Abstract
NK cell activation is controlled by the integration of signals from cytokine receptors and germ-line encoded activation and inhibitory receptors. NK cells undergo two distinct phases of activation during MCMV infection: a nonselective phase mediated by pro-inflammatory cytokines and a specific phase driven by signaling through Ly49H, an NK cell activation receptor that recognizes infected cells. We sought to delineate cell surface markers that could distinguish NK cells that had been activated nonselectively from those that had been specifically activated through NK cell receptors. We demonstrated that Sca-1 is highly upregulated during viral infections (to an even greater extent than CD69) and serves as a novel marker of early, nonselective NK cell activation. Indeed, a greater proportion of Sca-1+ NK cells produced IFN-γ compared to Sca-1− NK cells during MCMV infection. In contrast to the universal upregulation of Sca-1 (as well as KLRG1) on NK cells early during MCMV infection, differential expression of Sca-1, as well as CD27 and KLRG1, was observed on Ly49H+ and Ly49H− NK cells late during MCMV infection. Persistently elevated levels of KLRG1 in the context of down regulation of Sca-1 and CD27 were observed on NK cells that expressed Ly49H. Furthermore, the differential expression patterns of these cell surface markers were dependent on Ly49H recognition of its ligand and did not occur solely as a result of cellular proliferation. These findings demonstrate that a combination of Sca-1, CD27, and KLRG1 can distinguish NK cells nonselectively activated by cytokines from those specifically stimulated through activation receptors.
Keywords: NK Cells, Ly49H, MCMV, Sca-1, KLRG1, CD27, CD69
Introduction
NK cells are innate immune lymphocytes, which were initially characterized by their ability to kill transformed and infected cells without prior sensitization. Studies of rare humans with isolated NK cell deficiencies (1) as well as mice depleted of NK cells (2) have demonstrated that NK cells are a critical component of host defense against viruses, particularly herpesviruses. Murine cytomegalovirus (MCMV), a beta herpesvirus, has been an invaluable tool for delineating the role of NK cell activation receptors in early host defense.
NK cell responses are controlled by cytokines and signals from activation and inhibitory receptors. Previous studies have shown that mouse strains that express the Ly49H activation receptor, such as C57BL/6 (B6), are resistant to MCMV infection, while those that do not, such as BALB/c, are susceptible to infection (3–5). Furthermore, transgenic expression of Ly49H in susceptible mouse strains results in resistance to MCMV (6), and congenic B6 mouse strains that lack the Ly49H locus are susceptible to MCMV (3, 7). Ly49H recognizes the MCMV-encoded protein m157, resulting in NK cell activation and the killing of infected cells (8, 9). Moreover, when infected with an m157-deficient virus, B6 mice become susceptible to MCMV infection (10, 11), demonstrating the non-redundant role of Ly49H in mediating resistance to MCMV.
NK cells undergo two distinct phases of activation during viral infections, such as MCMV. Early during MCMV infection, there is nonselective activation of both Ly49H+ and Ly49H− subsets of NK cells, which results in NK cell proliferation and IFN-γ production. This phase is driven by pro-inflammatory cytokines, such as IL-15 and IL-12 (12, 13). Subsequently, there is specific activation of Ly49H+ NK cells, which is dependent on Ly49H recognition of m157 and results in preferential expansion of the Ly49H+ subset of NK cells (14–16). Other activation receptor/ligand pairs have been identified in a variety of different infection systems and/or mouse strains (17–20). Moreover, preferential expansion of specific NK cell subsets has also been observed following a number of human infections (21–23), implicating a role for specific NK cell activation in human health and disease.
To our knowledge, there have been no reports of distinct cell surface proteins (“markers”) associated with the specific activation of NK cells. In contrast, several markers of early, nonspecific NK cell activation have been described, including the C-type lectin-like cell receptors CD69 (also called very early antigen) and the killer cell lectin-like receptor G1 (KLRG1) (24, 25). While CD69 is not expressed on resting NK cells (26), approximately 30–40% of resting NK cells express KLRG1 (24, 27, 28). Despite this difference, both CD69 and KLRG1 are highly upregulated on NK cells following activation by a variety of stimuli, including viral infections (26, 28–30). However, CD69 is only transiently upregulated on NK cells following MCMV infection while there is prolonged upregulation of KLRG1 expression for more than a week (28, 31). Previous studies have shown that KLRG1+ NK cells are less activated (28) and more prone to apoptosis following MCMV infection (31) than KLRG1− NK cells. However, none of these studies have examined the relationship between KLRG1 and Ly49H expression during the specific phase of NK cell activation during MCMV infection.
Although stem cell antigen 1 (Sca-1, also called Ly-6A/E) has been best characterized as a marker of hematopoietic stem cells (reviewed in (32)), it was initially identified on activated lymphocytes (33). Interestingly, there is conflicting evidence as to whether Sca-1 has an activating or inhibitory effect on lymphocytes. Initial studies demonstrated that antibody cross-linking of Sca-1 resulted in T and B cell activation in vitro (34–37). However, subsequent experiments demonstrated that effector T cells from Sca-1 deficient mice had increased proliferative responses (38) and that double negative regulatory T cells from Sca-1 deficient mice are unable to suppress skin allograft rejection (39). However, the expression and function of Sca-1 on NK cells is unknown.
We hypothesized that the nonselective and specific phases of NK cell activation would result in characteristic expression patterns of cell surface proteins (or markers). Markers of early, nonselective activation would be highly expressed early following infection on most NK cells, but have negligible expression on naïve NK cells (similar to that of CD69). Conversely, markers of specific activation would be differentially modulated on cells expressing the appropriate activation receptor. For example, one would expect differential expression of the markers on Ly49H+ and Ly49H− subsets of NK cells during MCMV infection of B6 mice. The identification of such a panel of cell surface proteins will facilitate the study and identification of activation receptor/ligand pairs in other systems. In this study, we have identified and characterized cell surface markers of both nonselective and specific NK cell activation.
Materials and Methods
Mice
C57BL/6 mice were obtained from National Cancer Institute (Charles River, MA). 129.IFNαβR-deficient mice (IFNαβR−/−, (40)) were backcrossed onto a C57BL/6 background for 10 generations as previously described (16). C57BL/6.Sca-1EGFP/EGFP mice (Sca-1−/−, (41)), which have an enhanced GFP cassette knocked into the Sca-1 locus, were a generous gift from Timothy Graubert (Washington University, St. Louis, MO). C57BL/6.DAP12 loss-of-function knock-in (DAP12KI; (42)) mice were a kind gift from Eric Vivier (CNRS-INSERM-Universite de la Mediterranee, France). Mice were maintained under specific pathogen-free conditions and used between 8 and 14 weeks of age. All experiments were conducted in accordance with institutional guidelines for animal care and use.
Antibodies
Biotinylated anti-Ly49H Ab (3D10) was a kind gift from Wayne Yokoyama (Washington University). The following antibodies and PE-streptavidin were purchased from eBioscience (San Diego, CA), unless indicated otherwise: biotinylated anti-KLRG1 (2F1); FITC-conjugated anti-KLRG1 (2F1), -Ly49H (3D10), and -NKp46 (29A1.4, R&D Systems); PE-conjugated anti-CD27 (LG.7F9), -CD69 (H1.2F3), -IFN-γ (XMG1.2), and -KLRG1 (2F1); PerCP/Cy5.5-conjugated anti-CD3 (145-2C11) and -NK1.1 (PK136); PE/Cy7-conjugated anti-CD27 (LG.7F9) and -Sca-1 (D7); eFluor® 450-conjugated anti-NKp46 (29A1.4); allophycocyanin-conjugated anti-Ly49H (3D10); allophycocyanin/eFluor® 780-conjugated anti-CD3 (17A2); allophycocyanin/Cy7-conjugated anti-CD3e (145-2C11, BD Pharmingen, San Diego, CA); V500-conjugated anti-Sca-1 (D7, BD Biosciences, San Jose, CA) and V450-conjugated anti-CD69 (H1.2F3, BD Biosciences).
