyamoto Y, Portoghese PS, Takemori AE. Involvement of delta 2 opioid receptors in acute dependence on morphine in mice. J Pharmacol Exp Ther. 1993a;265:1325–7. [PubMed] [Google Scholar]
- Miyamoto Y, Portoghese PS, Takemori AE. Involvement of delta 2 opioid receptors in the development of morphine dependence in mice. J Pharmacol Exp Ther. 1993b;264:1141–5. [PubMed] [Google Scholar]
- Mizoguchi H, Narita M, Nagase H, Suzuki T, Quock RM, Tseng LF. Use of antisense oligodeoxynucleotide to determine delta-opioid receptor involvement in [D-Ala2]deltorphin II-induced locomotor hyperactivity. Life Sci. 1996;59:PL69–73. doi: 10.1016/0024-3205(96)00307-4. [DOI] [PubMed] [Google Scholar]
- Morin-Surun MP, Boudinot E, Gacel G, Champagnat J, Roques BP, Denavit-Saubie M. Different effects of mu and delta opiate agonists on respiration. Eur J Pharmacol. 1984;98:235–40. doi: 10.1016/0014-2999(84)90594-6. [DOI] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Aibak H, Rymar VV, Pradhan A, Hoffert C, Mennicken F, Stroh T, Sadikot AF, O Donnell D, Clarke PB, Collier B, Henry JL, Vincent JP, Beaudet A. Morphine-induced changes in delta opioid receptor trafficking are linked to somatosensory processing in the rat spinal cord. J Neurosci. 2004a;24:5549–59. doi: 10.1523/JNEUROSCI.2719-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL, Beaudet A. Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice. J Neurosci. 2003;23:4888–98. doi: 10.1523/JNEUROSCI.23-12-04888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Kieffer B, Collier B, Beaudet A. Mu-opioid receptor knockout prevents changes in delta-opioid receptor trafficking induced by chronic inflammatory pain. Pain. 2004b;109:266–73. doi: 10.1016/j.pain.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Mosberg HI, Hurst R, Hruby VJ, Gee K, Yamamura HI, Galligan JJ, Burks TF. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci U S A. 1983;80:5871–4. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosberg HI, Kroona HB. Incorporation of a novel conformationally restricted tyrosine analog into a cyclic, delta opioid receptor selective tetrapeptide (JOM-13) enhances delta receptor binding affinity and selectivity. J Med Chem. 1992;35:4498–500. doi: 10.1021/jm00101a030. [DOI] [PubMed] [Google Scholar]
- Moss IR, Scott SC, Inman JD. Mu- vs. delta-opioid influence on respiratory and sleep behavior during development. Am J Physiol. 1993;264:R754–60. doi: 10.1152/ajpregu.1993.264.4.R754. [DOI] [PubMed] [Google Scholar]
- Murray CW, Cowan A. [D-Pen2, D-Pen5]enkephalin, the standard delta opioid agonist, induces morphine-like behaviors in mice. Psychopharmacology (Berl) 1990;102:425–6. doi: 10.1007/BF02244117. [DOI] [PubMed] [Google Scholar]
- Nagase H, Fujii H. Opioids in preclinical and clinical trials. Top Curr Chem. 2011;299:29–62. doi: 10.1007/128_2010_74. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Inoue A, Kita T, Nakamura M, Chang AC, Cohen SN, Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979;278:423–7. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Negri L, Broccardo M, Lattanzi R, Melchiorri P. Effects of antisense oligonucleotides on brain delta-opioid receptor density and on SNC80-induced locomotor stimulation and colonic transit inhibition in rats. Br J Pharmacol. 1999;128:1554–60. doi: 10.1038/sj.bjp.0702931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri L, Noviello V, Angelucci F. Behavioural effects of deltorphins in rats. Eur J Pharmacol. 1991a;209:163–8. doi: 10.1016/0014-2999(91)90165-m. [DOI] [PubMed] [Google Scholar]
- Negri L, Potenza RL, Corsi R, Melchiorri P. Evidence for two subtypes of delta opioid receptors in rat brain. Eur J Pharmacol. 1991b;196:335–6. doi: 10.1016/0014-2999(91)90450-5. [DOI] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- Nielsen CK, Simms JA, Li R, Mill D, Yi H, Feduccia AA, Santos N, Bartlett SE. delta-Opioid Receptor Function in the Dorsal Striatum Plays a Role in High Levels of Ethanol Consumption in Rats. J Neurosci. 2012;32:4540–52. doi: 10.1523/JNEUROSCI.5345-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Teranishi Y, Takahashi H, Toyosato M, Notake M, Nakanishi S, Numa S. Isolation and structural organization of the human preproenkephalin gene. Nature. 1982;297:431–4. doi: 10.1038/297431a0. [DOI] [PubMed] [Google Scholar]
