Abstract
Benign prostatic hyperplasia (BPH) is one of the most common diseases among elderly men. As the old-age population is increasing recently, it is to our interest to observe the growing BPH within them. In BPH, the dihydrotestosterone (DHT) acts as promotes prostate growth. It inhibits enzyme 5α-reductase that is involved in the conversion of testosterone to the DHT activity which reduces the excessive prostate growth. Through experiments, the effects of Phellius linteus water extract performed on the BPH rats were induced by testosterone treatments. For 12 weeks, Sprague-Dawley rats were treated with testosterone for the induction of BPH. Rats were divided into four experimental groups: the not treated group (N), the testosterone injection and D.W treatment group (TN), the testosterone injection and Phellinus linteus treatment group (TP) and testosterone injection and finasteride treatment group (TF). Prostate weight, volume and weight ratio in the TP group and the TF group were significantly lower than the TN group. Testosterone and DHT levels in the TN group were significantly higher than that of the N group. And the TP group was significantly decreased than that of the TN group. While prostates of control rats revealed severe acinar gland atrophy and stromal proliferation; the TP and TF groups showed trophic symptoms and were lined by flattened epithelial cells, thus, the stromal proliferation is relatively low as compared to the TN group. These suggest that Phellinus linteus water extracts may be an useful remedy for treating the benign prostatic hyperplasia.
Keywords: Phellius lineteus, benign prostatic hyperplasia, acne symptoms, hair growth, dihydrotestosterone
Introduction
Benign prostatic hyperplasia (BPH) is a common urological disorder in men. Its prevalence increases with age and may affect every 3 out of 4 men in their sixties [1-3]. As the main endocrine changes, sexual hormones and aging act as elements of enlarged prostate [4]. Recently, there has been a continuous increase in the number of patients with benign prostatic hyperplasia in South Korea due to an increase in the number of old-aged people, and thus, prostate has emerged as important medical concerns [5].
The molecular mechanism of benign prostatic hyperplasia has not yet been clearly revealed. However, the hypertrophy of prostate, caused by excessive dihydrotestosterone (DHT) is estimated as the mechanism that oversupplies testosterone in blood, and leads to vast amount of DHT synthesis via the action of 5α-reductase in the prostate; the synthesized DHT combines with androgen receptor with consequent generation of benign prostatic hyperplasia [6,7]. Furthermore, secretion from androgen of prostate cells decreases as the male patients become old while the receptors of prostate cells increase to balance endocrine system as DHT is combined with other parts [8]. This process is estimated to be related to the increased histological, ocular and clinical prevalence rate cases due to an increase [9,10] in age, which leads to cell hyper-plasia and changes in tissues. In other words, the level of oxidative stress increases with an increase in age. If the antioxidant system inside the body cannot protect the body from oxidative damages [9], the senile change occurs in the prostate with consequent developments of benign prostatic hyperplasia.
Phellinus linteus, also called as Phellinus baumii, is a white-colored rotting fungi which belongs to the hymenochaetaceae family, and is grown or planted in China, Cambodia and Japan. Phellinus linteus contains various nutrients including saccharides, proteins, vitamins, minerals and large amounts of β-glucan. Recently, Phellinus linteus has been used as health food and medicine for cancer treatment [11-13]. Currently, there are ongoing researches on benign prostatic hyperplasia in Korea and other countries. The antioxidant [14,15], anticancer and immunopotentiation [16,17] effects of various functional mushrooms which contain large amounts of psychological active substances are being studied actively. However, sufficient research on benign prostatic hyperplasia is still lacking. Hence, in this regard, the present study is conducted to verify the stability and efficacy of Phellinus linteus on benign prostatic hyperplasia. By doing so, rats with such symptoms generated by injecting testosterone were being used to verify the efficacy of Phellinus linteus.