Viruses and injection of mice
Salivary gland stock of Smith strain MCMV (American Type Culture Collection [ATCC]; Manassas, VA) was prepared using young BALB/c mice that were i.p. injected with 1 × 106 PFU of tissue-culture propagated MCMV. The titer of the stock was determined using a standard plaque assay (3) in permissive NIH3T12 fibroblasts (ATCC). MCMV-AT1.5 (G881A mutation creating a premature stop codon) is an m157-deficient isolate of MCMV (MCMV-Δm157), which has been previously described (11). Unless otherwise indicated, mice were injected i.p. with 5 × 104 PFU/mouse of salivary gland stock MCMV.
Vaccinia virus (VV, ATCC) was propagated as previously described (43). All VV infections were done by i.p. injection with 5 × 106 PFU/mouse. HSV strain 17 was a kind gift from David Leib (Dartmouth Medical School, Lebanon, NH). HSV was propagated and titered as previously described (43). All HSV infections were done by i.p. injection of 1.5 × 106 PFU/mouse.
Splenocyte preparation, intracellular staining and flow cytometry
Single-cell suspensions of splenocytes were prepared using standard techniques (14). To block non-specific binding of antibodies to Fc receptors, splenocytes were incubated in 2.4G2 (anti-Fcγ II/III receptor) supernatants (hybridoma from ATCC) prior to staining with labeled Abs. Data acquisition was performed with a FACSCalibur flow cytometer (BD Pharmingen) using CellQuest software (BD Biosciences) or with a FACScan (BD Pharmingen) with DxP multi-color upgrade (Cytek Development, Fremont, CA) and FlowJo CE software (Tree Star, Ashland Organ). Data analysis was performed using FlowJo (Tree Star). Intracellular IFN-γ staining was done as previously described (14). NK cells were identified as CD3−, NK1.1+, or as CD3−, NK1.1+, NKp46+. All histograms have been scaled for the purpose of comparison.
Quantification of MCMV viral loads using real-time PCR
MCMV viral loads were determined by quantifying the copies of the IE1 gene using qRT-PCR (TaqMan) as previously described (44), with the following modifications. DNA was isolated using the QIAmp DNA Blood MiniKit (Qiagen, Valencia, CA) from homogenized spleens diluted 1:10 in PBS. Five microliters of 100 and 1000 fold dilutions of each sample were run in triplicate. Samples were run in 96-well plates on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Inc., Foster City, CA).
In vitro IL-15 proliferation assay
Single-cell suspensions of splenocytes were enriched for NK cells by negative selection with magnetic bead (Miltenyi Biotec, Auburn, CA). Splenocytes were labeled with 0.5 µM CFSE (Molecular Probes, Eugene, OR) as previously described (45). CFSE-labeled splenocytes were cultured in 96-well plates for 72 h with various concentrations of murine IL-15 (PeproTech, Rocky Hill, NJ).
Statistical analysis
Data analysis was done with Microsoft Excel and GraphPad Prism (GraphPad Software, La Jolla, CA). Unless otherwise noted, unpaired, two-tailed t tests were used to determine statistically significant differences. Error bars in figures represent standard deviations from mean value.
Results
Sca-1 is a novel marker of early, nonselective NK cell activation
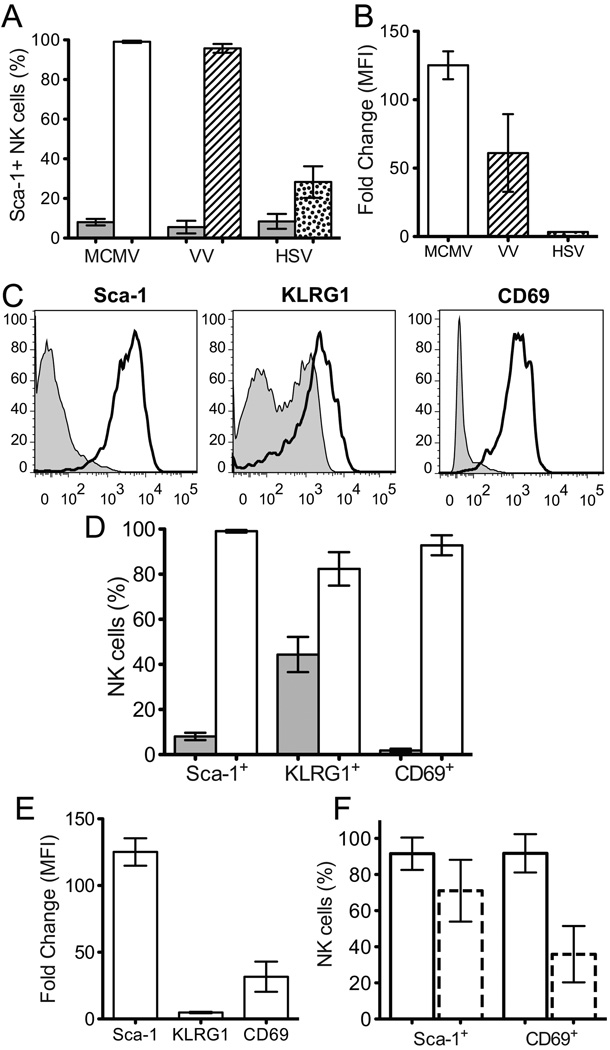
We used flow cytometry to analyze the expression of various proteins on NK cells from naïve mice and from mice 2 days post infection (p.i.) with MCMV. In these experiments, we observed that there was negligible expression of Sca-1 on NK cells from uninfected mice (6.7 ± 2.7%) while it was highly expressed on almost all NK cells 2 days p.i. with MCMV (99.1 ± 0.6%) (Fig. 1A and B; Table I). Sca-1 is also upregulated following other viral infections, including vaccinia virus (VV) and herpes simplex virus type 1 (HSV) infections (Fig. 1A and B).
Figure 1. Sca-l is upregulated on NK cells 2 days p.i. MCMV to a greater extent than other identified activation markers.
A) Percentage of NK cells that express Sca-1 in naïve B6 mice (grey fill) or B6 mice 2 days p.i. MCMV (no fill), VV (stripes), or HSV (dots). B) Fold change of the MFI of Sca-1 between NK cells from naïve B6 mice and B6 mice 2 days p.i. MCMV (no fill), VV (stripes), or HSV (dots). C) Representative histograms showing Sca-1, KLRG1, or CD69 expression on splenic NK cells from naïve B6 mice (thin line, grey fill) or from B6 mice 2 days p.i. wt MCMV (thick line, no fill). D) Percentage of NK cells that express Sca-1, KLRG1, or CD69 in naïve B6 mice (grey fill) or B6 mice 2 days p.i. MCMV (no fill). E) Fold change of the MFI of Sca-1, KLRG1, or CD69 between NK cells from naïve B6 mice and B6 mice 2 days p.i. MCMV. F) Frequency of Sca-1+ or CD69+ NK cells from wt B6 (solid outline) or IFNαβR−/− (dashed outline) mice 2 days p.i. MCMV. Composite data from 2 independent experiments each with 3–4 mice/genotype. B and E) MFI values are for the entire NK cell population. A), B), D), and E) show composite results from 2–3 independent experiments with a total of 3–5 naïve mice and 7–9 infected mice.
