Abstract
Background
Helicobacter pylori (H. pylori) seropositivity is a potential risk for poor cognition among US adults.
Methods
Cross-sectional data from the National Health and Nutrition Examination Survey III, phase 1 (1988–91) were used. Measures included age-group specific neuropsychological test batteries and two measures of H. pylori seropositivity (IgG and IgG-CagA) (20–59yo:n=2,090–2,248; 60–90yo:n=2,123–2,388). We explored sex- and race-specific associations.
Results
On the basis of multiple OLS and zero-inflated Poisson regression models, we detected a poorer performance among those 60–90yo with H. pylori IgG+ vs. IgG− on a verbal memory test (story recall, correct items), overall (β=−0.04±0.01, p=0.010). NH blacks and women (20–59yo) performed worse on the serial digits learning errors (SDL-TE) when H. pylori IgG+(vs. IgG−), another verbal memory test (β=+0.94±0.40, p=0.029 and β=+1.19±0.44, p=0.012, respectively); (p<0.10 for interaction by sex and race). More trials to completion on this test (SDL-TTC) were also required among H. pylori IgG+ overall (20–59yo, β=+0.30±0.13, p=0.033). Other race-specific associations without significant interaction by race that were detected in the same direction of worse performance with seropositivity included H. pylori IgG+ in NH blacks with the symbol digits substitution test-number of errors (SDS-E) (20–59yo, p=0.038) and with the incorrect items on the orientation test (ORIENT-INC) (60–90yo, p=0.038). H. pylori IgG CagA+ was associated with poorer Word Recall test performance(WR-CORR) among Mexican Americans(60–90yo, p=0.048), WR-TRIALS among NH whites(60–90yo, p=0.047) and ORIENT-INC among NH blacks(60–90yo, p=0.025).
Conclusions
H. pylori seropositivity markers were associated with poor cognition among US adults. Longitudinal research is needed to extrapolote those findings to cognitive decline, incident dementia and Alzheimer’s Disease.
Keywords: Helicobacter pylori, seropositivity, cognitive function, adults, aging
INTRODUCTION
Dementia affects 6% to 10% of adults above 65y, most of whom are diagnosed with Alzheimer’s Disease (AD)(1). AD is a progressive neurodegenerative disorder with multi-factorial etiology(2) that is the main cause of disability and dependency in old age(3), and the leading socio-economic burden for health care in developed countries.(4) Despite lack of curative treatment, epidemiological evidence reveals important risk factors for sporadic AD, many of which are non-modifiable (e.g. ApoE ε4, age and sex). A recent review has attributed 2,866,951 of prevalent AD among US older adults to low education, smoking, physical inactivity, depression, mid-life obesity, mid-life hypertension and type 2 diabetes, accounting for 54% of total AD cases.(5) Thus, half of AD occurrence remains unexplained by commonly studied modifiable risk factors. Therefore, it is urgent to identify those factors and cost-effective interventions to address them, preferably at earlier ages before the onset of AD symptoms. Recent evidence points to possible involvement of infectious agents in the etiology of AD, though many failed to show an association, possibly due to methodological differences in assessing infection.(4) More recently, research has implicated bacterial agents in incidence and survival from AD, mainly Helicobacter pylori (H. pylori). (6–13)
H. pylori is a curved spiral gram-negative bacterium found in the gastric mucosa of a large proportion of humans worldwide (>50%).(14) Infection is often acquired during childhood and becomes chronic during adulthood if untreated. Its prevalence increases with age (cohort rather than age effect) mainly explained by changes in socioeconomic conditions. H. pylori, a heterogeneous bacterial species, includes a highly pathogenic strain named CagA which promotes a strong inflammatory response.(15) H. pylori infection is commonly known to cause a number of upper digestive disease, particularly peptic ulcer, a degenerative disease (as for AD). In fact, peptic ulcer is an outcome of infection, stress, chemical irritants and genetic susceptibility.(16) In addition, H. pylori infection was linked to extra-digestive disorders, including atherosclerosis,(17) hypertension and stroke(18), all of which were associated with AD, an effect mediated by impairment of the blood-brain barrier.(19–21)
Recently, a number of biological mechanisms have been suggested to provide evidence for a causal link between H. pylori infection and AD. Those included the following: (1) Reduced absorption of folate and vitamin B-12 and increased level of homocysteine, a neurotoxic substance (22–23) (2) Apoptosis caused by T cell-mediated immune response, overexpression of Nitric oxide (NO) or molecular mimicry of host structures. (24–25) (3) Other inflammatory responses, including cytokines, platelet activation, acute phase proteins and eicosanoids (25) and (4) The infection possibly crossing the blood-brain barrier and contributing to amyloid deposition. (26)
However, the limited number of epidemiological studies examining this important research question (6–13) failed to assess H. pylori’s relationship with cognitive performance during normal aging and therefore to provide evidence for a putative intervention at an earlier stage of this neurodegenerative disease. Moreover, even though many of those studies found a positive association between H. pylori and AD, those that found no association had a number of methodological limitations that are outlined in the discussion section. (12–13)
In addition, recent analyses examining secular trends indicate that there are major racial and ethnic differences in H. pylori seroprevalence that are sustained over time with the largest burden observed in Non-Hispanic(NH) blacks and Mexican Americans, and that there was a significant drop in prevalence over time observed only among NH Whites particularly at older ages.(27) Other studies have replicated these racial and ethnic disparities and have additionally shown differences in seroprevalence by sex, whereby women were at higher risk for H. pylori seropositivity.(28)
Given the paucity of studies linking H. pylori infection to various cognitive outcomes and the fact that most studies had focused on AD-type of dementia as the primary outcome, it is particularly important to evaluate H. pylori seropositivity’s association with cognitive performance during normal aging, taking into account potential differences by sex and by race/ethnicity. The present study used cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III to examine associations between serum measures of H. pylori infection (or seropositivity) and cognitive performance in various domains among US younger and older adults (Objective A), and to explore if those associations were sex and/or race-specific (Objective B). Secondary objectives were to describe the prevalence of H. pylori seropositivity and cognitive function across sex and race (Objective C) and determine independent socio-demographic, lifestyle and health-related predictors of H. pylori seropositivity (Objective D).
METHODS
Database
NHANES III phase 1 (1988–1991), a multistage, stratified sampling design, over-sampled older adults (60–90yo) and minorities (African Americans and Mexican Americans). Following a home-interview, selected individuals were invited to a mobile examination center (MEC) for body measurements, clinical evaluations and laboratory testing.(29) The present study was approved for ethical treatment of participants by the Institutional Review board of the National Institute on Aging, Intramural Research Program.
Study Sample
Among adults (aged 20–90yo) interviewed in phase 1 with semi-complete socio-demographics, 5,927 were 20–59yo and 3,468 were 60–90yo. Two separate batteries of cognitive function tests were administered to these two age groups. Cognitive testing was available on a smaller proportion (n=2,267 to 2,438 of 5,927) of 20–59yo compared to 60–90yo (n=2,569 to 3,382 out of 3,468). Among participants with complete cognitive data, measures of H. pylori exposure were available only for n=2,090 to 2,248 for 20–59yo and n=2,123 to 2,388 for 60–90yo, depending on the cognitive measure. Missing data on other covariates ranged between <1% and ~6% of the final sample with complete cognitive, socio-demographic and H. pylori data (See Figure, Supplemental Digital Content).
Cognitive assessment
Three cognitive tests were administered to participants aged 20–59yo, simple reaction time (SRT), symbol-digit substitution test (Errors: SDS-E and Latencies: SDS-L) and serial digits learning (Trials to completion: SDL-TTC and total errors: SDL-TE) from which five cognitive test scores were computed. Among those aged 60–90yo, three cognitive tests were administered, word recall (Correct: WR-CORR, number of trials: WR-TRIALS), story recall, correct items (SR-CORR), a math test (also termed serial 3’s; incorrect items: MATH-INC) and orientation to time from the Mini-Mental State Examination, incorrect items (ORIENT-INC) (See Supplemental Digital Content 2).
H. pylori antibody measurement
H. pylori antibodies were assayed in 1996 on participants aged ≥20y using H. pylori IgG ELISA (Wampole Laboratories, Cranbury, NJ). An immune status ratio was computed by dividing the optical density for each specimen by the mean optical density of the cutoff controls. Cutoffs chosen were similar to other analyses whereby, H. pylori− individuals had an immune status ratio ranging from 0–0.90; “equivocal” seropositivity ranged from 0.91–1.09 and H. pylori+ had a value greater than 1.10.(27) Of 7,465 participants aged ≥20 years with available H. pylori seropositivity status in NHANES III, 207 (2.8%) screened “equivocal”.(27) More details on this assay and its performance characteristics among US adults are available for the more recent NHANES data (1999–2000).(30) H. pylori anti-CagA IgG (CagA strains increase risk of stomach adenocarcinoma, hypertension, atherosclerosis and stroke), as described elsewhere.(17–21, 31)
Covariates
In multiple age group-specific (20–59yo; 60–90yo) regression models, covariates considered as potential confounders of the hypothesized associations included age (continuous); sex; race as Non-Hispanic White (NHW), Non-Hispanic black (NHB), Mexican-American (MA), other ethnicity (OTHER); marital status as currently unmarried or currently married; educational level as <High school, High school, >High school; poverty income ratio as ≤100%, >100–≤200%, >200%; interview language as English or Spanish; smoking status as never, former or current.; self-reported physical activity compared to age peers as more active, about the same, or less active; and, weight status body mass index defined by weight(kg) divided by height squared (m2)).