- O'Donovan MR, Johns S, Wilcox P. The effect of PHA stimulation on lymphocyte sub-populations in whole-blood cultures. Mutagenesis. 1995;10:371–4. doi: 10.1093/mutage/10.4.371. [DOI] [PubMed] [Google Scholar]
- Ochi T, Fujii T, Motoyama Y, Goto T. Antinociceptive properties of FR140423 mediated through spinal delta-, but not mu- and kappa-, opioid receptors. Eur J Pharmacol. 1999;380:73–9. doi: 10.1016/s0014-2999(99)00522-1. [DOI] [PubMed] [Google Scholar]
- Onofrio BM, Yaksh TL. Intrathecal delta-receptor ligand produces analgesia in man. Lancet. 1983;1:1386–7. doi: 10.1016/s0140-6736(83)92170-0. [DOI] [PubMed] [Google Scholar]
- Pacheco DF, Reis GM, Francischi JN, Castro MS, Perez AC, Duarte ID. delta-Opioid receptor agonist SNC80 elicits peripheral antinociception via delta(1) and delta(2) receptors and activation of the l-arginine/nitric oxide/cyclic GMP pathway. Life Sci. 2005;78:54–60. doi: 10.1016/j.lfs.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Pairet M, Ruckebusch Y. Opioid receptor agonists in the rabbit colon: comparison of in vivo and in vitro studies. Life Sci. 1984;35:1653–8. doi: 10.1016/0024-3205(84)90176-0. [DOI] [PubMed] [Google Scholar]
- Pakarinen ED, Woods JH, Moerschbaecher JM. Repeated acquisition of behavioral chains in squirrel monkeys: comparisons of a mu, kappa and delta opioid agonist. J Pharmacol Exp Ther. 1995;272:552–9. [PubMed] [Google Scholar]
- Parkhill AL, Bidlack JM. Several delta-opioid receptor ligands display no subtype selectivity to the human delta-opioid receptor. Eur J Pharmacol. 2002;451:257–64. doi: 10.1016/s0014-2999(02)02241-0. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Multiple opiate receptors: deja vu all over again. Neuropharmacology. 2004;47(Suppl 1):312–23. doi: 10.1016/j.neuropharm.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Patel HH, Head BP, Petersen HN, Niesman IR, Huang D, Gross GJ, Insel PA, Roth DM. Protection of adult rat cardiac myocytes from ischemic cell death: role of caveolar microdomains and delta-opioid receptors. Am J Physiol Heart Circ Physiol. 2006;291:H344–50. doi: 10.1152/ajpheart.01100.2005. [DOI] [PubMed] [Google Scholar]
- Pazos A, Florez J. Interaction of naloxone with mu- and delta-opioid agonists on the respiration of rats. Eur J Pharmacol. 1983;87:309–14. doi: 10.1016/0014-2999(83)90343-6. [DOI] [PubMed] [Google Scholar]
- Pazos A, Florez J. A comparative study in rats of the respiratory depression and analgesia induced by mu- and delta-opioid agonists. Eur J Pharmacol. 1984;99:15–21. doi: 10.1016/0014-2999(84)90427-8. [DOI] [PubMed] [Google Scholar]
- Peart JN, Hoe LE, Gross GJ, Headrick JP. Sustained ligand-activated preconditioning via delta-opioid receptors. J Pharmacol Exp Ther. 2011;336:274–81. doi: 10.1124/jpet.110.172593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SA, Hoshaw BA, Unterwald EM. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br J Pharmacol. 2006;147:864–72. doi: 10.1038/sj.bjp.0706686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pert CB, Snyder SH. Opiate receptor: demonstration in nervous tissue. Science. 1973;179:1011–4. doi: 10.1126/science.179.4077.1011. [DOI] [PubMed] [Google Scholar]
- Petrillo P, Angelici O, Bingham S, Ficalora G, Garnier M, Zaratin PF, Petrone G, Pozzi O, Sbacchi M, Stean TO, Upton N, Dondio GM, Scheideler MA. Evidence for a selective role of the delta-opioid agonist [8R-(4bS*,8aalpha,8abeta,12bbeta)]7,10-Dimethyl-1-methoxy-11-(2-methylpropyl)oxycarbonyl 5,6,7,8,12,12b-hexahydro-(9H)-4,8-methanobenzofuro[3,2-e]pyrrolo[2,3-g]iso quinoline hydrochloride (SB-235863) in blocking hyperalgesia associated with inflammatory and neuropathic pain responses. J Pharmacol Exp Ther. 2003;307:1079–89. doi: 10.1124/jpet.103.055590. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Wenger CD, Dorow JD. Naltrexone effects on ethanol drinking acquisition and on established ethanol consumption in C57BL/6J mice. Alcohol Clin Exp Res. 1997;21:691–702. [PubMed] [Google Scholar]