Table I.
Marker expression on NK cells from naïve mice or from mice 1.5 or 2 days p.i. MCMV
| Sca-1 | KLRG1 | CD69 | ||
|---|---|---|---|---|
| Frequency of Marker+ cells (%) |
Naïve | 6.7 ± 2.7 | 42.8 ± 7.8 | 1.9 ± 0.6 |
| 1.5 days p.i. |
44.7 ± 11.9 | 59.5 ± 4.0 | 65.9 ± 17.3 | |
| 2 days p.i. | 99.1 ± 0.6 | 83.8 ± 5.7 | 92.8 ± 4.4 |
Frequency of Sca-1, KLRG1, and CD69 expressing splenic NK cells from naïve mice or from mice 1.5 or 2 days p.i. with MCMV. Data is average from 3–4 independent experiments with a total of 8–13 mice/time point.
Subsequently, we compared the expression of Sca-1 with the expression of previously identified activation markers, CD69 (26, 29, 30) and KLRG1 (28) (Fig. 1C and D). Like Sca-1, the frequency of KLRG1+ and CD69+ NK cells increased between naïve and infected mice (Table I). Moreover, there was an increase in the abundance of each marker per cell as measured by an increase in the mean fluorescence intensity (MFI) (Fig. 1E). Sca-1 abundance increased by an average of 125 ± 10 fold, KLRG1 by 4.9 ± 0.5 fold, and CD69 by 31.7 ± 11 fold. Similarly, there is increased expression of Sca-1, KLRG1, and CD69 on hepatic NK cells 2 days p.i. MCMV (Fig. S1A and B). Together these results demonstrate that Sca-1 is more strongly induced on NK cells following MCMV infection than other previously described markers of early NK cell activation, KLRG1 and CD69.
Prior studies have shown that type I interferons (IFNs) can induce the expression of both Sca-1 (46) and CD69 (29). To determine whether type I IFNs are required for the induction of Sca-1 and CD69 during the early, nonspecific response to MCMV infection, we analyzed Sca-1 and CD69 expression on NK cells from wt B6 or IFNαβR−/− mice 2 days p.i MCMV (Fig. 1F). While the increased frequency of Sca-1+ NK cells from IFNαβR−/− mice 2 days p.i. MCMV was only slightly reduced compared to that seen in wt B6 mice (71.0 ± 17.1% and 91.6 ± 9.0%, respectively), the frequency of CD69+ NK cells was markedly less in IFNαβR−/− mice (35.9 ± 15.6%) compared to that seen in wt B6 mice (91.8 ± 10.6%). These results suggest that type I IFNs are not necessary for the induction of either marker during MCMV infection. However, based on the significant decrease in the frequency of CD69+ NK cells from IFNαβR−/− mice compared to wt B6 mice, type I IFNs contribute substantially to the induction of CD69 expression during MCMV infection. In contrast, it appears that other factors can compensate for the absence of type I IFN signaling in inducing Sca-1 expression during MCMV infection, consistent with previous reports that IFN-γ, IL-6, and −9 can also induce expression of Sca-1 (47–49).
Functional consequences of Sca-1 upregulation
We investigated whether the upregulation of these activation markers had any functional implications. We looked at marker expression with respect to IFN-γ production in both splenic (Fig. 2A, 2B, and S1G) and hepatic (Fig. S1C, D, and F) NK cells 1.5 days p.i. MCMV, the time point of maximal production of IFN-γ by splenic NK cells (14) following MCMV infection (Fig. S1E). There was a statistically significant increase in the proportion of splenic Sca-1+ and CD69+ NK cells that made IFN-γ (51.3 ± 9.0% and 56.5 ± 8.0%, respectively) compared to Sca-1− and CD69− NK cells (39.2 ± 11.2% and 20.1 ± 5.4%, respectively). Contrary to a previous report by Robbins and colleagues (28), we did not observe a difference between the proportion of KLRG1+ and KLRG1− NK cells producing IFN-γ (45.3 ± 13% and 43.5 ± 11%, respectively, p = 0.22). Similar patterns were seen for hepatic NK cells (Fig. S1C and D), although the overall magnitude was lower than that seen in spleen (Fig. S1E). From these results, we concluded that Sca-1 and CD69 upregulation specifically identify activated NK cells early during MCMV infection.
Figure 2. Sca-1 and CD69 expression correlate with IFN-γ production.
A) Representative histograms showing relative proportions of splenic NK cells making IFN-γ 1.5 days p.i. MCMV based on expression of Sca-1 (left), KLRG1 (middle), or CD69 (right). B) Proportion of splenic NK cells that make IFN-γ 1.5 days p.i. MCMV based on Sca-1, KLRG1, or CD69 expression. Marker negative cells (◊) and marker expressing cells (♦). Composite data from 2 independent experiments with 3–5 mice/group. Statistical significance was determined using paired, two-tailed t-tests. C) IFN-γ production by splenic NK cells from wt B6 mice or Sca-1−/− mice 1.5 days p.i. MCMV. Composite data from 2 independent experiments with 4–5 mice/group. **p < 0.01, ***p < 0.001.
To investigate the function of Sca-1 on NK cells, we examined NK cell responses in Sca-1−/− mice (41). We did not observe any difference in splenic viral titers or the frequency of proliferating NK cells during MCMV infection (data not shown). Furthermore, no differences were seen in NK cell mediated killing by cytokine stimulated LAK cells generated from Sca-1−/− or wt splenic NK cells (data not shown). However, 1.5 days p.i. with MCMV, there was a significant increase in the frequency of NK cells producing IFN-γ from Sca-1−/− mice (65.3 ± 7.3%) compared to those from wt B6 mice (44.1 ± 12.6%) (Fig. 2C). These differences in IFN-γ production occurred in the absence of any significant differences in serum concentrations of IL-12 from wt B6 or Sca-1−/− mice 1.5 days p.i. with MCMV (data not shown). These results suggest that Sca-1 has an inhibitory function on NK cells during MCMV infection.