USDA’s 1995-Health Eating Index (HEI)(32) (range:0–100) and entered as quintiles, assessed overall dietary quality. Participants self-rated their health as follows: Would you say your health in general is “Excellent”, “Very good”, “Good”, “Fair” or “Poor”? We entered self-rated health into models as four dummy variables with “Excellent” as the common referent. Finally, we created a comorbidity index as a count (0–20) of self-reported conditions namely “arthritis,” “congestive heart failure,” “stroke,” “asthma,” “chronic bronchitis,” “emphysema,” “hay fever,” “cataracts,” “goiter,” “thyroid disease,” “lupus,” “gout,” “skin cancer,” “other cancer,” “diabetes,” “hypertension,” “chest pain/possible angina,” “heart attack/myocardial infarction,” “osteoporosis” and “kidney stones“, as described in a previous study.(33)
Statistical analysis
We used Stata release 11.0(34) for analyzing survey data. Two design variables were specified for the phase 1 of NHANES III (1988–1991), the primary sampling unit and the stratum. Sampling weights used were those for cognitive testing variables (phase 1) for the 20–59y subgroup and those for the MEC exam (phase 1) for the 60–90y group. Means, proportions and regression coefficients were estimated, accounting for design complexity and sampling weights. The main exposures were transformed into binary variables as follows: (1) H. pylori antibody IgG (positive; negative or equivocal), (2) H. pylori CagA (positive; negative).
First, study sample characteristics were described and compared between the two age groups under study (by assessing 95% CI overlap and using two-samples t-test comparing means and proportions across age groups). Second, proportions seropositive for H. pylori (IgG and IgG CagA) were graphically represented using bar diagrams, comparing age group-sex categories in one analysis and age group-race in another. To test statistical differences between categories in each analysis and for each exposure variable, design-based F-tests were conducted, taking into account sampling design complexity. We also explored cognitive performance test weighted means across sex and race groups using dot plots and tested differences across groups using one-way ANOVA and Bonferroni corrected t-test, taking sampling weights into account. We used a similar approach to compare prevalence proportions of H. pylori seropositivity by race and sex groups, using a design-based F-test (Objectives C). Third, sample characteristics were entered into a multiple logistic regression model as predictors for the two exposure variables (H. pylori IgG and CagA); (Objective D). Fourth, we used multiple OLS and zero-inflated Poisson regression models to test the primary hypotheses for each age sub-group with their respective cognitive function scores as outcomes and the two exposure variables entered separately in each model with potentially confounding socio-demographic, lifestyle and health-related factors. We used OLS models for continuous pseudo-normal cognitive scores (usually involving time elapsed to complete the test; 20–59yo) and zero-inflated Poisson regression models in the tests for 60–90yo, which were counts with a large proportion of zero scores (Objective A). Finally, we explored whether the hypothesized associations were sex and/or race-specific by conducting stratified analyses and tested sex and racial differences by adding interaction terms alternatively with sex and race to the main effects model (Objective B). A type I error of 0.05 was considered for statistical significance in all analyses, except for interaction terms where significance level was set at 0.10 (35).
RESULTS
Table 1 displays estimated means and proportions of main study characteristics (phase 1 of NHANES III). Generally, younger participants (20–59yo) were more likely than their older counterparts (60–90yo) to use Spanish as their interview language (4.2% vs. 1.7%, p<0.001), more likely to smoke currently (34.2% vs.16.1%, p<0.001), have excellent self-rated health (24.6% vs. 12.8%, p<0.001) and report more activity compared to age peers (22.7% vs. 16.8%, p<0.001). However, the older age group under study (60–90yo) had a healthier diet (1995-HEI: 68.0 vs. 61.6, p<0.001) but reported, on average, a larger number of co-morbid conditions (2.8 vs. 1.1, p<0.001). The prevalence of H. pylori IgG exposure was significantly higher in the 60–90yo group compared to the 20–59yo (52.6% vs. 26.5%, p<0.001). The proportion of CagA IgG was also higher in the older age group (25.8% vs. 16.7%, p<0.001).
Table 1.
Study sample characteristics, among participants with complete basic socio-demographics and cognitive dataa (20–59yo and 60–90yo), NHANES III phase 1 (1988–1991)
| 20–59yo | 60–90yo | |||||
|---|---|---|---|---|---|---|
| N | Mean or % | SE | N | Mean or % | SE | |
|
|
|
|||||
| Socio-demographic | ||||||
| Women, % | 2,438 | 51.0 | 1.5 | 57.6 | 1.2 | |
| Age, y | 2,438 | 36.9 | 0.3 | 3,382 | 70.3 | 0.2 |
| Race, % | 2,438 | 3,382 | ||||
| NH White | 78.0 | 2.4 | 86.3 | 1.8 | ||
| NH Black | 11.0 | 1.2 | 8.2 | 1.1 | ||
| Mexican-American | 5.2 | 0.7 | 2.1 | 0.4 | ||
| Other ethnicity | 5.8 | 1.3 | 3.3 | 0.9 | ||
| Married, % | 2,438 | 61.2 | 1.9 | 3,382 | 59.2 | 1.5 |
| Education, % | 2,438 | 3,336 | ||||
| <High School | 7.1 | 0.8 | 25.2 | 2.3 | ||
| High School | 49.1 | 1.9 | 47.1 | 1.4 | ||
| >High School | 43.8 | 2.2 | 27.7 | 2.1 | ||
| Poverty income ratio, % | 2,438 | 3,382 | ||||
| 0–100% | 11.0 | 1.0 | 10.6 | 1.1 | ||
| >100%–200% | 17.9 | 2.0 | 24.8 | 1.9 | ||
| >200% | 71.1 | 2.1 | 64.5 | 2.2 | ||
| Language of interview | 2,438 | 3,348 | ||||
| English | 95.8 | 0.9 | 98.3 | 0.3 | ||
| Spanish | 4.2 | 0.9 | 1.7 | 0.3 | ||
| Lifestyle and health-related | ||||||
| Cigarette smoking status | 2,438 | 3,382 | ||||
| Never | 43.3 | 1.7 | 43.8 | 1.3 | ||
| Former | 22.5 | 1.5 | 40.1 | 1.6 | ||
| Current | 34.2 | 1.7 | 16.1 | 0.9 | ||
| Self-rated health | 2,438 | 3,382 | ||||
| Excellent | 24.6 | 1.7 | 12.8 | 1.3 | ||
| Very good | 31.5 | 1.7 | 23.3 | 1.3 | ||
| Good | 31.8 | 1.6 | 34.7 | 1.6 | ||
| Fair | 10.8 | 1.3 | 21.1 | 1.6 | ||
| Poor | 1.7 | 0.4 | 7.8 | 0.6 | ||
| 1995-Healthy Eating Index, % | 2,399 | 3,382 | ||||
| Mean | 61.6 | 0.5 | 68.0 | 0.5 | ||
| Q1:poor quality diet | 6.8 | 0.7 | 3,382 | 4.3 | 0.4 | |
| Q5:better quality diet | 16.2 | 1.5 | 35.5 | 1.7 | ||
| Physical activity | 2,395 | 3,236 | ||||
| More active | 22.7 | 1.4 | 16.8 | 1.2 | ||
| About the same | 46.2 | 1.2 | 41.1 | 1.2 | ||
| Less active | 31.2 | 1.4 | 42.1 | 1.7 | ||
| Co-morbidity index, Mean | 2,412 | 1.10 | 0.04 | 3,369 | 2.82 | 0.07 |
| Body mass index, kg.m−2, Mean | 2,432 | 26.2 | 0.2 | 2,843 | 26.6 | 0.1 |
| H. pylori exposures | ||||||
| H. pylori IgG, % positive | 2,247 | 26.5 | 1.9 | 2,388 | 52.6 | 1.6 |
| H. pylori CagA IgG, % positive | 2,248 | 16.7 | 1.3 | 2,388 | 25.8 | 1.2 |
| Cognitive performance scores | ||||||
| SRT | 2,438 233.1 | 1.4 | __ | __ | __ | |
| SDS-L | 2,404 23.0 | 0.2 | __ | __ | __ | |
| SDS-E | 2,404 1.4 | 0.1 | __ | __ | __ | |
| SDL-TTC | 2,267 4.6 | 0.1 | __ | __ | __ | |
| SDL-TE | 2,335 4.5 | 0.2 | __ | __ | __ | |
| WR-CORR | __ | ____ | 3,109 | 5.50 | 0.03 | |
| WR-TRIALS | __ | ____ | 3,048 | 0.05 | 0.01 | |
| SR-CORR | __ | ____ | 2,569 | 4.09 | 0.04 | |
| MATH-INC | __ | ____ | 3,382 | 0.74 | 0.05 | |
| ORIENT-INC | __ | ____ | 2,922 | 1.85 | 0.01 | |
The largest available sample size per age group was used by selecting the most complete cognitive measure per age group.