Materials and Methods
Preparation of water extracts from Phellius linteus
For this experiment, Phellius linteus, which was cultivated in Kumhwang Bio, located in Samgok-ri, Munsan-eup, and Jinju-si, Korea, was purchased and it was used as experimental material by extraction and concentration after drying. After confirming whether the extraneous material was mixed, material was rinsed. After weighing Phellius linteus, the first extraction after 12 hours was conducted at 95℃ and adding water to generate 20 times more than the material weight. The second extraction was conducted 12 hours later at 95℃ by adding five times more water than the material weight. After mixing the first and the second extracts, filtration was performed, and the Phellius linteus extracts were obtained through 95℃ sterilization with a concentrator. The β-glucan content of Phellinus linteus, used in this experiment, was 6 g per 100 g in the fruiting body of Phellinus linteus.
Manufacturing of the animal model of BPH by the administration of testosterone to aged rats
The hypothesis that human Benign Prostatic Hyperplasia occurs due to aging or male hormone excess is dominant. Therefore, based on this hypothesis, this experiment divides groups into the aged group, the testosterone intervention group, the administration group of controlled drugs, and the administration group of olfactory materials by using age rates of over 12 weeks as test models of Benign Prostatic Hyperplasia under the judgment that this group division shall be effective in reducing prostate at the appropriate levels under the influence of drugs. Accordingly, in this experiment, the model of Benign Prostatic Hyperplasia caused by aging or the excess of testosterone was manufactured by giving subcutaneous injections with testosterone of 1.5 mg/corn oil 0.1 ml/kg for 30 days, after diluting testosterone of male Sprague-Dawley rats which reached 12 weeks and also completed the acclimatization period of the corn oil. The research was performed in accordance with the guidelines established by the International University of Korea Institutional Animal Care and Use Committee (IUK 2011-06).
The allocation of experimental groups and experimental methods
Ten rats were assigned to each group and the allocation of groups was divided into normal group, control group, the administration group of Phellinus linteus, and positive control group. Subcutaneous injection was given to the normal group with 0.1 ml/kg, and oral administration was made only with physiological saline of 5 ml/kg. While subcutaneous injection was given to the administration group of Phellinus linteus for 30 days with the mixture of testosterone, oral administration was made by using zonde one time per day with 1.725 ml/kg of the Phellinus linteus extract. In terms of the finasteride administration group, which is the positive control group, subcutaneous injections of testosterone were given for 30 days, oral administration was also made one time per day by using zonde with 1 ml/kg by suspending finasteride to physiological saline.
Body weight measurement
The first weight measurement was made on the commencement date of the experiment, and again in the last sampling, which was the closing date of the experiment. For weight measurements, an electronic scale was used for all rats before their morning feed. In order to minimize weight errors for weight movements, rats were placed into a plastic bowl where their weight could be recorded stably.
The measurement of prostate ratio according to volume and weight of the prostate
The day following the last administration, body weight measurements and blood samplings were made. When sacrificing a rat, fat or foreign materials around the prostate were removed to measure the maximum and minimum lengths of the prostate horizontally and vertically. The volume of the prostate was calculated according to the formula: Prostate volume (cm3): 1/2 (a × b2) (a, longer dimension; b, shorter dimension), while the size of the prostate was calculated with prostate weight ratio (mg/100 g of BW) = prostate weight (mg) × 100 (g) / Body weight (g). The weight of the prostate was measured by using the electronic scale after measuring the size of the prostate and after removing the moisture around the prostate.
Hematologic analysis
When measuring hematologic testosterone, blood tests were taken from the hearts of rats on the last day of the experiment. Configuration of blood was used at 3,000 rpm for 20 minutes and testosterone was measured by using the testosterone EIA kit (Cayman chemical company, USA) after storing at -40℃ through isolating serum. DHT concentrations were measured with BMG LABTECH EIA reader, which is the product of the FLUO star OPTIMA by using the rat dihydrotestosterone EIA kit (cusabio, USA).
Histological changes of the prostate
After fixing each prostate into the 10% neutral buffered formalin solution, a paraffin block was made after dehydration by inserting ethanol of 60%, 80%, 95%, 100% respectively. Thereafter, each prostate tissue was sliced with the thickness of 4 µm by using microtome and it was attached to the gelatincoated slide. To dye the tissue section, paraffin was removed by immersing each prostate into xylene and rehydration was performed by immersing each prostate in ethanol and distilled water of 100%, 95%, 80%, 70% one by one. Rehydrated tissue was evaluated in histological analysis under the optical microscope by dyeing it with Hematoxyline & Eosin (H&E).