KLRG1, CD27, and Sca-1 are markers of specific NK cell activation
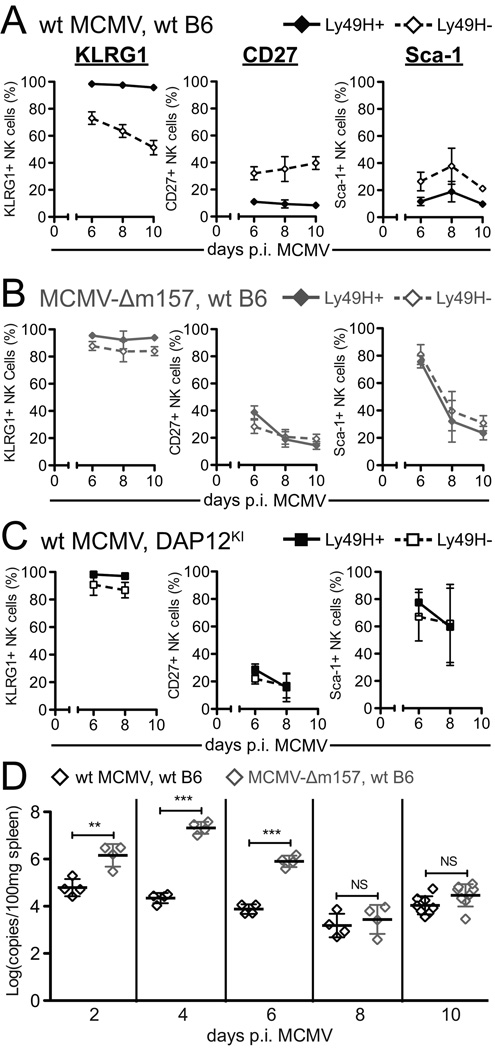
In contrast to markers of early, nonselective activation during MCMV infection, markers of specific activation should be differentially expressed on Ly49H+ and Ly49H− NK cells. Although early during MCMV infection Sca-1 and KLRG1 are uniformly upregulated on NK cells regardless of Ly49H expression (Fig. 3C), late during MCMV infection (6–10 days p.i.) we observed differential expression of both Sca-1 and KLRG1 on Ly49H+ and Ly49H− NK cells (Fig. 3A–C). In addition, Ly49H+ and Ly49H− NK cells differentially express CD27, a costimulatory molecule of the TNF receptor superfamily that has been described as a marker of NK cell maturation (reviewed in (50)). KLRG1 expression is maintained on Ly49H+ NK cells (98.8 ± 0.4% at 8 days p.i.), while it is gradually lost on Ly49H− NK cells (66.6 ± 5.7% at 8 days p.i.). Conversely, the expression of CD27 and Sca-1 decrease more rapidly on Ly49H+ NK cells (8.8 ± 6.3% and 17.8 ± 9.7%, respectively, at 8 days p.i.) than on Ly49H− NK cells (33.2 ± 8.3% and 42.8 ± 11.6%, respectively, at 8 days p.i.). The differential expression of KLRG1, CD27, and Sca-1 during MCMV infection suggests that these cell surface molecules function as markers of specific NK cell activation.
Figure 3. Expression of KLRG1, CD27, and Sca-1 is differentially regulated on Ly49H+ and Ly49H− NK cells during MCMV infection.
A) Representative dot plots showing KLRG1 (top), CD27 (middle), or Sca-1 (bottom) expression with respect to Ly49H expression and B) histograms showing the expression of these markers on Ly49H+ (solid line, no fill) or Ly49H− (dashed line, grey fill) splenic NK cells from B6 mice 8 days p.i. MCMV. C) Proportion of Ly49H+ (♦, solid line) or Ly49H− (◊, dashed line) splenic NK cells that express KLRG1, CD27, or Sca-1 from B6 mice 0, 2, 4, 6, 8, or 10 days p.i. MCMV. D) Frequency of CD27/Sca-1 subsets among Ly49H−KLRG1− or Ly49H+KLRG1+ NK cells from wt B6 mice 4, 6 or 8 days p.i. MCMV. Sca-1+CD27− (black), Sca-1+CD27+ (black stripes), Sca-1−CD27+ (black dots), Sca-1−CD27− (no fill). E) Representative histogram showing frequency of Ly49H+ NK cells among NK cell subsets based on KLRG1, CD27, and Sca-1 coexpression patterns from wt B6 mice 8 days p.i. MCMV, which is quantified on the graph to the right as the ratio of Ly49H+ to Ly49H− NK cells in each subset. All panels show cumulative results from 2, independent experiments with 4–5 mice/group/time point. Statistical significance was determined using paired, two-tailed t-tests. ***p < 0.001, **p < 0.01
The evaluation of the coexpression patterns of KLRG1, CD27, and Sca-1 supported this conclusion. The coexpression of these markers was compared on NK cells that had been specifically activated (Ly49H+KLRG1+ NK cells) and NK cells that had not been specifically activated (Ly49H−KLRG1−NK cells) (Fig. 3D). While there was no predominant CD27/Sca-1 subset among the Ly49H−KLRG1− NK cells, most Ly49H+KLRG1+ NK cells became CD27−Sca-1− over time (88.1 ± 1.8% at 8 days p.i.). Moreover, when we gated NK cells based on the expression of these three markers, we observed that the KLRG1+CD27−Sca-1− population was enriched for Ly49H+ cells (ratio of Ly49H+ to Ly49H− NK cells of 11.8 ± 3.4 at 8 days p.i.) while very few of the KLRG1−CD27+Sca-1+ NK cells were Ly49H+ (ratio of Ly49H+ to Ly49H− NK cells of 0.24 ± 0.1 at 8 days p.i.) (Fig. 3E). Therefore, it may be possible to use KLRG1, CD27, and Sca-1 expression on NK cells during viral infections to enrich for populations with high or low expression of receptors responsible for specific activation.
Differential expression of KLRG1, CD27, and Sca-1 is dependent on Ly49H recognition of m157
To determine whether the distinct expression patterns of KLRG1, CD27, and Sca-1 on Ly49H+ and Ly49H− NK cells depend on the binding of Ly49H to m157, we analyzed the expression of KLRG1, CD27, and Sca-1 on NK cells from mice infected with either wt MCMV or m157-deficient MCMV (MCMV-Δm157: (16)). In all previous experiments, mice were infected with 5 × 104 PFU wt MCMV; however, due to the increased virulence of MCMV-Δm157 in B6 mice (11, 16), we inoculated mice with 1 × 104 PFU of virus to provide sufficient stimulation but prevent excessive mortality. In contrast to the differential expression of KLRG1, CD27, and Sca-1 that we observed when mice were infected with wt MCMV (Fig. 3 and Fig. 4A), infection of mice with MCMV-Δm157 resulted in similar proportions of Ly49H+ and Ly49H− NK cells expressing KLRG1, CD27, or Sca-1 (Fig. 4B). Furthermore, studies with DAP12KI mice, which lack the ability to signal through Ly49H, revealed similar findings with no differential expression of Sca-1, KLRG1, or CD27 following infection with wt MCMV (Fig. 4C). Together, these studies demonstrated that the modulation of expression levels of KLRG1, CD27, and Sca-1 on Ly49H+ NK cells was dependent on the recognition of m157 by Ly49H and subsequent DAP12 mediated signaling.
Figure 4. Differential expression of KLRG1, CD27, and Sca-1 on Ly49H+ and Ly49H− NK cells during MCMV infection depends on Ly49H recognition of m157 protein.
A, B, and C) Proportion of Ly49H+ (filled symbol, solid line) or Ly49H− (open symbol, dashed line) splenic NK cells that express KLRG1 (left), CD27 (middle), or Sca-1 (right) from wt B6 mice (A and B, diamonds) or DAP12KI mice (C, squares) 6, 8, or 10 days p.i. with 1 × 104 PFU wt MCMV (A and C, black) or MCMV-Δm157 (B, grey). Figures show cumulative results from 2–3 independent experiments with 3–5 mice/group/time point. D) Viral loads from spleens of wt B6 mice 2, 4, 6, 8, or 10 days p.i. with 1 × 104 PFU wt MCMV (black) or MCMV-Δm157 (grey). Symbols represent viral loads for an individual mouse. **p<0.01, ***p<0.001.