- Picolo G, Cury Y. Peripheral neuronal nitric oxide synthase activity mediates the antinociceptive effect of Crotalus durissus terrificus snake venom, a delta- and kappa-opioid receptor agonist. Life Sci. 2004;75:559–73. doi: 10.1016/j.lfs.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Platt DM, Bano KM. Opioid receptors and the discriminative stimulus effects of ethanol in squirrel monkeys: Mu and delta opioid receptor mechanisms. Eur J Pharmacol. 2011;650:233–9. doi: 10.1016/j.ejphar.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohorecky LA, Skiandos A, Zhang X, Rice KC, Benjamin D. Effect of chronic social stress on delta-opioid receptor function in the rat. J Pharmacol Exp Ther. 1999;290:196–206. [PubMed] [Google Scholar]
- Pol O, Ferrer I, Puig MM. Diarrhea associated with intestinal inflammation increases the potency of mu and delta opioids on the inhibition of gastrointestinal transit in mice. J Pharmacol Exp Ther. 1994;270:386–91. [PubMed] [Google Scholar]
- Poole DP, Pelayo JC, Scherrer G, Evans CJ, Kieffer BL, Bunnett NW. Localization and regulation of fluorescently labeled delta opioid receptor, expressed in enteric neurons of mice. Gastroenterology. 2011;141:982–991. e1–8. doi: 10.1053/j.gastro.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoghese PS, Sultana M, Nelson WL, Klein P, Takemori AE. Delta opioid antagonist activity and binding studies of regioisomeric isothiocyanate derivatives of naltrindole: evidence for delta receptor subtypes. J Med Chem. 1992;35:4086–91. doi: 10.1021/jm00100a014. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gaveriaux-Ruff C, Kieffer BL. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS One. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gaveriaux-Ruff C, Kieffer BL. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci. 2011;32:581–90. doi: 10.1016/j.tips.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Walwyn W, Nozaki C, Filliol D, Erbs E, Matifas A, Evans C, Kieffer BL. Ligand-directed trafficking of the delta-opioid receptor in vivo: two paths toward analgesic tolerance. J Neurosci. 2010;30:16459–68. doi: 10.1523/JNEUROSCI.3748-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purington LC, Sobczyk-Kojiro K, Pogozheva ID, Traynor JR, Mosberg HI. Development and in vitro characterization of a novel bifunctional mu-agonist/delta-antagonist opioid tetrapeptide. ACS Chem Biol. 2011;6:1375–81. doi: 10.1021/cb200263q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahim RT, Meissler JJ, Jr, Cowan A, Rogers TJ, Geller EB, Gaughan J, Adler MW, Eisenstein TK. Administration of mu-, kappa- or delta2-receptor agonists via osmotic minipumps suppresses murine splenic antibody responses. Int Immunopharmacol. 2001;1:2001–9. doi: 10.1016/s1567-5769(01)00128-x. [DOI] [PubMed] [Google Scholar]
- Randall-Thompson JF, Pescatore KA, Unterwald EM. A role for delta opioid receptors in the central nucleus of the amygdala in anxiety-like behaviors. Psychopharmacology (Berl) 2010;212:585–95. doi: 10.1007/s00213-010-1980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasakham K, Liu-Chen LY. Sex differences in kappa opioid pharmacology. Life Sci. 2011;88:2–16. doi: 10.1016/j.lfs.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Hewson JM, Inan S, Cowan A. Brain delta2 opioid receptors mediate SNC-80-evoked hypothermia in rats. Brain Res. 2005;1049:61–9. doi: 10.1016/j.brainres.2005.04.074. [DOI] [PubMed] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol. 1994;45:330–4. [PubMed] [Google Scholar]