Statistical analysis
Results were analyzed and assessed by ANOVA and Tukey's honestly significant difference test [18]. Statistically determined differences were considered significant at P < 0.05.
Results
Measurement of weight change
Body weight change is shown in Table 1. Total body weight gains after 30 days, changes of Phellinus linteus extracts groups and positive control groups were significantly decreased compared to the TN group, 37.3% and 36.7% respectively. Total body weight gains after 30 days leading to changes of Phellinus linteus extracts groups and positive control groups were significantly decreased compared to the TN groups with 37.3% and 36.7%, respectively.
Table 1.
Body weight gains of each experimental group
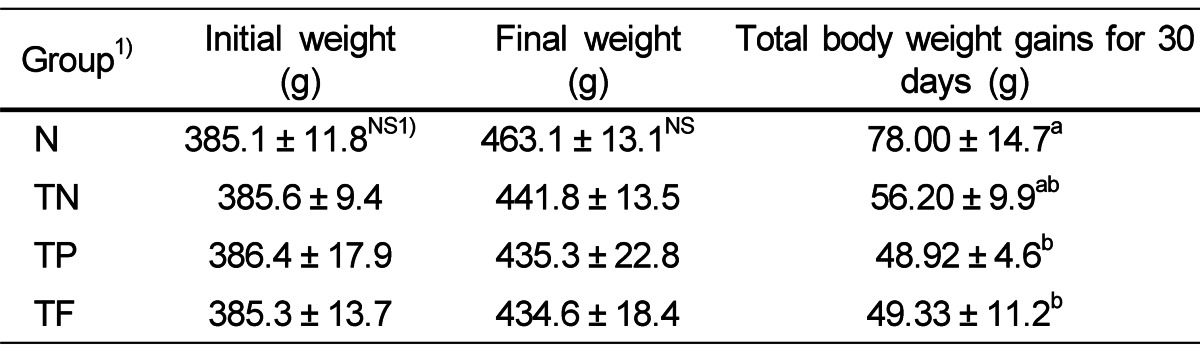
All values are mean ± SE (n = 10). Those with different superscripts in the same column are significantly different at P < 0.05 by Tukey's test.
1)NS: not significant
N: not treated group
TN: testosterone injection and D.W. 5 ml/kg orally treatment group
TP: testosterone injection and Phellinus linteus (1.725 mL/kg) orally treatment group
TF: testosterone injection and finasteride (1.0 mL/kg) orally treatment group
Prostate ratio according to volume and weight of the prostate
Prostate weight, volume and weight ratio is shown in Table 2. The prostate volume of TN group (1.369 ± 0.11 cm3) was significantly higher than that of N group (0.323 ± 0.04 cm3), and the administration groups of Phellinus linteus, and positive control group were significantly lower than that of TN group, 0.536 ± 0.57 cm3 and 0.437 ± 0.05 cm3 respectively.
Table 2.
Prostate volume, weight and prostate weight ratio of each experimental group
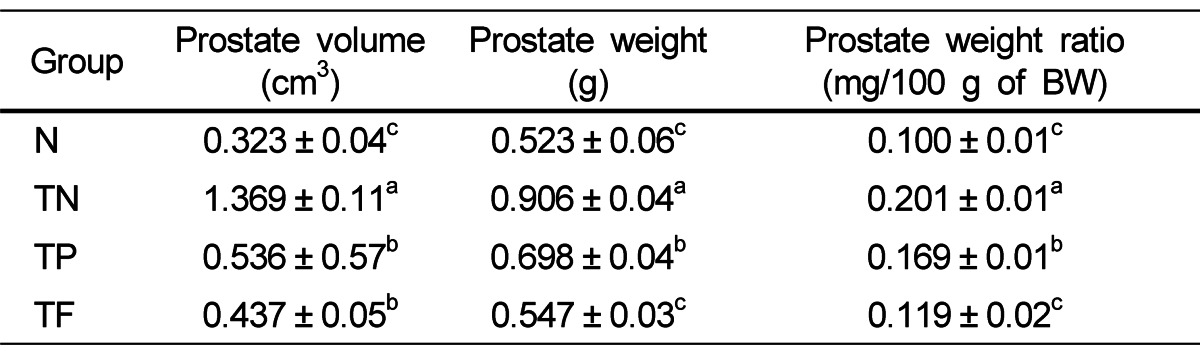
All values are mean ± SE (n = 10). Those with different superscripts in the same column are significantly different at P < 0.05 by Tukey's test.