Given that there was no stimulation through Ly49H in the mice infected with MCMV-Δm157, we expected that the expression levels of KLRG1, CD27, and Sca-1 on NK cells from mice infected with MCMV-Δm157 would resemble those on Ly49H− NK cells from mice infected with wt MCMV. However, the observed expression levels of KLRG1 and CD27 on NK cells from mice infected with MCMV-Δm157 were intermediate between the levels on Ly49H+ and Ly49H− NK cells from mice infected with wt MCMV, while the expression levels of Sca-1 were substantially higher at day 6 p.i. in mice infected with MCMV-Δm157 compared to either Ly49H+ and Ly49H− NK cells from mice infected with wt MCMV (Fig. 4B). These observations were consistent with the higher viral loads (100-fold at days 4 and 6 p.i.) found in mice infected with MCMV-Δm157 compared with mice infected with wt MCMV (Fig. 4D). The differences in viral loads following infection with wt MCMV and MCMV-Δm157 were abrogated by days 8 and 10 p.i. (Fig. 4D). We hypothesized that the prolonged elevation of viral loads in mice infected with MCMV-Δm157 resulted in persistent non-specific NK cell activation compared to infection with wt MCMV, reflected in higher than expected expression levels of KLRG1, CD27, and Sca-1. We verified this hypothesis in wt B6 mice infected with MCMV-Δm157 treated daily with either ganciclovir (GCV) or a control solution. The viral loads in mice infected with MCMV-Δm157 and treated with GCV were reduced to levels comparable to those seen in untreated mice infected with the wt MCMV (Fig. S2A and C). The higher than expected levels of Sca-1 observed in both Ly49H+ and Ly49H− NK cells on day 6 p.i. with MCMV-Δm157 (Fig 4B; and in mice treated with control solution, Fig. S2B) were abrogated in GCV treated mice (Fig. S2B). Importantly, no significant differences were observed in the proportions of Ly49H+ and Ly49H− NK cells that expressed KLRG1, CD27, or Sca-1 when GCV treatment lowered the viral loads in mice infected with MCMV-Δm157 to levels comparable to those seen in mice infected with the wt MCMV (Fig. S2B).
Differential expression of KLRG1, CD27, and Sca-1 is not the result of preferential proliferation
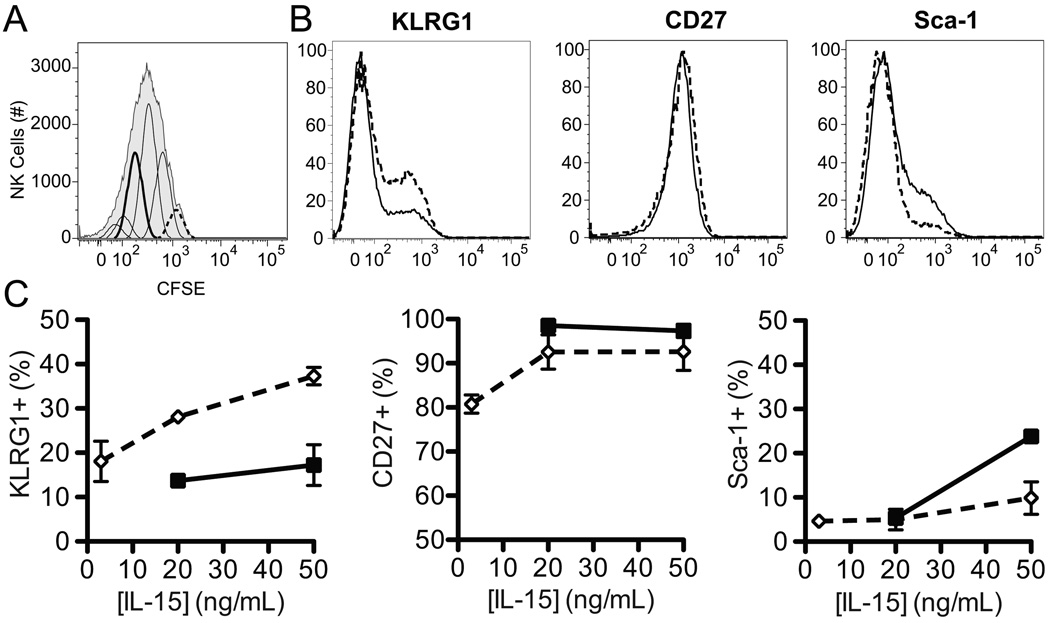
During the specific activation phase of MCMV infection, Ly49H+ NK cells proliferate substantially more than Ly49H− NK cells (12, 14, 16, 51), raising the possibility that the differential regulation of KLRG1, CD27, and Sca-1 expression on Ly49H+ and Ly49H− NK cells could reflect a by-product of cellular proliferation rather than NK cell receptor-mediated activation. Moreover, previous studies have shown that KLRG1 and CD27 are upregulated following adoptive transfer of NK cells into lymphopenic hosts (RAG/common gamma chain-deficient mice) and subsequent homeostatic proliferation (52, 53). To determine whether proliferation alone was sufficient to change expression of these markers, we analyzed their expression on CFSE-labeled NK cells cultured in vitro with various concentrations of IL-15 for 72 hours (Fig. 5). Based on CFSE dilution, we identified subsets of NK cells that had divided (diluted CFSE) and those that had not (no CFSE dilution) (Fig. 5A). While no differences (Fig. 5B and C) were seen in CD27 expression on subsets of NK cells that had divided versus those that had not (with 50 ng/mL IL-15, 97.4 ± 1.5% and 92.6 ± 4.2%, respectively), there was a decreased frequency of KLRG1+ NK cells among those that had divided compared to those that had not (with 50 ng/mL IL-15, 17.2 ± 4.6% and 37.3 ± 2.0%, respectively). In contrast, there was a slightly greater frequency of Sca-1+ NK cells that had divided compared to those that had not (with 50 ng/mL IL-15, 23.8 ± 1.3% and 9.88 ± 3.7%, respectively, Fig. 5B and C). These minor perturbations of KLRG1 and Sca-1 expression contrast with the modulation of these markers during MCMV infection. Furthermore, the patterns of change are the opposite of what we saw for Ly49H+ (analogous to the proliferative subset) and Ly49H− NK cells in vivo during MCMV infection. Taken together, these results demonstrate that the differential expression of KLRG1, CD27, and Sca-1 during MCMV infection is not a mere consequence of preferential proliferation.
Figure 5. Proliferation alone does not result in the differential expression of KLRG1, CD27, or Sca-1.
A) Representative histograms showing CFSE dilution and peak identification of NK cells that had (solid, thick line) or had not (dashed line) divided. B) Representative histograms showing relative expression of KLRG1, CD27, and Sca-1 on splenic NK cells that had (solid line) or had not divided (dashed line). C) Proportion of NK cells that had (■, solid line) or had not divided (◇, dashed line) that expressed KLRG1 (left), CD27 (middle), or Sca-1 (right) following 72 h of culture in 3, 20 or 50 ng/mL of IL-15. Data is composite of 2–3 independent experiments with pooled triplicates.
Discussion
While it is well-established that distinct mechanisms mediate the nonselective and specific activation of NK cells, we have for the first time demonstrated the presence of cell surface markers that distinguish NK cells that have been nonselectively activated by cytokines from those that have been specifically stimulated through activation receptors. Whereas markers of nonselective activation are universally upregulated on all NK cells early during viral infection, markers of specifically activated NK cells become differentially expressed at later times during infection on NK cells that express or lack expression of an activating receptor able to recognize infected cells. We have shown that Sca-1 is highly upregulated early during viral infections, and at later times can be used to distinguish cells that have been specifically activated through an activation receptor. Additionally, KLRG1 and CD27 expression levels are differentially modulated on specifically activated cells. Together, the differential expression patterns of KLRG1, CD27, and Sca-1 can identify a population enriched for specifically activated NK cells.