- Reinoso-Barbero F, de Andres I. Effects of opioid microinjections in the nucleus of the solitary tract on the sleep-wakefulness cycle states in cats. Anesthesiology. 1995;82:144–52. doi: 10.1097/00000542-199501000-00019. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Lonnberg P, Marjamaki P, Molsa P, Sako E, Paljarvi L. Brain methionine- and leucine-enkephalin receptors in patients with dementia. Neurosci Lett. 1993;161:77–80. doi: 10.1016/0304-3940(93)90144-a. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Bykov V, Jacobson AE, Rice KC, Long JE, Bowen WD. A study of the effect of the irreversible delta receptor antagonist [D-Ala2,Leu5,Cys6]-enkephalin on delta cx and delta ncx opioid binding sites in vitro and in vivo. Peptides. 1992a;13:691–4. doi: 10.1016/0196-9781(92)90174-2. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Mahboubi A, Bykov V, Kim CH, de Costa BR, Jacobson AE, Rice KC. Probing the opioid receptor complex with (+)-trans-SUPERFIT. II. Evidence that mu ligands are noncompetitive inhibitors of the delta cx opioid peptide binding site. Peptides. 1992b;13:1137–43. doi: 10.1016/0196-9781(92)90020-4. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Mahboubi A, Bykov V, Kim CH, Jacobson AE, Rice KC. Probing the opioid receptor complex with (+)-trans-superfit. I. Evidence that [D-Pen2,D-Pen5]enkephalin interacts with high affinity at the delta cx binding site. Peptides. 1991;12:359–64. doi: 10.1016/0196-9781(91)90026-l. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. Faseb J. 2007;21:2455–65. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh A, Sugiyama A, Nemoto T, Fujii H, Wada K, Oka J, Nagase H, Yamada M. The novel delta opioid receptor agonist KNT-127 produces antidepressant-like and antinociceptive effects in mice without producing convulsions. Behav Brain Res. 2011;223:271–9. doi: 10.1016/j.bbr.2011.04.041. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Sugiyama A, Nemoto T, Fujii H, Wada K, Oka J, Nagase H, Yamada M. The novel delta opioid receptor agonist KNT-127 produces antidepressant-like and antinociceptive effects in mice without producing convulsions. Behav Brain Res. 2012;223:271–9. doi: 10.1016/j.bbr.2011.04.041. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Yoshikawa Y, Onodera K, Kamei J. Role of delta-opioid receptor subtypes in anxiety-related behaviors in the elevated plus-maze in rats. Psychopharmacology (Berl) 2005;182:327–34. doi: 10.1007/s00213-005-0112-6. [DOI] [PubMed] [Google Scholar]
- Sakami S, Kawai K, Maeda M, Aoki T, Fujii H, Ohno H, Ito T, Saitoh A, Nakao K, Izumimoto N, Matsuura H, Endo T, Ueno S, Natsume K, Nagase H. Design and synthesis of a metabolically stable and potent antitussive agent, a novel delta opioid receptor antagonist, TRK-851. Bioorg Med Chem. 2008a;16:7956–67. doi: 10.1016/j.bmc.2008.07.065. [DOI] [PubMed] [Google Scholar]
- Sakami S, Maeda M, Kawai K, Aoki T, Kawamura K, Fujii H, Hasebe K, Nakajima M, Endo T, Ueno S, Ito T, Kamei J, Nagase H. Structure-antitussive activity relationships of naltrindole derivatives. Identification of novel and potent antitussive agents. J Med Chem. 2008b;51:4404–11. doi: 10.1021/jm701440h. [DOI] [PubMed] [Google Scholar]
- Saland LC, Abeyta A, Frausto S, Raymond-Stintz M, Hastings CM, Carta M, Valenzuela CF, Savage DD. Chronic ethanol consumption reduces delta-and mu-opioid receptor-stimulated G-protein coupling in rat brain. Alcohol Clin Exp Res. 2004;28:98–104. doi: 10.1097/01.ALC.0000108658.00243.BF. [DOI] [PubMed] [Google Scholar]
- Salemi S, Aeschlimann A, Wollina U, Gay RE, Michel BA, Gay S, Sprott H. Up-regulation of delta-opioid receptors and kappa-opioid receptors in the skin of fibromyalgia patients. Arthritis Rheum. 2007;56:2464–6. doi: 10.1002/art.22735. [DOI] [PubMed] [Google Scholar]
- Sales N, Riche D, Roques BP, Denavit-Saubie M. Localization of mu- and delta-opioid receptors in cat respiratory areas: an autoradiographic study. Brain Res. 1985;344:382–6. doi: 10.1016/0006-8993(85)90820-0. [DOI] [PubMed] [Google Scholar]
- Saloman JL, Niu KY, Ro JY. Activation of peripheral delta-opioid receptors leads to anti-hyperalgesic responses in the masseter muscle of male and female rats. Neuroscience. 2011;190:379–85. doi: 10.1016/j.neuroscience.2011.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Befort K, Contet C, Becker J, Matifas A, Kieffer BL. The delta agonists DPDPE and deltorphin II recruit predominantly mu receptors to produce thermal analgesia: a parallel study of mu, delta and combinatorial opioid receptor knockout mice. Eur J Neurosci. 2004;19:2239–48. doi: 10.1111/j.0953-816X.2004.03339.x. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–59. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gaveriaux-Ruff C, Kieffer BL. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A. 2006;103:9691–6. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PW. Opioid Peptide-Derived Delta Antagonists, Inverse Agonists, and Mixed Mu Agonists/Delta Agonists. Marcel Dekker, Inc; 2004. Marcel Dekker, Inc. [Google Scholar]