Abbreviations: M=Median; NHANES=National Health and Nutrition Examination Surveys; R=Range; SE=Standard error; NH=Non-Hispanic; SRT=Simple reaction time ;SDS-L=Symbol Digits Substitution tests-Latencies; SDS-E=Symbol Digits Substitution tests-Errors; Serial Digits Learning-Trials to completion; SDL-TE=Serial Digits Learning-Total Errors; WR-CORR=Word Recall-Correct; WR-TRIALS=Word Recall-Number of Trials; ; SR-CORR=Story recall-correct; MATH-INC=Math test-Incorrect; ORIENT-INC=Orientation to time, incorrect items.
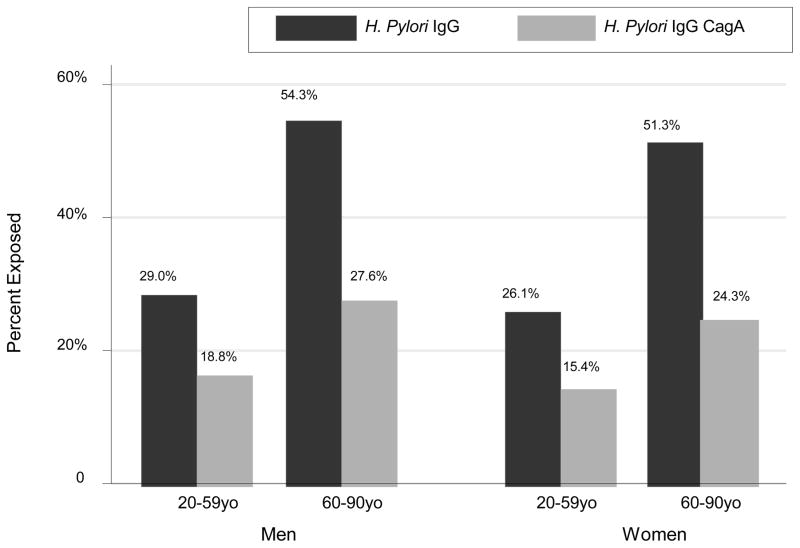
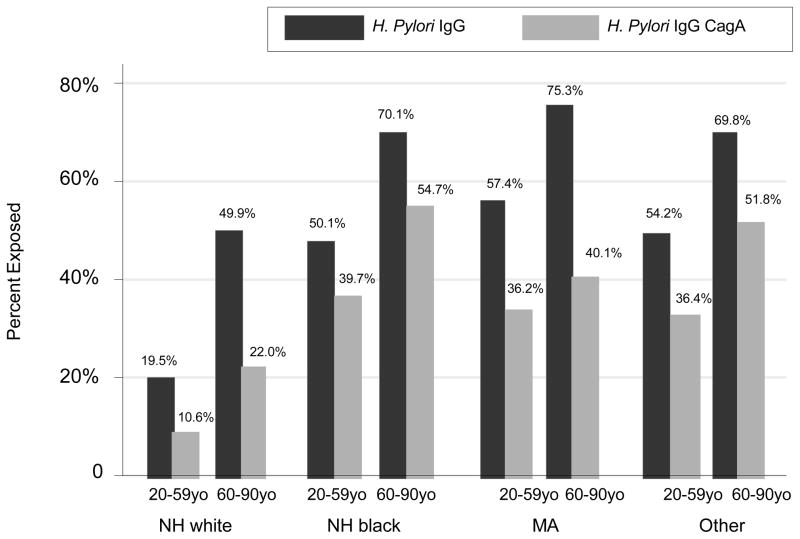
Using design-based F-test, the proportion of each H. pylori exposure was significantly different between each age group-sex category when weighted proportion of each H. pylori exposure variable were compared (Figure 1). In particular, within each sex, H. pylori seropositivity prevalence rate was higher in the older age group, on the basis of both IgG and IgG CagA exposures (p<0.001). Figure 2 further explores H. pylori exposures by age group and race. There were notable differences in prevalence particularly when comparing NH whites to other race groups within each age group and for both exposures, the differences among which were statistically significant overall according to design-based F-test comparing all age-race categories for each exposure(p<0.001).
Figure 1. Bar plot of H. pylori IgG and CagA exposures by age group and sex; NHANES III phase 1 (1988–1991).
Abbreviations: H. pylori=Helicobacter pylori; NHANES=National Health and Nutrition Examination Surveys.
Notes: Design based F-test indicated significant differences in proportions of each H. pylori exposure between age group-sex categories (p<0.001).
Figure 2. Bar plot of H. pylori IgG and CagA exposures by age group and race; NHANES III phase 1 (1988–1991).
Abbreviations: H. pylori=Helicobacter pylori; NHANES=National Health and Nutrition Examination Surveys.
Notes: Design based F-test indicated significant differences in proportions of each H. pylori exposure between age group-race categories (p<0.001).
On the basis of findings from multiple logistic regression analyses (Table 2), age was an independent predictor for H. pylori seropositivity for both exposures with increased odds of seropositivity by 2–4% per year increase in age (p<0.001). Women had a 33% lower odds than men to be seropositive for total H. pylori (p=0.009). NH whites had a significantly lower prevalence of seropositivity (total and CagA) compared to other groups, particularly when compared to NH blacks and Mexican-Americans(p<0.001 in most cases, with OR ranging from 3.08 to 5.03), though the disparity was non-significant (p>0.05) when contrasting the “Other ethnicity” group to NH whites. Education, particularly having >High school level, was inversely related to H. pylori seropositivity when taking <High school as the referent for both exposures and age groups (OR ranged between 0.38 and 0.46, p<0.01). Poverty income ratio was not independently related to lower H. pylori seropositivity rate. Current smoking status, self-rated health and the dietary quality (1995-Healthy Eating index) were not related to any of the two exposures. Although BMI was positively associated with H. pylori IgG CagA (OR=1.02, p=0.021), there was an inverse relationship between this H. pylori exposure and the co-morbidity index (OR=0.93, p=0.017). When we added two-way interaction terms of age×education and age×PIR in each model, we found that there was no significant interaction for both age groups combined and for H. pylori IgG or CagA exposures (p>0.05). In a model where H. pylori IgG CagA was a predictor for total H. pylori IgG exposure, controlling for all other covariates, the OR was estimated to be 9.53 with a 95% CI: 7.38–12.29, indicating a very strong association between the two exposures in the total population.
Table 2.
Multiple logistic regression analysis for predictors of the two H. pylori seropositivity exposures (H. pylori IgG, H. pylori CagA IgG) among participants with complete basic socio-demographics and cognitive dataa (both age groups combined), NHANES III phase 1 (1988–1991)
| H. pylori IgG | H. pylori CagA IgG | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR | 95%CI | p-value | OR | 95% CI | p-value | |
|
| ||||||
| (N=4,429) | (N=4,429) | |||||
| Age | 1.04 | (1.03;1.05) | <0.001 | 1.02 | (1.02;1.03) | <0.001 |
| Female | 0.77 | (0.64;0.93) | 0.009 | 0.85 | (0.68;1.06) | 0.144 |
| Race (Ref=NH White) | ||||||
| NH Black | 3.53 | (2.74;4.54) | <0.001 | 5.03 | (4.04;6.26) | <0.001 |
| Mexican-American | 4.39 | (3.11;6.21) | <0.001 | 3.08 | (1.99;4.77) | <0.001 |
| Other ethnicity | 4.41 | (2.56;7.59) | <0.001 | 4.95 | (2.76;8.87) | <0.001 |
| Married (Ref=unmarried) | 1.13 | (0.90;1.41) | 0.268 | 0.86 | (0.68;1.10) | 0.220 |
| Education (Ref=<High School) | ||||||
| High School | 0.64 | (0.47;0.87 | 0.007 | 0.75 | (0.54;1.04) | 0.088 |
| >High School | 0.38 | (0.26;0.55) | <0.001 | 0.46 | (0.31;0.66) | <0.001 |
| Poverty income ratio (Ref=0–100%) | ||||||
| >100%–200% | 0.94 | (0.64;1.40) | 0.764 | 1.23 | (0.92;1.64) | 0.149 |
| >200% | 0.76 | (0.55;1.04) | 0.088 | 1.08 | (0.78;1.50) | 0.630 |
| Language of interview (Ref=English) | ||||||
| Spanish | 1.00 | (0.56;1.78) | 0.997 | 1.47 | (0.70;3.08) | 0.289 |
| Cigarette smoking status (Ref=Never) | ||||||
| Former | 0.96 | (0.77;1.18) | 0.673 | 0.96 | (0.75;1.22) | 0.723 |
| Current | 1.06 | (0.80;1.40) | 0.683 | 1.06 | (0.79;1.40) | 0.701 |
| Missing | __ | __ | __ | __ | __ | |
| Self-rated health (Ref=Excellent) | ||||||
| Very good | 1.01 | (0.70;1.46) | 0.963 | 0.88 | (0.64;1.20) | 0.393 |
| Good | 1.00 | (0.73;1.35) | 0.974 | 0.93 | (0.72;1.20) | 0.574 |
| Fair | 1.15 | (0.79;1.67) | 0.445 | 0.82 | (0.60;1.10) | 0.170 |
| Poor | 1.03 | (0.52;2.03) | 0.922 | 0.79 | (0.42;1.51) | 0.465 |
| Missing | 0.25 | (0.03;1.81) | 0.163 | 0.29 | (0.04;1.98) | 0.197 |
| 1995-Healthy Eating Index (Ref=Q1) | ||||||
| Q2 | 0.99 | (0.63;1.55) | 0.959 | 1.18 | (0.78;1.79) | 0.407 |
| Q3 | 0.95 | (0.63;1.55) | 0.799 | 1.09 | (0.65;1.85) | 0.729 |
| Q4 | 0.91 | (0.57;1.44) | 0.664 | 0.90 | (0.58;1.40) | 0.634 |
| Q5 : better quality diet | 0.74 | (0.45;1.22) | 0.228 | 0.83 | (0.59;1.17) | 0.269 |
| Missing | 0.66 | (0.33;1.30) | 0.219 | 0.39 | (0.19;0.79) | 0.011 |
| Physical activity (Ref=More active) | ||||||
| About the same | 0.96 | (0.73;1.25) | 0.725 | 0.78 | (0.60;1.02) | 0.068 |
| Less active | 1.23 | (0.90;1.66) | 0.179 | 1.04 | (0.83;1.30) | 0.734 |
| Body mass index, kg.m−2 | 1.01 | (0.99;1.03) | 0.125 | 1.02 | (1.00;1.03) | 0.021 |
| Co-morbidity index | 0.99 | (0.92;1.06) | 0.759 | 0.93 | (0.87;0.98) | 0.017 |
Abbreviations: NH=Non-Hispanic; NHANES=National Health and Nutrition Examination Surveys; SE=Standard error.