We identified the upregulation of Sca-1 as a novel marker of nonselectively activated NK cells. Similar to CD69 and KLRG1, which have previously been shown to be upregulated following NK cell activation (28, 54), there is a dramatic increase in the frequency of Sca-1+ NK cells following MCMV infection, as well as VV and HSV infections (Fig. 1A and B). However, the increase in the abundance of Sca-1 per cell is substantially greater than that of either KLRG1 or CD69 (Fig. 1F). Our observations at the protein level are corroborated by similar findings at the transcript level in recent data from the Immunological Genome Project (55), which reveals that Sca-1 is the most highly upregulated transcript on NK cells (greater than 65 fold) at 1 day p.i. with MCMV. In contrast, CD69 transcript levels increase by only 6 fold.
Both Sca-1 and CD69 identify functionally active NK cells, as demonstrated by the increased proportion of Sca-1+ and CD69+ NK cells that make IFN-γ compared to NK cells that do not express these markers (Fig. 2A and B). Although a greater proportion of Sca-1+ NK cells made IFN-γ compared to Sca-1− NK cells in wt mice, NK cells from Sca-1-deficient mice produced more IFN-γ compared to those from wt B6 mice during MCMV infection (Fig. 2C). This observation suggests that Sca-1 may have an inhibitory function on activated cells. These findings are in concordance with the increased T cell responses observed in Sca-1 deficient mice (38). In addition, T cells from mice that overexpress Sca-1 have blunted responses to a variety of stimuli (56). Interestingly, similar observations have been made regarding CD69. While CD69 is highly expressed on activated NK cells, CD69-deficient mice manifest greater NK-cell dependent rejection of MHC class I-deficient tumor cells than wt mice (57). These results support the conclusion that Sca-1 is an inhibitory receptor that marks nonselectively activated NK cells.
Although there are other reports of an inhibitory function for Sca-1 (38, 56), the mechanism of this inhibition remains unclear. Sca-1 is a GPI anchored protein of the Ly-6 gene family (58). Sca-1 may bind to CD22 on B cells (59), but it is unclear whether this is the only ligand for Sca-1. Various mechanisms for Sca-1 activity have been proposed (reviewed in (60)). Sca-1 may act through association with an accessory signaling protein, such as the CD3 ζ chain (61, 62), allowing Sca-1 ligation to transduce a signal and potentially inhibit other cellular processes by competing for downstream signaling molecules. Alternatively, the function of Sca-1 might be dependent on its association with “lipid rafts” (63), specialized regions of the cell membrane that are enriched with saturated sphingolipids, cholesterol and a variety of proteins, including GPI-anchored proteins (reviewed in (64)). “Lipid rafts” and their associated proteins may regulate other protein-protein and protein-lipid interactions. For example, it has been shown that ligation of Sca-1 with plate bound antibody inhibits TCR-induced proliferation of T cells by preventing the translocation of the IL-2Rα chain from “lipid rafts” into the soluble membrane fraction where IL-2Rβ and γ chains are located (65). Future work will be required to determine the mechanism of action of Sca-1 on NK cell function.
During the specific phase of NK cell activation during MCMV infection, Ly49H signaling following recognition of m157 results in selective activation of Ly49H+ NK cells. We have shown that Ly49H signaling through DAP12 results in the differential expression of KLRG1, CD27, and Sca-1 on Ly49H+ NK cells compared with Ly49H− NK cells. Ly49H+ NK cells persistently express KLRG1 but downregulate CD27 and Sca-1 expression more rapidly than Ly49H− NK cells (Fig. 3). By 8 days p.i. MCMV, KLRG1+CD27−Sca-1− NK cells are predominately Ly49H+, while KLRG1−CD27+Sca-1+ NK cells are primarily Ly49H− (Fig. 3E). We demonstrated that the differential expression of these markers is dependent on Ly49H recognition of m157 and the subsequent signaling via DAP12 (Fig. 4). Interestingly, Chen and colleagues have observed a similar expression pattern for Sca-1 on B cells: non-specific activation of B cells with IFN-γ results in Sca-1 upregulation, while stimulation through the B cell receptor results in decreased expression of Sca-1 (66). Additionally, there is increased expression of KLRG1 and decreased expression of CD27 on virus-specific T cells from both humans and mice (67, 68). Thus, the context-dependent regulation of activation markers is not unique to NK cells, but rather, it seems to be a more general phenomenon also observed in other immune cells.
Furthermore, we have demonstrated that the differential expression of KLRG1, CD27, and Sca-1 on Ly49H+ and Ly49H− NK cells is not a direct consequence of preferential proliferation of the Ly49H+ NK cells (Fig. 5). In contrast with our in vitro experiments, two previous reports showed increased KLRG1 and CD27 expression on NK cells that had undergone homeostatic proliferation in vivo following adoptive transfer into lymphopenic hosts (52, 53). This discrepancy may reflect the influence of increased levels of other cytokines in the RAG/common gamma chain-deficient recipients. Although our in vitro system does not fully mirror in vivo homeostatic proliferation, it does provide a simplified system to be able to look directly at the effects of proliferation.
In summary, the identification of markers of nonselective and specific NK cell activation will significantly facilitate in vivo studies of NK cell activation during viral infections and has potential applications in other areas of NK cell research. For example, Sun and colleagues have suggested that increased expression of KLRG1 and decreased expression of CD27 are characteristic of memory NK cells (69). Although additional studies will be needed to determine how Sca-1 is related to memory NK cells, it appears that KLRG1 and CD27 expression correlate with both specific NK cell activation and memory response potential. The distinct expression patterns of KLRG1, CD27, and Sca-1 may also prove useful in isolating specifically activated NK cells during other viral infections and in identifying previously undiscovered activation receptors involved in responses to infections or other stimuli.
Supplementary Material
Acknowledgments
This research was supported by NIAID R01 AI078994 and AI073552 grants and Washington University
Institutional Training Grant T32-AI007172.
Abbreviations
- MCMV
murine cytomegalovirus
- KLRG1
killer cell lectin-like receptor G1
- Sca-1
stem cell antigen 1
- p.i.