- Schreiber G, Campa MJ, Prabhakar S, O'Briant K, Bepler G, Patz EF., Jr. Molecular characterization of the human delta opioid receptor in lung cancer. Anticancer Res. 1998;18:1787–92. [PubMed] [Google Scholar]
- Schultz JE, Gross GJ. Opioids and cardioprotection. Pharmacol Ther. 2001;89:123–37. doi: 10.1016/s0163-7258(00)00106-6. [DOI] [PubMed] [Google Scholar]
- Sezen SF, Kenigs VA, Kapusta DR. Renal excretory responses produced by the delta opioid agonist, BW373U86, in conscious rats. J Pharmacol Exp Ther. 1998;287:238–45. [PubMed] [Google Scholar]
- Sharp BM, Gekker G, Li MD, Chao CC, Peterson PK. Delta-opioid suppression of human immunodeficiency virus-1 expression in T cells (Jurkat) Biochem Pharmacol. 1998;56:289–92. doi: 10.1016/s0006-2952(98)00155-5. [DOI] [PubMed] [Google Scholar]
- Sharp BM, McAllen K, Gekker G, Shahabi NA, Peterson PK. Immunofluorescence detection of delta opioid receptors (DOR) on human peripheral blood CD4+ T cells and DOR-dependent suppression of HIV-1 expression. J Immunol. 2001;167:1097–102. doi: 10.4049/jimmunol.167.2.1097. [DOI] [PubMed] [Google Scholar]
- Shen J, Chan KW, Chen BT, Philippe J, Sehba F, Duttaroy A, Carroll J, Yoburn BC. The effect of in vivo ethanol consumption on cyclic AMP and delta-opioid receptors in mouse striatum. Brain Res. 1997;770:65–71. doi: 10.1016/s0006-8993(97)00747-6. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Chefer VI, Thompson AC. Delta-opioid receptor antagonists prevent sensitization to the conditioned rewarding effects of morphine. Biol Psychiatry. 2009;65:169–74. doi: 10.1016/j.biopsych.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers BD, Harlan RE, Romano GJ, Howells RD, Pfaff DW. Cellular localization of proenkephalin mRNA in rat brain: gene expression in the caudate-putamen and cerebellar cortex. Proc Natl Acad Sci U S A. 1986;83:6221–5. doi: 10.1073/pnas.83.16.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook JE, Lemcke PK, Gehrig CA, Hruby VJ, Burks TF. Antidiarrheal properties of supraspinal mu and delta and peripheral mu, delta and kappa opioid receptors: inhibition of diarrhea without constipation. J Pharmacol Exp Ther. 1989;249:83–90. [PubMed] [Google Scholar]
- Simon EJ, Hiller JM. The opiate receptors. Annu Rev Pharmacol Toxicol. 1978;18:371–94. doi: 10.1146/annurev.pa.18.040178.002103. [DOI] [PubMed] [Google Scholar]
- Simon EJ, Hiller JM, Edelman I. Stereospecific binding of the potent narcotic analgesic (3H) Etorphine to rat-brain homogenate. Proc Natl Acad Sci U S A. 1973;70:1947–9. doi: 10.1073/pnas.70.7.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Portoghese PS, Takemori AE. Differential antagonism of delta opioid agonists by naltrindole and its benzofuran analog (NTB) in mice: evidence for delta opioid receptor subtypes. J Pharmacol Exp Ther. 1991;257:676–80. [PubMed] [Google Scholar]
- Sofuoglu M, Portoghese PS, Takemori AE. 7-Benzylidenenaltrexone (BNTX): A selective [delta]1 opioid receptor antagonist in the mouse spinal cord. Life Sciences. 1993;52:769–775. doi: 10.1016/0024-3205(93)90240-4. [DOI] [PubMed] [Google Scholar]
- Stasinopoulos T, Goodchild AK, Christie MJ, Chalmers J, Pilowsky PM. Delta opioid receptor immunoreactive boutons appose bulbospinal CI neurons in the rat. Neuroreport. 2000;11:887–91. doi: 10.1097/00001756-200003200-00045. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Melchiorri P, Negri L, Hughes TK, Jr., Scharrer B. [D-Ala2]deltorphin I binding and pharmacological evidence for a special subtype of delta opioid receptor on human and invertebrate immune cells. Proc Natl Acad Sci U S A. 1992;89:9316–20. doi: 10.1073/pnas.89.19.9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PE, Hammond DL. Activation of spinal delta-1 or delta-2 opioid receptors reduces carrageenan-induced hyperalgesia in the rat. J Pharmacol Exp Ther. 1994;268:701–8. [PubMed] [Google Scholar]