Prostate weight of the TN group (0.906 ± 0.04 g) was significantly higher than that of the N group (0.523 ± 0.06 g), and the administration groups of Phellinus linteus, and positive control group were significantly lower than that of the TN group, 0.698 ± 0.04 g and 0.547 ± 0.03 g respectively. Finally, prostate weight ratio of the TN group (0.201 ± 0.01 mg/100 g of BW) was significantly higher than that of the N group (0.100 ± 0.01 mg/100 g of BW), and the administration groups of Phellinus linteus, and positive control group were significantly lower than that of the TN group, 0.169 ± 0.01 mg/100 g of BW and 0.119 ± 0.02 mg/100 g of BW respectively.
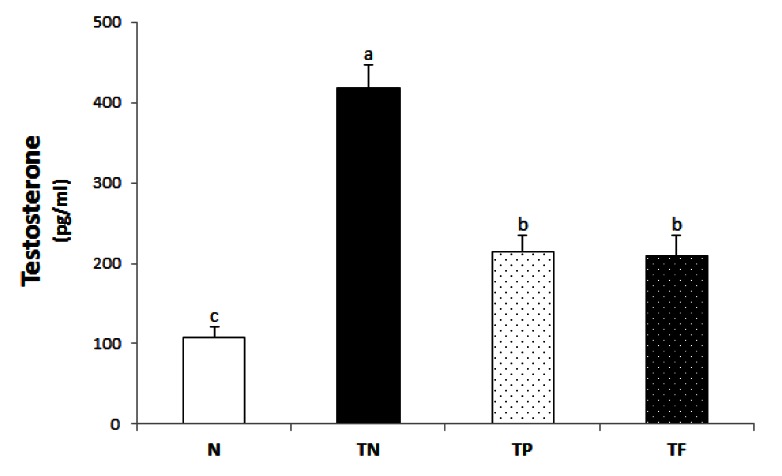
Serum on testosterone concentration
The serum testosterone concentration (Fig. 1) in the TN group (417.9 ± 28.9 pg/mg) were significantly higher than that of the N group (102.7 ± 16.9 pg/mg). And the administration groups of Phellinus linteus (216.8 ± 18.4 pg/mg) and the positive control group (209.5 ± 25.5 pg/mg) were significantly lower than that of TN group, 48.1% and 49.8% respectively.
Fig. 1.
Testosterone levels of each experimental group. All values are mean ± SE (n = 10). Those with different superscripts in the same column are significantly different at P < 0.05 by Tukey's test.