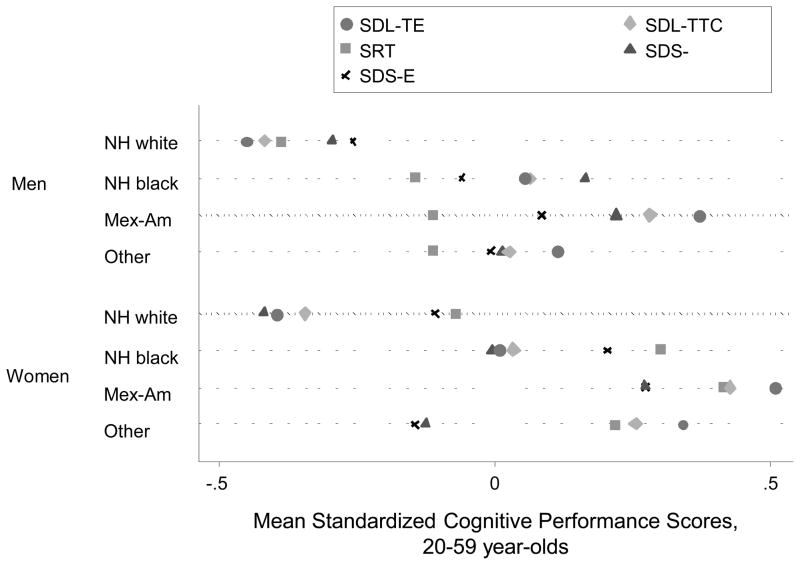
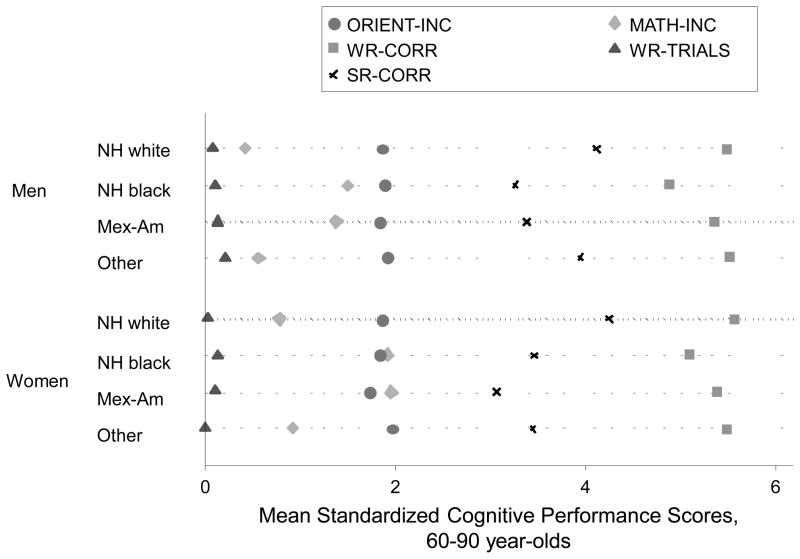
Figure 3 shows weighted means of cognitive performance scores (standardized z-scores) for the tests administered in the 20–59yo group by sex and race. One-way ANOVA indicated significant differences in standardized cognitive performance scores administered to younger age groups by sex-race categories (p<0.001). Bonferroni-corrected multiple comparisons showed that differences within each sex were consistently significant between NH whites and other race groups, indicating worse performance in other race groups among both men and women (p<0.001). A similar pattern of significant differences upon multiple comparisons was observed when comparing means of cognitive performance scores by sex and race for tests administered in the older age group (60–90yo) (Figure 4), particularly for MATH-INC and SR-CORR.
Figure 3. Dot plot for means of standardized cognitive performance scores in the younger age group (20–59yo) by sex and race; NHANES III phase 1 (1988–1991).
Abbreviations: ANOVA=Analysis of Variance; NH=Non-Hispanic; NHANES=National Health and Nutrition Examination Surveys; SDL-TTC=Serial Digits Learning-Trials to completion; SDL-TE=Serial Digits Learning-Total Errors; SDS-E=Symbol Digits Substitution tests-Errors; SDS-L=Symbol Digits Substitution tests-Latencies; SRT=Simple reaction time.
Notes: One-way ANOVA test indicated that there were significant differences in the standardized cognitive performance scores administered to the younger age groups by sex-race categories (p<0.001). Bonferroni-corrected multiple comparisons showed that differences within each sex were consistently significant between NH whites and the other race groups, indicating poorer performance in the other race groups among both men and women (p<0.001).
Figure 4. Dot plot for means of cognitive performance scores in the older age group (60–90yo) by sex and race; NHANES III phase 1 (1988–1991).
Abbreviations: ANOVA=Analysis of Variance; NHANES=National Health and Nutrition Examination Surveys; NH=Non-Hispanic; MATH-INC=Math test-Incorrect; ORIENT-INC=Orientation to time, incorrect items; SR-CORR=Story recall-correct; WR-CORR=Word Recall-Correct; WR-TRIALS=Word Recall-Number of Trials.
Notes: One-way ANOVA test indicated that there were significant differences in the cognitive performance scores administered to the older age groups by sex-race categories (p<0.001). Bonferroni-corrected multiple comparisons showed that differences within each sex were consistently significant between NH whites and the other race groups, indicating poorer performance in the other race groups among both men and women (p<0.001), particularly for MATH-INC and SR-CORR.
Table 3 displays associations between the two H. pylori exposures and age-group specific cognitive test performance, as determined by multiple regression analyses. Among the 20–59yo group who were given a fixed and distinctive set of cognitive tests (compared to the older group), seropositivity for H. pylori (H. pylori IgG+) was positively associated with time to completion on the serial digits learning test, indicating poorer cognition among the exposed (compared to the unexposed; β=+0.30±0.13, p=0.033). This association was detected only in women (β=+0.60±0.20, p<0.01) with no significant interaction between sex×H. pylori IgG (p>0.10). Similarly, the number of errors on this test (SDL-TE) was on average higher among the exposed group (H. pylori IgG+; β=+0.51±0.26, p=0.058), particularly among women (β=+1.19±0.44, p=0.012) and NH black(β=+0.94±0.40, p=0.029), with a significant interaction detected by race and sex (p<0.10). H. pylori IgG+ was linked to more errors on the symbol digits substitution test among NH blacks (β=+0.76±0.34, p=0.038), but to a shorter latency period on that test among Mexican Americans (β= −1.97±0.8, p=0.035). The latter finding was also observed for H. pylori CagA exposure (β= −1.80±0.58, p=0.005). However, no significant interaction by race was detected in those instances.
Table 3.