- Sugiyama A, Saitoh A, Yamada M, Inagaki M, Oka J, Nagase H, Yamada M. The anxiolytic-like effect of a novel delta opioid receptor agonist KNT-127 in rats Society for Neuroscience. New Orleans: 2012. [Google Scholar]
- Suzuki T, Tsuji M, Mori T, Misawa M, Endoh T, Nagase H. Effect of the highly selective and nonpeptide delta opioid receptor agonist TAN-67 on the morphine-induced place preference in mice. J Pharmacol Exp Ther. 1996;279:177–85. [PubMed] [Google Scholar]
- Suzuki T, Tsuji M, Mori T, Misawa M, Nagase H. Involvement of delta 1 and delta 2 opioid receptor subtypes in the development of physical dependence on morphine in mice. Pharmacol Biochem Behav. 1997;57:293–9. doi: 10.1016/s0091-3057(96)00319-x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yoshiike M, Mizoguchi H, Kamei J, Misawa M, Nagase H. Blockade of delta-opioid receptors prevents morphine-induced place preference in mice. Jpn J Pharmacol. 1994;66:131–7. doi: 10.1254/jjp.66.131. [DOI] [PubMed] [Google Scholar]
- Swegle JM, Logemann C. Management of common opioid-induced adverse effects. Am Fam Physician. 2006;74:1347–54. [PubMed] [Google Scholar]
- Szeto HH, Soong Y, Wu D, Olariu N, Kett A, Kim H, Clapp JF. Respiratory depression after intravenous administration of delta-selective opioid peptide analogs. Peptides. 1999;20:101–5. doi: 10.1016/s0196-9781(98)00141-7. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Kappa- and delta-opioids block sympathetically dependent hyperalgesia. J Neurosci. 1991;11:928–32. doi: 10.1523/JNEUROSCI.11-04-00928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MJ, Yin C, Tang CP, Ke CQ, Lin G, Ye Y. Antitussive indole alkaloids from Kopsia hainanensis. Planta Med. 2010;77:939–44. doi: 10.1055/s-0030-1250631. [DOI] [PubMed] [Google Scholar]
- Tejedor-Real P, Mico JA, Smadja C, Maldonado R, Roques BP, Gilbert-Rahola J. Involvement of delta-opioid receptors in the effects induced by endogenous enkephalins on learned helplessness model. Eur J Pharmacol. 1998;354:1–7. doi: 10.1016/s0014-2999(98)00423-3. [DOI] [PubMed] [Google Scholar]
- Terenius L. Characteristics of the “receptor” for narcotic analgesics in synaptic plasma membrane fraction from rat brain. Acta Pharmacol Toxicol (Copenh) 1973;33:377–84. doi: 10.1111/j.1600-0773.1973.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Thompson RC, Mansour A, Akil H, Watson SJ. Cloning and pharmacological characterization of a rat mu opioid receptor. Neuron. 1993;11:903–13. doi: 10.1016/0896-6273(93)90120-g. [DOI] [PubMed] [Google Scholar]
- Thorat SN, Hammond DL. Modulation of Nociception by Microinjection of Delta-1 and Delta-2 Opioid Receptor Ligands in the Ventromedial Medulla of the Rat. J Pharmacol Exp Ther. 1997;283:1185–1192. [PubMed] [Google Scholar]
- Torregrossa MM, Jutkiewicz EM, Mosberg HI, Balboni G, Watson SJ, Woods JH. Peptidic delta opioid receptor agonists produce antidepressant-like effects in the forced swim test and regulate BDNF mRNA expression in rats. Brain Res. 2006;1069:172–81. doi: 10.1016/j.brainres.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortella FC, Echevarria E, Robles L, Mosberg HI, Holaday JW. Anticonvulsant effects of mu (DAGO) and delta (DPDPE) enkephalins in rats. Peptides. 1988;9:1177–81. doi: 10.1016/0196-9781(88)90104-0. [DOI] [PubMed] [Google Scholar]
- Tortella FC, Robles L, Mosberg HI, Holaday JW. Electroencephalographic assessment of the role of delta receptors in opioid peptide - induced seizures. Neuropeptides. 1984;5:213–6. doi: 10.1016/0143-4179(84)90065-9. [DOI] [PubMed] [Google Scholar]
- Tsushima H, Mori M, Matsuda T. Effects of D-Ala2-D-Leu5-enkephalin, microinjected into the supraoptic and paraventricular nuclei, on urine outflow rate. Jpn J Pharmacol. 1993;63:181–6. doi: 10.1254/jjp.63.181. [DOI] [PubMed] [Google Scholar]
- Ukai M, Takada A, Sasaki Y, Kameyama T. Stimulation of delta1- and delta2-opioid receptors produces amnesia in mice. Eur J Pharmacol. 1997;338:1–6. doi: 10.1016/s0014-2999(97)01310-1. [DOI] [PubMed] [Google Scholar]