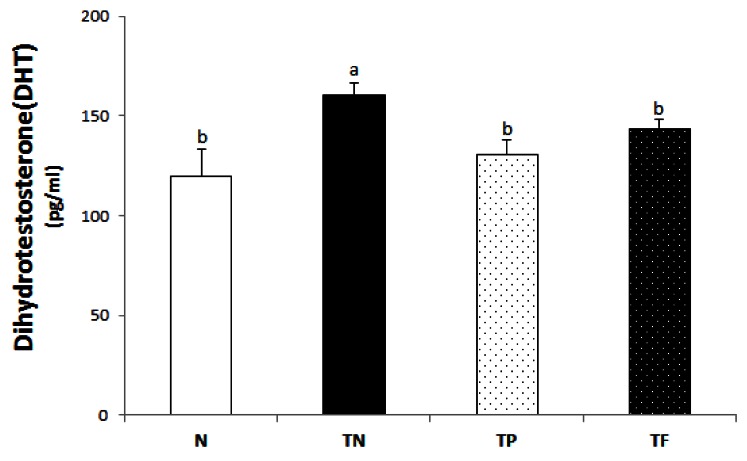
Serum on dihydrotestosterone (DHT) concentration
In terms of the function of DHT in the prostate, DHT has titer of male hormones corresponding to about five times the amount of testosterone. Because such concentration of DHT in the prostate is about twice the amount of testosterone, the function of DHT in the prostate can correspond to about 10 times the amount of testosterone [11]. DHT makes structural change and dimerization by binding to androgen receptors (AR) presented in the nucleus of epithelial cells and stromal cells in the prostate. The serum DHT concentration is shown in Fig. 2. The DHT concentration of the TN group (160.5 ± 5.8 pg/mg) was significantly higher than that of the N group (119.6 ± 13.8 pg/mg). And the administration group of Phelinus liteus (130.6 ± 7.2 pg/mg) and the positive control group (143.9 ± 4.2 pg/mg) were significantly lower than that of the TN group, 18.6% and 10.4% respectively.
Fig. 2.
Dihydrotestosterone (DHT) levels of each experimental group. All values are mean ± SE (n = 10). Those with different superscripts in the same column are significantly different at P < 0.05 by Tukey's test.
Histological changes of the prostate
In order to examine the effects of the administration of Phellinus linteus extracts on histological changes of the prostate, H&E staining was being performed. The optical microscopy observations of H&E staining samples were compared as shown in Fig. 3. A normal group maintained the size and shape of the acinar gland well without atrophy. Ductal epithelial cells also maintained the normal shape of the nucleus of cells and the glands in columnar shape and the amount of interstitial tissues were shown appropriately. In contrast, within the control group, either the cystic dilatation was observed or the depression towards lumen due to proliferation of ductal epithelial cell was shown. The acinar gland was contracted in oval and linear shapes and was irregularly distributed. Fibrosis and edema of interstitial tissue were also being observed. In a group with administration of Phellinus linteus extracts, atrophy of the acinar gland showing cystic dilation was noticeably reduced as compared with a control group. Less fibrosis of connective and interstitial tissues was observed. In a positive control, luminal cells in the epithelial component showed a relatively round shape which resembled the normal group. Duct epithelial cell were also maintained in columnar shape. Fibrosis of interstitial tissue was decreased, but the aspect of edema was shown.
Fig. 3.
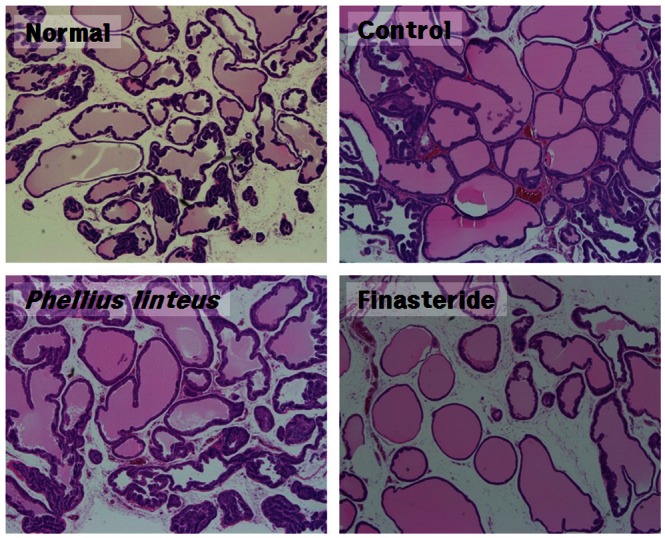
H&E stains of protate tissue from the rats (× 40). Normal prostate (N): Tubular glands are lined by simple tall columnar epithelium and supported by a connective tissue stroma. Control prostate (TN): The glands are cystically-dilated and highly-infolded, and are lined by simple columnar epithelium, which is supported by a fibrous and edematous stroma. Phellius linteus prostate (TP): The glands show atrophy and are lined by flattened epithelial cells and stromal proliferation is relatively decreased compared to TN group. Finasteride prostate (TF): The glands show atrophy and the epithelium is flattened and stromal fibrosis is not observed, but stromal edema is not decreased.