Multiple OLS and zero-inflated poisson regression analysis for predictors (H. pylori IgG and IgG CagA) of cognitive performance, by age group and effect modification by sex and race; NHANES III phase 1 (1988–1991) a
| 60–90y | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| SRT
|
SDS-E
|
SDS-L
|
SDL-TTC
|
SDL-TE
|
|||||||||||
| β | SEE | p-value | β | SEE | p-value | β | SEE | p-value | β | SEE | p-value | β | SEE | p-value | |
|
|
|
|
|
|
|||||||||||
| Model 1: H. pylori IgG | |||||||||||||||
|
| |||||||||||||||
| (N=2,178) | (N=2,147) | (N=2,147) | (N=2,027) | (N=2,086) | |||||||||||
| All | +0.43 | 4.14 | 0.919 | +0.17 | 0.12 | 0.172 | +0.60 | 0.48 | 0.219 | +0.30 | 0.13 | 0.033 | +0.51 | 0.26 | 0.058 |
|
| |||||||||||||||
| (N=1,092) | (N=1,077) | (N=1,077) | (N=1,026) | (N=1,051) | |||||||||||
| Men | +4.92 | 3.71 | 0.198 | +0.13 | 0.14 | 0.356 | +0.78 | 0.54 | 0.161 | +0.07 | 0.20 | 0.746 | −0.03 | 0.44 | 0.943┼ |
|
| |||||||||||||||
| (N=1,086) | (N=1,070) | (N=1,070) | (N=1,001) | (N=1,034) | |||||||||||
| Women | −4.09 | 6.15 | 0.513 | +0.18 | 0.18 | 0.341 | +0.48 | 0.47 | 0.322 | +0.60 | 0.20 | 0.007 | +1.19 | 0.44 | 0.012 |
|
| |||||||||||||||
| (N=871) | (N=869) | (N=869) | (N=861) | (N=862) | |||||||||||
| NH white | +3.72 | 4.62 | 0.430 | +0.04 | 0.19 | 0.845 | +0.73 | 0.45 | 0.115 | +0.29 | 0.16 | 0.096 | +0.24 | 0.30 | 0.431§ |
|
| |||||||||||||||
| (N=606) | (N=594) | (N=594) | (N=564) | (N=580) | |||||||||||
| NH black | +4.24 | 6.36 | 0.512 | +0.76 | 0.34 | 0.038 | +1.36 | 0.96 | 0.174 | +0.22 | 0.17 | 0.197 | +0.94 | 0.40 | 0.029 |
|
| |||||||||||||||
| (N=628) | (N=610) | (N=610) | (N=535) | (N=574) | |||||||||||
| Mexican-American | −6.33 | 4.04 | 0.133 | −0.02 | 0.36 | 0.947 | −1.97 | 0.88 | 0.035 | −0.01 | 0.14 | 0.911 | +0.11 | 0.40 | 0.773 |
|
| |||||||||||||||
| Model 2: H. pylori IgG CagA | |||||||||||||||
|
| |||||||||||||||
| (N=2,179) | (N=2,148) | (N=2,148) | (N=2,028) | (N=2,086) | |||||||||||
| All | +3.03 | 4.53 | 0.510 | +0.02 | 0.15 | 0.912 | +0.16 | 0.40 | 0.689 | +0.20 | 0.16 | 0.237 | +0.33 | 0.36 | 0.356 |
|
| |||||||||||||||
| (N=1,093) | (N=1,078) | (N=1,078) | (N=1,027) | (N=1,052) | |||||||||||
| Men | +6.77 | 4.49 | 0.146 | +0.07 | 0.2 | 0.724 | −0.10 | 0.58 | 0.819 | −0.06 | 0.26 | 0.811 | −0.12 | 0.50 | 0.812 |
|
| |||||||||||||||
| (N=1,086) | (N=1,070) | (N=1,070) | (N=1,001) | (N=1,034) | |||||||||||
| Women | −0.26 | 6.48 | 0.968 | −0.07 | 0.26 | 0.792 | +0.48 | 0.51 | 0.353 | +0.53 | 0.23 | 0.030 | +0.94 | 0.46 | 0.055 |
|
| |||||||||||||||
| (N=871) | (N=869) | (N=869) | (N=861) | (N=862) | |||||||||||
| NH white | +7.91 | 5.90 | 0.193 | −0.04 | 0.26 | 0.881 | −0.2 | 0.54 | 0.786 | +0.20 | 0.21 | 0.350 | +0.12 | 0.38 | 0.754 |
|
| |||||||||||||||
| (N=606) | (N=594) | (N=594) | (N=564) | (N=580) | |||||||||||
| NH black | +0.21 | 5.40 | 0.969 | +0.53 | 0.29 | 0.088 | +1.52 | 0.78 | 0.066 | +0.19 | 0.14 | 0.184 | +0.80 | 0.40 | 0.056 |
|
| |||||||||||||||
| (N=629) | (N=611) | (N=611) | (N=536) | (N=575) | |||||||||||
| Mexican-American | −9.00 | 4.79 | 0.075 | +0.07 | 0.41 | 0.867 | −1.80 | 0.58 | 0.005 | −0.06 | 0.13 | 0.637 | −0.19 | 0.46 | 0.685 |
|
| |||||||||||||||
|
60–90y
|
|||||||||||||||
|
WR-CORR
|
WR-TRIALS
|
SR-CORR
|
MATH-INC
|
ORIENT-INC
|
|||||||||||
| β | SEE | p-value | β | SEE | p-value | β | SEE | p-value | β | SEE | p-value | β | SEE | p-value | |
|
| |||||||||||||||
| Model 1: H. pylori IgG | |||||||||||||||
|
| |||||||||||||||
| (N=2,108) | (N=2,068) | (N=2,163) | (N=2,223) | (N=1,994) | |||||||||||
| All | +0.00 | 0.01 | 0.866 | +0.07 | 0.30 | 0.810 | −0.04 | 0.01 | 0.010 | +0.13 | 0.09 | 0.141 | +0.01 | 0.01 | 0.176 |
|
| |||||||||||||||
| (N=1,056) | (N=1,037) | (N=1,090) | (N=1,121) | (N=997) | |||||||||||
| Men | +0.00 | 0.02 | 0.877 | +0.46 | 0.37 | 0.230 | −0.07 | 0.02 | 0.001 | +0.14 | 0.11 | 0.223┼ | −0.01 | 0.02 | 0.547 |
|
| |||||||||||||||
| (N=1,052) | (N=1,031) | (N=1,073) | (N=1,102) | (N=997) | |||||||||||
| Women | +0.00 | 0.01 | 0.696 | −0.75 | 0.84 | 0.378 | −0.03 | 0.02 | 0.086 | +0.11 | 0.10 | 0.259 | +0.03 | 0.01 | 0.062 |
|
| |||||||||||||||
| (N=1,320) | (N=1,296) | (N=1,354) | (N=1,373) | (N=1,290) | |||||||||||
| NH white | +0.00 | 0.01 | 0.814 | +0.34 | 0.26 | 0.202§ | −0.04 | 0.01 | 0.015 | +0.16 | 0.10 | 0.128 | +0.01 | 0.01 | 0.351 |
|
| |||||||||||||||
| (N=402) | (N=400) | (N=407) | (N=428) | (N=387) | |||||||||||
| NH black | +0.00 | 0.02 | 0.790 | +0.60 | 0.69 | 0.388 | −0.05 | 0.04 | 0.231 | +0.02 | 0.06 | 0.713 | +0.05 | 0.02 | 0.038 |
|
| |||||||||||||||
| (N=354) | (N=344) | (N=373) | (N=386) | (N=284) | |||||||||||
| Mexican-American | −0.02 | 0.02 | 0.468 | −0.05 | 0.18 | 0.798 | −0.01 | 0.04 | 0.745 | +0.00 | 0.08 | 0.991 | +0.01 | 0.02 | 0.626 |
|
| |||||||||||||||
| Model 2: H. pylori IgG CagA | |||||||||||||||
|
| |||||||||||||||
| (N=2,108) | (N=2,068) | (N=2,163) | (N=2,223) | (N=1,994) | |||||||||||
| All | +0.00 | 0.01 | 0.712 | +0.34 | 0.28 | 0.232 | +0.00 | 0.02 | 0.900 | +0.06 | 0.07 | 0.365 | +0.00 | 0.01 | 0.880 |
|
| |||||||||||||||
| (N=1,056) | (N=1,037) | (N=1,090) | (N=1,121) | (N=997) | |||||||||||
| Men | −0.01 | 0.02 | 0.684 | +0.53 | 0.39 | 0.187 | −0.02 | 0.03 | 0.457 | −0.08 | 0.06 | 0.164 | −0.02 | 0.02 | 0.302 |
|
| |||||||||||||||
| (N=1,052) | (N=1,031) | (N=1,073) | (N=1,102) | (N=997) | |||||||||||
| Women | +0.00 | 0.01 | 0.640 | −0.57 | 0.92 | 0.543 | +0.00 | 0.02 | 0.908 | +0.06 | 0.09 | 0.519 | +0.02 | 0.01 | 0.188 |
|
| |||||||||||||||
| (N=1,320) | (N=1,296) | (N=1,354) | (N=1,373) | (N=1,290) | |||||||||||
| NH white | +0.00 | 0.01 | 0.729 | +0.64 | 0.31 | 0.047 | +0.00 | 0.02 | 0.938 | +0.10 | 0.08 | 0.198 | +0.00 | 0.01 | 0.755 |
|
| |||||||||||||||
| (N=402) | (N=400) | (N=407) | (N=428) | (N=387) | |||||||||||
| NH black | +0.01 | 0.02 | 0.644 | +0.17 | 0.52 | 0.748 | +0.00 | 0.05 | 0.986 | −0.07 | 0.08 | 0.347 | +0.06 | 0.02 | 0.025 |
|
| |||||||||||||||
| (N=354) | (N=344) | (N=373) | (N=386) | (N=284) | |||||||||||
| Mexican-American | −0.05 | 0.02 | 0.048 | +0.11 | 0.34 | 0.755 | −0.04 | 0.05 | 0.421 | +0.03 | 0.09 | 0.762 | −0.03 | 0.04 | 0.384 |
P<0.10 for null hypothesis that sex×exposure (H. pylori IgG and IgG CagA) interaction term γ =0 in model with sex among main effects;
P<0.10 for null hypothesis that race×exposure (H. pylori IgG and IgG CagA) interaction term γ =0 in model with race among main effects.