- Ukai M, Toyoshi T, Kameyama T. Multi-dimensional analysis of behavior in mice treated with the delta opioid agonists DADL (D-Ala2-D-Leu5-enkephalin) and DPLPE (D-Pen2-L-Pen5-enkephalin) Neuropharmacology. 1989;28:1033–9. doi: 10.1016/0028-3908(89)90114-7. [DOI] [PubMed] [Google Scholar]
- van Rijn RM, Brissett DI, Whistler JL. Dual efficacy of delta opioid receptor selective ligands for ethanol drinking and anxiety. J Pharmacol Exp Ther. 2010;335:133–139. doi: 10.1124/jpet.110.170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Brissett DI, Whistler JL. Distinctive modulation of ethanol place preference by delta opioid receptor-selective agonists. Drug Alcohol Depend. 2012a;122:156–9. doi: 10.1016/j.drugalcdep.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Brissett DI, Whistler JL. Emergence of functional spinal delta opioid receptors after chronic ethanol exposure. Biol Psychiatry. 2012b;71:232–8. doi: 10.1016/j.biopsych.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Whistler JL. The delta(1) opioid receptor is a heterodimer that opposes the actions of the delta(2) receptor on alcohol intake. Biol Psychiatry. 2009;66:777–84. doi: 10.1016/j.biopsych.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah TW, Wild KD, Takemori AE, Sultana M, Portoghese PS, Bowen WD, Mosberg HI, Porreca F. Mediation of swim-stress antinociception by the opioid delta 2 receptor in the mouse. J Pharmacol Exp Ther. 1992;262:190–7. [PubMed] [Google Scholar]
- Vaughn LK, Wire WS, Davis P, Shimohigashi Y, Toth G, Knapp RJ, Hruby VJ, Burks TF, Yamamura HI. Differentiation between rat brain and mouse vas deferens delta opioid receptors. Eur J Pharmacol. 1990;177:99–101. doi: 10.1016/0014-2999(90)90556-l. [DOI] [PubMed] [Google Scholar]
- Ventura C, Bastagli L, Bernardi P, Caldarera CM, Guarnieri C. Opioid receptors in rat cardiac sarcolemma: effect of phenylephrine and isoproterenol. Biochim Biophys Acta. 1989;987:69–74. doi: 10.1016/0005-2736(89)90456-2. [DOI] [PubMed] [Google Scholar]
- Wade PR, Palmer JM, McKenney S, Kenigs V, Chevalier K, Moore BA, Mabus JR, Saunders PR, Wallace NH, Schneider CR, Kimball ES, Breslin HJ, He W, Hornby PJ. Modulation of Gastrointestinal Function by MuDelta, a mixed Mu Opioid Receptor Agonist/Delta Opioid Receptor Antagonist. Br J Pharmacol. 2012;167:1111–1125. doi: 10.1111/j.1476-5381.2012.02068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Guan JS, Bao L, Zhang X. Distinct subcellular distribution of delta-opioid receptor fused with various tags in PC12 cells. Neurochem Res. 2008;33:2028–34. doi: 10.1007/s11064-008-9678-9. [DOI] [PubMed] [Google Scholar]
- Wang JB, Imai Y, Eppler CM, Gregor P, Spivak CE, Uhl GR. mu opiate receptor: cDNA cloning and expression. Proc Natl Acad Sci U S A. 1993;90:10230–4. doi: 10.1073/pnas.90.21.10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MJ, Holt JD, O'Neill SJ, Wei K, Pendergast W, Gross GJ, Gengo PJ, Chang KJ. ARD-353 [4-((2R,5S)-4-(R)-(4-diethylcarbamoylphenyl)(3-hydroxyphenyl)methyl)-2,5-d imethylpiperazin-1-ylmethyl)benzoic acid], a novel nonpeptide delta receptor agonist, reduces myocardial infarct size without central effects. J Pharmacol Exp Ther. 2006;316:423–30. doi: 10.1124/jpet.105.092742. [DOI] [PubMed] [Google Scholar]
- Wei ZY, Brown W, Takasaki B, Plobeck N, Delorme D, Zhou F, Yang H, Jones P, Gawell L, Gagnon H, Schmidt R, Yue SY, Walpole C, Payza K, St-Onge S, Labarre M, Godbout C, Jakob A, Butterworth J, Kamassah A, Morin PE, Projean D, Ducharme J, Roberts E. N,N-Diethyl-4-(phenylpiperidin-4-ylidenemethyl)benzamide: a novel, exceptionally selective, potent delta opioid receptor agonist with oral bioavailability and its analogues. J Med Chem. 2000;43:3895–905. doi: 10.1021/jm000229p. [DOI] [PubMed] [Google Scholar]
- Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, Murray SR, Von Zastrow M. Modulation of postendocytic sorting of G protein-coupled receptors. Science. 2002;297:615–20. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- Wild KD, Carlisi VJ, Mosberg HI, Bowen WD, Portoghese PS, Sultana M, Takemori AE, Hruby VJ, Porreca F. Evidence for a single functional opioid delta receptor subtype in the mouse isolated vas deferens. J Pharmacol Exp Ther. 1993;264:831–8. [PubMed] [Google Scholar]