Discussion
As a result, according to Noh et al. [19], Phellinus linteus has appeared to be more dependent on concentration as compared to highly fat cholesterols in regard to decreases in body weight; and, it has been reported by Choi et al. [20] that the higher the amount of glucan, the lower the body weight.
According to Kim et al. [13], the amount of body fat found in males increase as the person ages and inversely to male hormones. Furthermore, obesity has been reported to increase activities of sympathetic nerves and such activities affect the generation of benign prostatic hyperplasia. Therefore, with respect to testosterone, which causes benign prostatic hyperplasia, the increased amount of body fat caused by increase in age can augment the attack rates of benign prostatic hyperplasia. The decrease in body weight as observed in the present research was due to the action of β-glucan, which is an element of Phellinus linteus. Thus, it is proposed that Phellinus linteus extracts could be useful in promoting decreases in body weight with subsequent protections from the development of benign prostatic hyperplasia. This result shows benign prostatic hyperplasia occurring in the experiment model. In addition, the Phellinus linteus-injected group showed decreases in the bulk and weight of prostate by 61% and 23%, respectively, when comparing to the control group. This result signifies that the Phellinus linteus is effective in reducing the bulkiness and weight of corpulent prostate. Previously, it has been reported that various mushrooms, including Phellinus linteus are effective in caring for benign prostatic hyperplasia, as they contain large amounts of β-glucan, oxyphenyl carbon and polyphenol. In addition, the flavonoid, which is a physiological active substance, has been found to inhibit 5α-reductase and hence it is transferred to the DHT and inhibits the increase in DHT concentrations. Therefore, the capacity of the prostate is decreased [21,22]. Phellinus linteus can also be seen as a natural antioxidant and anti-cancer food as it contains large amounts of polyphenol ingredients including β-glucan and flavonoid [23,24]. Accordingly, it appears that various antioxidant ingredients of Phellinus linteus extract and the physiological chemical action caused by β-glucan are valid in the rat model; hence, statistically, significant decreases in the bulk and weight of the prostate in Phellinus linteus-injected group was observed when compared to the benign prostatic hyperplasia induced group. It is estimated that both Phellinus linteus and finasteride affected the transfer of testosterone, as testosterone in prostate cells is transferred into DHT by 5α-reductase. It would be appropriate to state that Phellinus linteus extract, used in this research, is absorbed in the intestine when it comes through the mouth, preventing testosterone to be over-injected when converted into DHT by preventing activation of 5α-reductase enzyme in prostate due to the benign prostatic hyperplasia. It was observed that the DHT concentration in blood was higher in the control group when compared to the experimental groups. There has been significant decreases in the TP group for Phellinus linteus injected groups and positive control groups than control groups. Even though the cause of benign prostatic hyperplasia is not being clearly revealed, this result verifies that the changes according to age, male hormone, especially DHT, is strongly related to such disease. The Phellinus linteus has been considered to prevent benign prostatic hyperplasia as it reduces DHT concentrations. The observation and bee venom [25] on H&E histological specimen through an optical microscope has been carried out to investigate whether injection of Phellinus linteus extract affects organizational changes in the prostate or not; thus indicating that 80% of a normal rat's ventral prostate consists of the dependent columnar cell and the male hormone has been found to influence the cell's proliferation and natural death directly. Therefore, it is contemplated that prostate hypertrophy occurs largely in the control group than in the normal group, as the prostate secretion is increased by highly concentrated testosterone. Furthermore, improvement in benign prostate hypertrophy was observed in Phehllinus linteus-injected group than the control group as the mushroom not only protects the shape and function of the prostate, but also structurally inhibits overgrowth of connective tissues hence preventing pathologic damages to the tissues caused by benign prostatic hyperplasia.