post infection
- MCMV-Δm157
m157-deficient MCMV
- B6
C57BL/6
Footnotes
The authors have no relevant financial disclosures
References
- 1.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 2.Bukowski JF, Woda BA, Habu S, Okumura K, Welsh RM. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J. Immunol. 1983;131:1531–1538. [PubMed] [Google Scholar]
- 3.Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, Dubbelde CE, Stone LR, Scalzo AA, Yokoyama WM. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- 4.Lee SH, Girard S, Macina D, Busà M, Zafer A, Belouchi A, Gros P, Vidal SM. Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat. Genet. 2001;28:42–45. doi: 10.1038/ng0501-42. [DOI] [PubMed] [Google Scholar]
- 5.Daniels KA, Devora G, Lai WC, O'Donnell CL, Bennett M, Welsh RM. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J. Exp. Med. 2001;194:29–44. doi: 10.1084/jem.194.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SH, Zafer A, de Repentigny Y, Kothary R, Tremblay ML, Gros P, Duplay P, Webb JR, Vidal SM. Transgenic expression of the activating natural killer receptor Ly49H confers resistance to cytomegalovirus in genetically susceptible mice. J. Exp. Med. 2003;197:515–526. doi: 10.1084/jem.20021713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng TP, French AR, Plougastel BFM, Pingel JT, Orihuela MM, Buller ML, Yokoyama WM. Ly49h is necessary for genetic resistance to murine cytomegalovirus. Immunogenetics. 2008;60:565–573. doi: 10.1007/s00251-008-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 9.Smith HRC, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, Scalzo AA, Fremont DH, Yokoyama WM. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bubic I, Wagner M, Krmpotic A, Saulig T, Kim S, Yokoyama WM, Jonjic S, Koszinowski UH. Gain of virulence caused by loss of a gene in murine cytomegalovirus. J. Virol. 2004;78:7536–7544. doi: 10.1128/JVI.78.14.7536-7544.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.French AR, Pingel JT, Wagner M, Bubic I, Yang L, Kim S, Koszinowski U, Jonjic S, Yokoyama WM. Escape of mutant double-stranded DNA virus from innate immune control. Immunity. 2004;20:747–756. doi: 10.1016/j.immuni.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 12.French AR, Sjölin H, Kim S, Koka R, Yang L, Young DA, Cerboni C, Tomasello E, Ma A, Vivier E, Kärre K, Yokoyama WM. DAP12 signaling directly augments proproliferative cytokine stimulation of NK cells during viral infections. J. Immunol. 2006;177:4981–4990. doi: 10.4049/jimmunol.177.8.4981. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei Xq, Liew FY, Caligiuri MA, Durbin JE, Biron CA. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 14.Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat. Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu. Rev. Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 16.Geurs TL, Zhao YM, Hill EB, French AR. Ly49H Engagement Compensates for the Absence of Type I Interferon Signaling in Stimulating NK Cell Proliferation During Murine Cytomegalovirus Infection. J. Immunol. 2009;183:5830–5836. doi: 10.4049/jimmunol.0901520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, Achdout H, Hanna J, Qimron U, Landau G, Greenbaum E, Zakay-Rones Z, Porgador A, Mandelboim O. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 18.Pyzik M, Charbonneau B, Gendron-Pontbriand EM, Babić M, Krmpotic A, Jonjic S, Vidal SM. Distinct MHC class I-dependent NK cell-activating receptors control cytomegalovirus infection in different mouse strains. J. Exp. Med. 2011;208:1105–1117. doi: 10.1084/jem.20101831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang M, Lanier LL, Sigal LJ. A role for NKG2D in NK cell-mediated resistance to poxvirus disease. PLoS Pathog. 2008;4:e30. doi: 10.1371/journal.ppat.0040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang M, Orr MT, Spee P, Egebjerg T, Lanier LL, Sigal LJ. CD94 is essential for NK cell-mediated resistance to a lethal viral disease. Immunity. 2011;34:579–589. doi: 10.1016/j.immuni.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuijpers TW, Baars PA, Dantin C, van den Burg M, van Lier RAW, Roosnek E. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008;112:914–915. doi: 10.1182/blood-2008-05-157354. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang S-M, Norris PJ, Nixon DF, Lanier LL. Expansion of a unique CD57□NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Björkström NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, Michaëlsson J, Malmberg KJ, Klingström J, Ahlm C, Ljunggren HG. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J. Exp. Med. 2011;208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaser C, Kaufmann M, Pircher H. Virus-activated CD8 T cells and lymphokine-activated NK cells express the mast cell function-associated antigen, an inhibitory C-type lectin. J. Immunol. 1998;161:6451–6454. [PubMed] [Google Scholar]
- 25.López-Cabrera M, Santis AG, Fernández-Ruiz E, Blacher R, Esch F, Sánchez-Mateos P, Sánchez-Madrid F. Molecular cloning, expression, and chromosomal localization of the human earliest lymphocyte activation antigen AIM/CD69, a new member of the C-type animal lectin superfamily of signal-transmitting receptors. J. Exp. Med. 1993;178:537–547. doi: 10.1084/jem.178.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlhofer FM, Yokoyama WM. Stimulation of murine natural killer (NK) cells by a monoclonal antibody specific for the NK1.1 antigen. IL-2-activated NK cells possess additional specific stimulation pathways. J. Immunol. 1991;146:3662–3673. [PubMed] [Google Scholar]
- 27.Hanke T, Corral L, Vance RE, Raulet DH. 2F1 antigen, the mouse homolog of the rat “mast cell function-associated antigen”, is a lectin-like type II transmembrane receptor expressed by natural killer cells. Eur. J. Immunol. 1998;28:4409–4417. doi: 10.1002/(SICI)1521-4141(199812)28:12<4409::AID-IMMU4409>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Robbins SH, Nguyen KB, Takahashi N, Mikayama T, Biron CA, Brossay L. Cutting edge: inhibitory functions of the killer cell lectin-like receptor G1 molecule during the activation of mouse NK cells. J. Immunol. 2002;168:2585–2589. doi: 10.4049/jimmunol.168.6.2585. [DOI] [PubMed] [Google Scholar]
- 29.Gerosa F, Scardoni M, Tommasi M, Benati C, Snelli L, Gandini G, Libonati M, Tridente G, Carra G. Interferon alpha induces expression of the CD69 activation antigen in human resting NK cells, while interferon gamma and tumor necrosis factor alpha are ineffective. Int. J. Cancer. 1991;48:473–475. doi: 10.1002/ijc.2910480328. [DOI] [PubMed] [Google Scholar]
- 30.Lanier LL, Buck DW, Rhodes L, Ding A, Evans E, Barney C, Phillips JH. Interleukin 2 activation of natural killer cells rapidly induces the expression and phosphorylation of the Leu-23 activation antigen. J. Exp. Med. 1988;167:1572–1585. doi: 10.1084/jem.167.5.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robbins SH, Tessmer MS, Mikayama T, Brossay L. Expansion and contraction of the NK cell compartment in response to murine cytomegalovirus infection. J. Immunol. 2004;173:259–266. doi: 10.4049/jimmunol.173.1.259. [DOI] [PubMed] [Google Scholar]
- 32.Bradfute SB, Graubert TA, Goodell MA. Roles of Sca-1 in hematopoietic stem/progenitor cell function. Exp. Hematol. 2005;33:836–843. doi: 10.1016/j.exphem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Yutoku M, Grossberg AL, Pressman D. A cell surface antigenic determinant present on mouse plasmacytes and only about half of mouse thymocytes. J. Immunol. 1974;112:1774–1781. [PubMed] [Google Scholar]
- 34.Malek TR, Ortega G, Chan C, Kroczek RA, Shevach EM. Role of Ly-6 in lymphocyte activation. II. Induction of T cell activation by monoclonal anti-Ly-6 antibodies. J. Exp. Med. 1986;164:709–722. doi: 10.1084/jem.164.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeh ET, Reiser H, Daley J, Rock KL. Stimulation of T cells via the TAP molecule, a member in a family of activating proteins encoded in the Ly-6 locus. J. Immunol. 1987;138:91–97. [PubMed] [Google Scholar]