- Williams SA, Abbruscato TJ, Hruby VJ, Davis TP. Passage of a delta-opioid receptor selective enkephalin, [D-penicillamine2,5] enkephalin, across the blood-brain and the blood-cerebrospinal fluid barriers. J Neurochem. 1996;66:1289–99. doi: 10.1046/j.1471-4159.1996.66031289.x. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Milner TA. Delta opioid receptors colocalize with corticotropin releasing factor in hippocampal interneurons. Neuroscience. 2011;179:9–22. doi: 10.1016/j.neuroscience.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Mascagni F, McDonald AJ. Sex differences in delta opioid receptor immunoreactivity in rat medial amygdala. Neurosci Lett. 2002;328:160–4. doi: 10.1016/s0304-3940(02)00481-0. [DOI] [PubMed] [Google Scholar]
- Xie CW, Lewis DV. Endogenous opioids regulate long-term potentiation of synaptic inhibition in the dentate gyrus of rat hippocampus. J Neurosci. 1995;15:3788–95. doi: 10.1523/JNEUROSCI.15-05-03788.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Lu YF, Rice KC, Ananthan S, Rothman RB. SoRI 9409, a non-peptide opioid mu receptor agonist/delta receptor antagonist, fails to stimulate [35S]-GTP-gamma-S binding at cloned opioid receptors. Brain Res Bull. 2001;55:507–11. doi: 10.1016/s0361-9230(01)00550-0. [DOI] [PubMed] [Google Scholar]
- Xu H, Ni Q, Jacobson AE, Rice KC, Rothman RB. Preliminary ligand binding data for subtypes of the delta opioid receptor in rat brain membranes. Life Sci. 1991;49:PL141–6. doi: 10.1016/0024-3205(91)90204-o. [DOI] [PubMed] [Google Scholar]
- Yajima Y, Narita M, Takahashi-Nakano Y, Misawa M, Nagase H, Mizoguchi H, Tseng LF, Suzuki T. Effects of differential modulation of mu-, delta- and kappa-opioid systems on bicuculline-induced convulsions in the mouse. Brain Res. 2000;862:120–6. doi: 10.1016/s0006-8993(00)02096-5. [DOI] [PubMed] [Google Scholar]
- Yamamura S, Ohoyama K, Nagase H, Okada M. Zonisamide enhances delta receptor-associated neurotransmitter release in striato-pallidal pathway. Neuropharmacology. 2009;57:322–31. doi: 10.1016/j.neuropharm.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Yang L, Wang H, Shah K, Karamyan VT, Abbruscato TJ. Opioid receptor agonists reduce brain edema in stroke. Brain Res. 2011;1383:307–16. doi: 10.1016/j.brainres.2011.01.083. [DOI] [PubMed] [Google Scholar]
- Yang S, Kawamura Y, Yoshikawa M. Effect of rubiscolin, a delta opioid peptide derived from Rubisco, on memory consolidation. Peptides. 2003;24:325–8. doi: 10.1016/s0196-9781(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Zhang J, Qian H, Zhao P, Hong SS, Xia Y. Rapid hypoxia preconditioning protects cortical neurons from glutamate toxicity through delta-opioid receptor. Stroke. 2006a;37:1094–9. doi: 10.1161/01.STR.0000206444.29930.18. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Guan JS. Role of delivery and trafficking of delta-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol Sci. 2006b;27:324–9. doi: 10.1016/j.tips.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Pan ZZ. Synaptic mechanism for functional synergism between {delta}-and {micro}-opioid receptors. J Neurosci. 2010;30:4735–45. doi: 10.1523/JNEUROSCI.5968-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Cho YK, Li CS. Activation of delta-opioid receptors reduces excitatory input to putative gustatory cells within the nucleus of the solitary tract. J Neurophysiol. 2009;101:258–68. doi: 10.1152/jn.90648.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hsu MS, Pintar JE. Developmental expression of the mu, kappa, and delta opioid receptor mRNAs in mouse. J Neurosci. 1998;18:2538–49. doi: 10.1523/JNEUROSCI.18-07-02538.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AGP, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Retention of Supraspinal Delta-like Analgesia and Loss of Morphine Tolerance in [delta] Opioid Receptor Knockout Mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]