Abbreviations: MATH-INC=Math test-Incorrect; NH=Non-Hispanic; NHANES=National Health and Nutrition Examination Surveys; ORIENT-INC=Orientation to time, incorrect items; SDL-TTC=Serial Digits Learning-Trials to completion; SDL-TE=Serial Digits Learning-Total Errors; SDS-E=Symbol Digits Substitution tests-Errors; SDS-L=Symbol Digits Substitution tests-Latencies; SE=Standard error; SR-CORR=Story recall-correct; SRT=Simple reaction time ;WR-CORR=Word Recall-Correct; WR-TRIALS=Word Recall-Number of Trials.
Models were adjusted for age (continuous), sex, marital status (married vs. not); education (<High School; High School; >High School), poverty income ratio (0–100%; >100–200%; >200%), language of interview (English vs. Spanish), cigarette smoking status (never, former, current), self-rated health (excellent, very good, good, fair, poor),1995-healthy eating index (quintiles), physical activity (more active, about the same, less active), body mass index and a co-morbidity index. Models for the 20–59y age group were multiple OLS regression models, while those for the 60–90y age group were poisson or zero-inflated poisson regression models.
Among the older group (60–90yo), H. pylori IgG+ was linked to worse performance on the story recall test with fewer correct items (β= −0.04±0.01, p=0.010). This association was stronger in men(β= −0.07±0.02, p=0.001) and was detected only in NH whites(β= −0.04±0.01, p=0.015), though no significant interaction by sex or race was found. NH blacks who were H. pylori IgG+ also had worse performance on orientation to time task with a larger number of incorrect items (β=+0.05±0.02, p=0.038). A similar pattern was observed for NH blacks when considering H. pylori IgG CagA exposure in relation to ORIENT-INC score(β=+0.06±0.02, p=0.025), though in both cases interaction with race was non-significant. In addition, H. pylori IgG CagA+ was associated with worse performance on the word recall test with smaller number of correct items (i.e. WR-CORR) among Mexican-Americans (β= −0.05±0.02, p=0.048), and worse performance on word recall test with a larger number of trials to completion(β=+0.64±0.31, p=0.047) among NH whites with no significant interaction by race detected(p>0.10 for interaction terms with race).
DISCUSSION
We detected a worse cognitive performance in verbal memory among participants who were 60–90yo and H. pylori IgG+. Among NHBs and in women within the younger age group, we found worse cognitive performance in the H. pylori IgG+ group for serial digits learning task, a test that taps verbal memory. In addition, a larger number of trials to complete in serial digits learning were related to being H. pylori IgG+. Other significant race-specific associations were in the same direction (H. pylori IgG+, with SDS-E in NH blacks, with SR-CORR in men and NH whites, with ORIENT-INC among NH blacks; H. pylori IgG CagA with WR-CORR among Mexican Americans, WR-TRIALS among NH whites and ORIENT-INC among NH blacks), though all of these associations that were detected were not significantly different from the other strata when conducting interaction analyses. In addition, we found that H. pylori IgG and IgG CagA seropositivity was related to better performance on SDS-L among Mexican-Americans, an unexpected finding. All other associations were non-significant.
This cross-sectional study of a nationally representative sample is to our knowledge the first to examine the associations of two H. pylori seropositivity exposures (IgG and IgG CagA) with cognitive test performance in 20–59yo and 60–90yo in a US setting. We also evaluated those associations within socio-demographic groups, mainly sex and race. A few recent studies have indicated that H. pylori may play an important role in the pathogenesis of AD, but findings are controversial. One of the earliest case-control studies conducted in Greece (n=50 AD cases and n=30 iron deficiency anemic control without AD) showed that AD cases had a significantly higher prevalence of histologically confirmed H. pylori infection compared to controls (88% vs. 46.7%, p<0.001).(6) The same group of researchers also conducted another case-control study in the same setting comparing cases with a prodromal phase of dementia known as mild cognitive impairment (MCI) (n=60) to aneamic controls (n=35). Histologically confirmed H. pylori infection was significantly more prevalent in cases than in controls (89% vs. 49%, OR= 8.47, 95% CI:3.03–23.67, p<0.001) and anti-H. pylori IgG titer was significantly higher in the MCI cases (74.86±57.22 vs. 17.37±9.30 U/ml, p<0.001).(9) In another case-control study, 27 AD patients were compared to 27 age-matched controls in terms of CSF levels of anti-H. pylori IgG. The findings suggested greater mean concentration in AD cases compared to controls (10.53±12.54 U/mL vs. 8.63±8.01 U/mL, p=0.047), and a higher CSF titer also positively correlated with the degree of severity of the disease among AD cases (10). Using the same data,(6) Kountouras and colleagues conducted an intervention study among H. pylori positive AD cases in which eradication was attempted using a combination of two antibiotics and one acid reducer (omeprazole, clarithromycin and amoxicillin), and were followed-up for two years while taking cholinesterase inhibitors. They found that around 85% of AD patients subjected to treatment had a successful eradication. In that subgroup, cognitive function as assessed on MMSE, CAMCOG, a concise neuropsychological test to assist dementia diagnosis and the Functional Rating Scale for Symptoms of Dementia (FRSSD) tests improved significantly over time, but that was not the case in the other group where eradication was not successful. They concluded that H. pylori eradication can reduce cognitive decline in AD over time, which further re-inforces the possible link between H. pylori and AD occurrence.(7) In another intervention study in which the end point was survival, it was shown that the group with successful H. pylori eradication, when compared to those with unsuccessful eradication had a significantly lower mortality risk [HR (95% CI)=0.287 (0.114–0.725), P=0.008], after adjusting for baseline age and MMSE score.(8) In a more recent study conducted in France using a sample of AD patients (n=53), Roubaud-Baudron and colleagues found that H. pylori infection was associated with decreased MMSE score (p=0.024), higher CSF pTau(181) (p=0.014) and tau (p=0.021) levels.(11) Nevertheless, two other studies conducted in Japan did not find an association between H. pylori infection and AD or cognitive impairment (12–13), though in one study(13) H. pylori infection was determined by urinary IgG which is an unreliable method of diagnosis.(36) In addition, in the latter study(13), age and sex were not matched between cases and controls and thus comparisons between the two study groups (i.e., AD patients and controls) cannot be expected to establish any firm conclusions. Another concern is the very high H. pylori infection prevalence in the general Japanese population around 70 years old, renders the study underpowered, requiring potentially thousands of participants in order to prove the existence of an association between AD and H. pylori infection. In view of the aforementioned methodological limitations, this study can neither confirm the lack of association between the H. pylori infection status and AD in the Japanese population, nor is it comparable with the European studies indicating such an association (e.g. (6, 11)). Lack of age-matching between cases and controls was noted in the other Japanese study as well. (12)
The potential link of H. pylori with AD and poor cognition in normal aging was suggested to be triggered by a number of mechanisms with end target being the brain and its vascular system which can be summarized as follows: (1) Endothelial damage caused by atrophic gastritis which leads to reduced absorption of folate and vitamin B12 and increased level of homocysteine (Hcy). In fact, H. pylori infection often results in chronic gastritis, which can lead to malabsorption of vitamins B-12 and folate, resulting in failure of the methylation reaction which involves 5-methyl-tetrahydrofolic acid and the increased concentration of Hcy.(22–23) Hcy has been shown to precipitate endothelial damage and neurodegeneration through the pathway of oxidative injury leading to both vascular disease and dementia.(37) (2) Apoptosis caused by T cell-mediated immune response, overexpression of Nitric oxide (NO) or molecular mimicry of host structures. In fact, in H. pylori-related autoimmune gastritis, cytolitic T lymphocytes that are activated around the gastric mucosa may cross-recognize various H. pylori epitopes and gastric H+/K+-ATPase autoantigen, via molecular mimicry.(24) It was also shown that serum parietal cell auto-antibodies correlated with the serological titer of H. pylori offering further evidence for the phenomenon of molecular mimicry (38). By analogy, Campylobacter jejuni infection, the main cause of gastroenteritis, is now known to be the most common antecedent to the autoimmune neuropathy Guillain-Barre syndrome, through the pathway of molecular mimicry.(39) New evidence suggests that IgG against VacA type of H. pylori in the cerebrospinal fluid may cross-react with human Na+/K+ ATPase A subunits in abaxonal Schwann cells resulting in demyelination in some patients.(40) This finding might suggest the possibility that H. pylori may also cross-react with ganglion cells in AD neuropathy.(25) (3) Other inflammatory responses including cytokines (IL-1, IL-6, IL-8, IL-10, IL-12, TNF-α, IFN-γ), platelet activation, acute phase proteins (fibrinogen, C-reactive protein) and eicosanoids (leukotrienes, prostaglandins, catalyzed by cyclo-oxygenase enzymes) which lead to both apoptosis and thrombosis or vascular lesion.(25) (4) Apart from the mentioned mechanisms, additional recent evidence suggest that Hp-n, a histidine-rich protein abundant in H. pylori and which forms amyloid-like oligomers, could be transported through the blood-brain barrier (BBB) by the main low density lipoprotein receptor-related protein-1 (LRP-1) and receptor for advanced glycation end products (RAGE) transporters for amyloid-β-peptide (Aβ) across the BBB from brain to blood and blood to brain, thereby playing a role in AD pathophysiology; H. pylori infection, by releasing the aforelisted inflammatory mediators leads to BBB disruption, which, in turn, apart from LRP-1 and RAGE, might promote free entry of Hp-n proteins into the brain. This then results in the development of AD pathologies by inducing Aβ fibril formation or aggregating synergistically with Aβ.(26)
On the other hand, H. pylori infection was shown to be associated with other extra-digestive diseases which might mediate its effect on AD or poor cognitive performance, including atherosclerosis, hypertension and stroke (17–21). Three potential mechanisms may be involved: (1) H. pylori infection (particularly CagA+ cytotoxic strains), influences the development of atherosclerosis and increases the risk for cardiovascular and cerebrovascular diseases; (2) CagA-positive H. pylori strains may play a role in the natural history of atherosclerotic stroke; and (3) The links between hypertensive disease and H. pylori infection may involve the activation of the cytokine cascade with the release of vasoactive substances from the primary site of infection, or molecular mimicry between the CagA antigens of H. pylori and specific peptides expressed by endothelial cells and smooth muscle cells.