- 36.Codias EK, Malek TR. Regulation of B lymphocyte responses to IL-4 and IFN-gamma by activation through Ly-6A/E molecules. J. Immunol. 1990;144:2197–2204. [PubMed] [Google Scholar]
- 37.Snapper CM, Yamada H, Mond JJ, June CH. Cross-linkage of Ly-6A/E induces Ca2+ translocation in the absence of phosphatidylinositol turnover and mediates proliferation of normal murine B lymphocytes. J. Immunol. 1991;147:1171–1179. [PubMed] [Google Scholar]
- 38.Stanford WL, Haque S, Alexander R, Liu X, Latour AM, Snodgrass HR, Koller BH, Flood PM. Altered proliferative response by T lymphocytes of Ly-6A (Sca-1) null mice. J. Exp. Med. 1997;186:705–717. doi: 10.1084/jem.186.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang ZX, Stanford WL, Zhang L. Ly-6A is critical for the function of double negative regulatory T cells. Eur. J. Immunol. 2002;32:1584–1592. doi: 10.1002/1521-4141(200206)32:6<1584::AID-IMMU1584>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Hwang SY, Hertzog PJ, Holland KA, Sumarsono SH, Tymms MJ, Hamilton JA, Whitty G, Bertoncello I, Kola I. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc. Natl. Acad. Sci. U. S. A. 1995;92:11284–11288. doi: 10.1073/pnas.92.24.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanson P, Mathews V, Marrus SH, Graubert TA. Enhanced green fluorescent protein targeted to the Sca-1 (Ly-6A) locus in transgenic mice results in efficient marking of hematopoietic stem cells in vivo. Exp. Hematol. 2003;31:159–167. doi: 10.1016/s0301-472x(02)01021-4. [DOI] [PubMed] [Google Scholar]
- 42.Tomasello E, Desmoulins PO, Chemin K, Guia S, Cremer H, Ortaldo J, Love P, Kaiserlian D, Vivier E. Combined natural killer cell and dendritic cell functional deficiency in KARAP/DAP12 loss-of-function mutant mice. Immunity. 2000;13:355–364. doi: 10.1016/s1074-7613(00)00035-2. [DOI] [PubMed] [Google Scholar]
- 43.Geurs TL, Hill EB, Lippold DM, French AR. Sex differences in murine susceptibility to systemic viral infections. J. Autoimmun. 2012;38:J245–J253. doi: 10.1016/j.jaut.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang-Feldman YJ, Wojtowicz A, Lochhead GR, Hale MA, Li Y, Pomeroy C. Use of quantitative real-time PCR (qRT-PCR) to measure cytokine transcription and viral load in murine cytomegalovirus infection. J. Virol. Methods. 2006;131:122–129. doi: 10.1016/j.jviromet.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 45.Zhao YM, French AR. Two-Compartment Model of NK Cell Proliferation: Insights from Population Response to IL-15 Stimulation. J. Immunol. 2012;188:2981–2990. doi: 10.4049/jimmunol.1102989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan KD, Shuai K, Lindwall G, Maher SE, Darnell JE, Bothwell AL. Induction of the Ly-6A/E gene by interferon alpha/beta and gamma requires a DNA element to which a tyrosine-phosphorylated 91-kDa protein binds. Proc. Natl. Acad. Sci. U. S. A. 1993;90:6806–6810. doi: 10.1073/pnas.90.14.6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dumont FJ, Dijkmans R, Palfree RG, Boltz RD, Coker L. Selective up-regulation by interferon-gamma of surface molecules of the Ly-6 complex in resting T cells: the Ly-6A/E and TAP antigens are preferentially enhanced. Eur. J. Immunol. 1987;17:1183–1191. doi: 10.1002/eji.1830170816. [DOI] [PubMed] [Google Scholar]
- 48.Demoulin JB, Maisin D, Renauld JC. Ly-6A/E induction by interleukin-6 and interleukin-9 in T cells. Eur. Cytokine Netw. 1999;10:49–56. [PubMed] [Google Scholar]
- 49.Khan KD, Lindwall G, Maher SE, Bothwell AL. Characterization of promoter elements of an interferon-inducible Ly-6E/A differentiation antigen, which is expressed on activated T cells and hematopoietic stem cells. Mol. Cell. Biol. 1990;10:5150–5159. doi: 10.1128/mcb.10.10.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol. Rev. 2006;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 51.Sun JC, Ma A, Lanier LL. Cutting edge: IL-15-independent NK cell response to mouse cytomegalovirus infection. J. Immunol. 2009;183:2911–2914. doi: 10.4049/jimmunol.0901872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun JC, Beilke JN, Bezman NA, Lanier LL. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J. Exp. Med. 2011;208:357–368. doi: 10.1084/jem.20100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huntington ND, Tabarias H, Fairfax K, Brady J, Hayakawa Y, Degli-Esposti MA, Smyth MJ, Tarlinton DM, Nutt SL. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J. Immunol. 2007;178:4764–4770. doi: 10.4049/jimmunol.178.8.4764. [DOI] [PubMed] [Google Scholar]
- 54.Krug A, French AR, Barchet W, Fischer JAA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Heng SP, Painter MW, Consortium IGP. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 56.Henderson SC, Kamdar MM, Bamezai A. Ly-6A.2 expression regulates antigen-specific CD4+ T cell proliferation and cytokine production. J. Immunol. 2002;168:118–126. doi: 10.4049/jimmunol.168.1.118. [DOI] [PubMed] [Google Scholar]
- 57.Esplugues E, Sancho D, Vega-Ramos J, Martínez C, Syrbe U, Hamann A, Engel P, Sánchez-Madrid F, Lauzurica P. Enhanced antitumor immunity in mice deficient in CD69. J. Exp. Med. 2003;197:1093–1106. doi: 10.1084/jem.20021337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LeClair KP, Palfree RG, Flood PM, Hammerling U, Bothwell A. Isolation of a murine Ly-6 cDNA reveals a new multigene family. EMBO J. 1986;5:3227–3234. doi: 10.1002/j.1460-2075.1986.tb04633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pflugh DL, Maher SE, Bothwell ALM. Ly-6 superfamily members Ly-6A/E, Ly-6C, and Ly-6I recognize two potential ligands expressed by B lymphocytes. J. Immunol. 2002;169:5130–5136. doi: 10.4049/jimmunol.169.9.5130. [DOI] [PubMed] [Google Scholar]
- 60.Bamezai A. Mouse Ly-6 proteins and their extended family: markers of cell differentiation and regulators of cell signaling. Arch. Immunol. Ther. Exp. (Warsz) 2004;52:255–266. [PubMed] [Google Scholar]
- 61.Wegener AM, Letourneur F, Hoeveler A, Brocker T, Luton F, Malissen B. The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell. 1992;68:83–95. doi: 10.1016/0092-8674(92)90208-t. [DOI] [PubMed] [Google Scholar]
- 62.Hermans MH, Malissen B. The cytoplasmic tail of the T cell receptor zeta chain is dispensable for antigen-mediated T cell activation. Eur. J. Immunol. 1993;23:2257–2262. doi: 10.1002/eji.1830230931. [DOI] [PubMed] [Google Scholar]
- 63.Bohuslav J, Cinek T, Horejsí V. Large, detergent-resistant complexes containing murine antigens Thy-1 and Ly-6 and protein tyrosine kinase p56lck. Eur. J. Immunol. 1993;23:825–831. doi: 10.1002/eji.1830230409. [DOI] [PubMed] [Google Scholar]
- 64.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat. Rev. Mol. Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 65.Marmor MD, Julius M. Role for lipid rafts in regulating interleukin-2 receptor signaling. Blood. 2001;98:1489–1497. doi: 10.1182/blood.v98.5.1489. [DOI] [PubMed] [Google Scholar]
- 66.Chen H-C, Frissora F, Durbin JE, Muthusamy N. Activation induced differential regulation of stem cell antigen-1 (Ly-6A/E) expression in murine B cells. Cell. Immunol. 2003;225:42–52. doi: 10.1016/j.cellimm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 67.Thimme R, Appay V, Koschella M, Panther E, Roth E, Hislop AD, Rickinson AB, Rowland-Jones SL, Blum HE, Pircher H. Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation. J. Virol. 2005;79:12112–12116. doi: 10.1128/JVI.79.18.12112-12116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur. J. Immunol. 2005;35:1113–1123. doi: 10.1002/eji.200425534. [DOI] [PubMed] [Google Scholar]
- 69.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.