Our study has many strengths, including the use of a large nationally representative sample, the inclusion of a wide age range of adults (20–59y and 60–90y) and tests that span a variety of cognitive domains. In addition, the large sample size and the distribution of the exposure variable (~30% H. pylori IgG+, around half of which are CagA) allowed sufficient power to conduct stratified analysis by sex and race, in addition to the combined analysis. However, our study is limited because it is cross-sectional; thus, no causal link can be ascertained between the exposures and outcomes. Prospective cohort studies, and ultimately a randomized controlled trial, are needed to better examine the effects of H. pylori eradication on various aspects of cognitive decline in different age groups. Another limitation is that the type of cognitive tests differed between the two age groups despite spanning a number of common domains in both, which did not allow us to examine effect modification of the main associations of interest by age per se. In terms of exposure assessment, the serological test has limitations as it fails to discriminate between current and past H. pylori infection, as the antibody remains detectable even after several months since eradication of H. pylori.(11, 25, 41) Distinguishing between the two states is essential, particularly if H. pylori is indeed responsible for inducing both humoral and cellular immune responses which cross-react with nerve components given shared epitopes (or molecular mimicry, as described earlier).(42) In fact, the gold standard for determining current infection is the histological analysis of gastric mucosa, which is a relatively invasive method to use in population-based studies.(25) In addition, information on the cognitive status of individuals, particularly for the older group, was limited. This information could only be abstracted based on use of medication which would not have incorporated everyone with AD or MCI. Thus, it was not possible to accurately exclude individuals with cognitive impairment in older individuals. Finally, residual confounding cannot be ruled out even though major potential confounders were adjusted for in our analyses. However, some genetic risk factors, including the ApoE genotype which was not readily available in our data, may have acted as an effect modifier in the main association of interest.
In conclusion, our study findings suggest that there are significant associations of H. pylori seropositivity markers with cognitive function among adults, though some of those associations differ markedly between the two age groups of interest by sex and race. It is important to conduct prospective cohort studies to examine age-related cognitive decline in relation to exposures of interest that would provide further insight into potential interventions, including eradication of H. pylori among adults, in order to prevent cognitive decline and delay onset of dementia, particularly AD.
Supplementary Material
Study sample selection
Abbreviations: NHANES=National Health and Nutrition Examination Surveys.
Acknowledgments
This research was fully supported by the Intramural Research Program of the National Institute on Aging. The authors would like to thank Drs. Angelina Sutin and Lori L. Beason-Held for their internal review of this manuscript.
ABBREVIATIONS
- Aβ
Amyloid beta
- AD
Alzheimer’s Disease
- ANOVA
Analysis of Variance
- ApoE
Apolipoprotein E
- BBB
Blood brain barrier
- CSF
Cerebrospinal Fluid
- FRSSD
Functional Rating Scale for Symptoms of Dementia
- HEI
Healthy Eating Index
- Hp-n
histidine-rich protein
- H. pylori
Helicobacter pylori
- IgG
Immunoglobulin G
- IL
interleukin
- INF
Interferon
- LRP-1
Low density lipoprotein Receptor-related Protein-1
- MCI
Mild Cognitive Impairment
- MATH-INC
Math test-Incorrect
- MEC
Medical Examination Center
- MMSE
Mini-Mental State Examination
- NH
Non-Hispanic
- MA
Mexican-American
- NHB
Non-Hispanic Black
- NHW
Non-Hispanic White
- pTau
phosphorylated Tau protein
- Other
Other ethnicity
- NHANES
National Health and Nutrition Examination Surveys
- NO
Nitric Oxide
- OLS
Ordinary Least Square
- ORIENT-INC
Orientation to time, incorrect items
- SE
Standard error
- NH
Non-Hispanic
- OR
Odds Ratio
- RAGE
Receptor for Advanced Glycation End-products
- SRT
Simple reaction time
- SDS-L
Symbol Digits Substitution tests-Latencies
- SDS-E
Symbol Digits Substitution tests-Errors
- SDL-TE
Serial Digits Learning-Total Errors
- SDL-TTC
Serial Digits Learning-Trials to completion
- SR-CORR
Story recall-correct
- TNF
Tumor Necrosis Factor
- USDA
US Department of Agriculture
- WR-CORR
Word Recall-Correct
- WR-TRIALS
Word Recall-Number of Trials
Footnotes
Conflicts of Interest and Source of Funding: None.
Disclosure: (a) Sources of funding: This study was entirely supported by the National Institute on Aging, Intramural Research Program (NIA/NIH/IRP).
(b) May A. Beydoun: “No conflict of interest”; Hind A. Beydoun: “No conflict of interest”; Monal R. Shroff: “No conflict of interest”; Melissa H. Kitner-Triolo: “No conflict of interest”; Alan B. Zonderman: “No conflict of interest”.
References
- 1.Hendrie HC. Epidemiology of dementia and Alzheimer’s disease. Am J Geriatr Psychiatry. 1998;6:S3–18. doi: 10.1097/00019442-199821001-00002. [DOI] [PubMed] [Google Scholar]
- 2.Roth M. The natural history of mental disorder in old age. J Ment Sci. 1955;101:281–301. doi: 10.1192/bjp.101.423.281. [DOI] [PubMed] [Google Scholar]
- 3.Helmer C, Pasquier F, Dartigues JF. Epidemiology of Alzheimer disease and related disorders. Med Sci (Paris) 2006;22:288–96. doi: 10.1051/medsci/2006223288. [DOI] [PubMed] [Google Scholar]
- 4.Honjo K, van Reekum R, Verhoeff NP. Alzheimer’s disease and infection: do infectious agents contribute to progression of Alzheimer’s disease? Alzheimers Dement. 2009;5:348–60. doi: 10.1016/j.jalz.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–28. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kountouras J, Tsolaki M, Gavalas E, Boziki M, Zavos C, Karatzoglou P, Chatzopoulos D, Venizelos I. Relationship between Helicobacter pylori infection and Alzheimer disease. Neurology. 2006;66:938–40. doi: 10.1212/01.wnl.0000203644.68059.5f. [DOI] [PubMed] [Google Scholar]
- 7.Kountouras J, Boziki M, Gavalas E, Zavos C, Grigoriadis N, Deretzi G, Tzilves D, Katsinelos P, Tsolaki M, Chatzopoulos D, Venizelos I. Eradication of Helicobacter pylori may be beneficial in the management of Alzheimer’s disease. J Neurol. 2009;256:758–67. doi: 10.1007/s00415-009-5011-z. [DOI] [PubMed] [Google Scholar]
- 8.Kountouras J, Boziki M, Gavalas E, Zavos C, Deretzi G, Chatzigeorgiou S, Katsinelos P, Grigoriadis N, Giartza-Taxidou E, Venizelos I. Five-year survival after Helicobacter pylori eradication in Alzheimer disease patients. Cogn Behav Neurol. 2010;23:199–204. doi: 10.1097/WNN.0b013e3181df3034. [DOI] [PubMed] [Google Scholar]
- 9.Kountouras J, Tsolaki M, Boziki M, Gavalas E, Zavos C, Stergiopoulos C, Kapetanakis N, Chatzopoulos D, Venizelos I. Association between Helicobacter pylori infection and mild cognitive impairment. Eur J Neurol. 2007;14:976–82. doi: 10.1111/j.1468-1331.2007.01827.x. [DOI] [PubMed] [Google Scholar]
- 10.Kountouras J, Boziki M, Gavalas E, Zavos C, Deretzi G, Grigoriadis N, Tsolaki M, Chatzopoulos D, Katsinelos P, Tzilves D, Zabouri A, Michailidou I. Increased cerebrospinal fluid Helicobacter pylori antibody in Alzheimer’s disease. Int J Neurosci. 2009;119:765–77. doi: 10.1080/00207450902782083. [DOI] [PubMed] [Google Scholar]
- 11.Roubaud-Baudron C, Krolak-Salmon P, Quadrio I, Megraud F, Salles N. Impact of chronic Helicobacter pylori infection on Alzheimer’s disease: preliminary results. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Nagga K, Rajani R, Mardh E, Borch K, Mardh S, Marcusson J. Cobalamin, folate, methylmalonic acid, homocysteine, and gastritis markers in dementia. Dement Geriatr Cogn Disord. 2003;16:269–75. doi: 10.1159/000072812. [DOI] [PubMed] [Google Scholar]
- 13.Shiota S, Murakami K, Yoshiiwa A, Yamamoto K, Ohno S, Kuroda A, Mizukami K, Hanada K, Okimoto T, Kodama M, Abe K, Yamaoka Y, Fujioka T. The relationship between Helicobacter pylori infection and Alzheimer’s disease in Japan. J Neurol. 2011;258:1460–3. doi: 10.1007/s00415-011-5957-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–5. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 15.Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol. 2008;10:1573–81. doi: 10.1111/j.1462-5822.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 16.Kinoshita J. Pathogens as a cause of Alzheimer’s disease. Neurobiol Aging. 2004;25:639–40. doi: 10.1016/j.neurobiolaging.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Xu Q, Schett G, Perschinka H, Mayr M, Egger G, Oberhollenzer F, Willeit J, Kiechl S, Wick G. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation. 2000;102:14–20. doi: 10.1161/01.cir.102.1.14. [DOI] [PubMed] [Google Scholar]
- 18.Sawayama Y, Ariyama I, Hamada M, Otaguro S, Machi T, Taira Y, Hayashi J. Association between chronic Helicobacter pylori infection and acute ischemic stroke: Fukuoka Harasanshin Atherosclerosis Trial (FHAT) Atherosclerosis. 2005;178:303–9. doi: 10.1016/j.atherosclerosis.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 19.D’Andrea MR. Add Alzheimer’s disease to the list of autoimmune diseases. Med Hypotheses. 2005;64:458–63. doi: 10.1016/j.mehy.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 20.de la Torre JC, Stefano GB. Evidence that Alzheimer’s disease is a microvascular disorder: the role of constitutive nitric oxide. Brain Res Brain Res Rev. 2000;34:119–36. doi: 10.1016/s0165-0173(00)00043-6. [DOI] [PubMed] [Google Scholar]
- 21.Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–12. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- 22.Malaguarnera M, Bella R, Alagona G, Ferri R, Carnemolla A, Pennisi G. Helicobacter pylori and Alzheimer’s disease: a possible link. Eur J Intern Med. 2004;15:381–6. doi: 10.1016/j.ejim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Santarelli L, Gabrielli M, Cremonini F, Santoliquido A, Candelli M, Nista EC, Pola P, Gasbarrini G, Gasbarrini A. Atrophic gastritis as a cause of hyperhomocysteinaemia. Aliment Pharmacol Ther. 2004;19:107–11. doi: 10.1046/j.1365-2036.2003.01820.x. [DOI] [PubMed] [Google Scholar]
- 24.D’Elios MM, Amedei A, Benagiano M, Azzurri A, Del Prete G. Helicobacter pylori, T cells and cytokines: the “dangerous liaisons”. FEMS Immunol Med Microbiol. 2005;44:113–9. doi: 10.1016/j.femsim.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Kountouras J, Gavalas E, Zavos C, Stergiopoulos C, Chatzopoulos D, Kapetanakis N, Gisakis D. Alzheimer’s disease and Helicobacter pylori infection: Defective immune regulation and apoptosis as proposed common links. Med Hypotheses. 2007;68:378–88. doi: 10.1016/j.mehy.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 26.Kountouras J, Boziki M, Zavos C, Gavalas E, Giartza-Taxidou E, Veni0zelos I, Deretzi G, Grigoriadis N, Tsiaousi E, Vardaka E. A potential impact of chronic Helicobacter pylori infection on Alzheimer’s disease pathobiology and course. Neurobiol Aging. 2012;33:e3–4. doi: 10.1016/j.neurobiolaging.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol. 2012;175:54–9. doi: 10.1093/aje/kwr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mapel D, Roberts M, Overhiser A, Mason A. The Epidemiology, Diagnosis, and Cost of Dyspepsia and Helicobater pylori Gastritis: A Case-Control Analysis in the Southwestern United States. Helicobacter. 2012 doi: 10.1111/j.1523-5378.2012.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Center for Disease Control and Prevention (CDC) The Third National Health and Nutrition Examination Survey (NHANES III 1988–94) Reference Manuals and Reports (CD-ROM) Bethesda, MD: Centers for Disease Control and Prevention; 1996. [Google Scholar]
- 30.Wener M. National Health and Nutrition Examination laboratory protocol. Vol. 2012. Hyattsville, MD: National Center for Health Statistics; 2008. Helicobacter pylori IgG antibodies in serum by enzyme immunoassay. http://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/lab11_met_helicobacter_pylori.pdf. [Google Scholar]
- 31.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–5. [PubMed] [Google Scholar]
- 32.McCullough ML, Feskanich D, Rimm EB, Giovannucci EL, Ascherio A, Variyam JN, Spiegelman D, Stampfer MJ, Willett WC. Adherence to the Dietary Guidelines for Americans and risk of major chronic disease in men. Am J Clin Nutr. 2000;72:1223–31. doi: 10.1093/ajcn/72.5.1223. [DOI] [PubMed] [Google Scholar]
- 33.Beydoun MA, Beydoun HA, Shroff MR, Kitner-Triolo MH, Zonderman AB. Serum leptin, thyroxine and thyroid-stimulating hormone levels interact to affect cognitive function among US adults: evidence from a large representative survey. Neurobiol Aging. 2012;33:1730–43. doi: 10.1016/j.neurobiolaging.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.STATA. Statistics/Data Analysis: Release 11.0. Texas: Stata Corporation; 2009. [Google Scholar]
- 35.Selvin S. Statistical Analysis of Epidemiologic Data. 3. Oxford University Press; 2004. [Google Scholar]
- 36.Leodolter A, Vaira D, Bazzoli F, Schutze K, Hirschl A, Megraud F, Malfertheiner P. European multicentre validation trial of two new non-invasive tests for the detection of Helicobacter pylori antibodies: urine-based ELISA and rapid urine test. Aliment Pharmacol Ther. 2003;18:927–31. doi: 10.1046/j.1365-2036.2003.01761.x. [DOI] [PubMed] [Google Scholar]
- 37.White AR, Huang X, Jobling MF, Barrow CJ, Beyreuther K, Masters CL, Bush AI, Cappai R. Homocysteine potentiates copper- and amyloid beta peptide-mediated toxicity in primary neuronal cultures: possible risk factors in the Alzheimer’s-type neurodegenerative pathways. J Neurochem. 2001;76:1509–20. doi: 10.1046/j.1471-4159.2001.00178.x. [DOI] [PubMed] [Google Scholar]
- 38.Sheu BS, Shiesh SC, Yang HB, Su IJ, Chen CY, Lin XZ. Implications of Helicobacter pylori serological titer for the histological severity of antral gastritis. Endoscopy. 1997;29:27–30. doi: 10.1055/s-2007-1004057. [DOI] [PubMed] [Google Scholar]
- 39.Moran AP, Prendergast MM. Molecular mimicry in Campylobacter jejuni and Helicobacter pylori lipopolysaccharides: contribution of gastrointestinal infections to autoimmunity. J Autoimmun. 2001;16:241–56. doi: 10.1006/jaut.2000.0490. [DOI] [PubMed] [Google Scholar]
- 40.Chiba S, Sugiyama T, Yonekura K, Tanaka S, Matsumoto H, Fujii N, Ebisu S, Sekiguchi K. An antibody to VacA of Helicobacter pylori in cerebrospinal fluid from patients with Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry. 2002;73:76–8. doi: 10.1136/jnnp.73.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fennerty MB. Helicobacter pylori. Arch Intern Med. 1994;154:721–7. [PubMed] [Google Scholar]
- 42.Kountouras J, Deretzi G, Zavos C, Karatzoglou P, Touloumis L, Nicolaides T, Chatzopoulos D, Venizelos I. Association between Helicobacter pylori infection and acute inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2005;12:139–43. doi: 10.1111/j.1468-1331.2004.00977.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study sample selection
Abbreviations: NHANES=National Health and Nutrition Examination Surveys.