References
- 1.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005;173:1256–1261. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Zhu C, Curado MP, Zheng T, Boyle P. Changing patterns of bladder cancer in the USA: evidence of heterogeneous disease. BJU Int. 2012;109:52–56. doi: 10.1111/j.1464-410X.2011.10283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsons JK. Benign prostatic hyperplasia and male lower urinary tract symptoms: epidemiology and risk factors. Curr Bladder Dysfunct Rep. 2010;5:212–218. doi: 10.1007/s11884-010-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arrighi HM, Metter EJ, Guess HA, Fozzard JL. Natural history of benign prostatic hyperplasia and risk of prostatectomy. The Baltimore Longitudinal Study of Aging. Urology. 1991;38:4–8. doi: 10.1016/0090-4295(91)80191-9. [DOI] [PubMed] [Google Scholar]
- 5.Miano R, De Nunzio C, Asimakopoulos AD, Germani S, Tubaro A. Treatment options for benign prostatic hyperplasia in older men. Med Sci Monit. 2008;14:RA94–RA102. [PubMed] [Google Scholar]
- 6.Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5α-reductase inhibitor. J Clin Endocrinol Metab. 2004;89:2179–2184. doi: 10.1210/jc.2003-030330. [DOI] [PubMed] [Google Scholar]
- 7.Isaacs JT. Antagonistic effect of androgen on prostatic cell death. Prostate. 1984;5:545–557. doi: 10.1002/pros.2990050510. [DOI] [PubMed] [Google Scholar]
- 8.Nixon P. New clinical trial of medical therapy for benign prostatic hyperplasia. Drug Benefit Trends. 1997;9:44–45. [Google Scholar]
- 9.Jang H, Ha US, Kim SJ, Yoon BI, Han DS, Yuk SM, Kim SW. Anthocyanin extracted from black soybean reduces prostate weight and promotes apoptosis in the prostatic hyperplasia-induced rat model. J Agric Food Chem. 2010;58:12686–12691. doi: 10.1021/jf102688g. [DOI] [PubMed] [Google Scholar]
- 10.Isaacs JT, Coffey DS. Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl. 1989;2:33–50. doi: 10.1002/pros.2990150506. [DOI] [PubMed] [Google Scholar]
- 11.Tsuji T, Du W, Nishioka T, Chen L, Yamamoto D, Chen CY. Phellinus linteus extract sensitizes advanced prostate cancer cells to apoptosis in athymic nude mice. PLoS One. 2010;5:e9885. doi: 10.1371/journal.pone.0009885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sliva D. Medicinal mushroom Phellinus linteus as an alternative cancer therapy. Exp Ther Med. 2010;1:407–411. doi: 10.3892/etm_00000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HM, Han SB, Oh GT, Kim YH, Hong DH, Hong ND, Yoo ID. Stimulation of humoral and cell mediated immunity by polysaccharide from mushroom Phellinus linteus. Int J Immunopharmacol. 1996;18:295–303. doi: 10.1016/0192-0561(96)00028-8. [DOI] [PubMed] [Google Scholar]
- 14.Kojima H, Tanigawa N, Kariya S, Komemushi A, Shomura Y, Sawada S, Arai E, Yokota Y. A case of spontaneous regression of hepatocellular carcinoma with multiple lung metastases. Radiat Med. 2006;24:139–142. doi: 10.1007/BF02493281. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki S, Ohshima S. In: Mori K, editor. Influence of shiitake (Lentinus edodes) on human serum cholesterol; Mushroom Science 9: Proceedings of the 9th International Scientific Congress on the Cultivation of Edible Fungi; Tokyo: Mushroom Research Institute in Japan; 1976. pp. 463–647. [Google Scholar]
- 16.Kim BC, Jeon WK, Hong HY, Jeon KB, Hahn JH, Kim YM, Numazawa S, Yosida T, Park EH, Lim CJ. The anti-inflammatory activity of Phellinus linteus (Berk. & M.A. Curt.) is mediated through the PKCdelta/Nrf2/ARE signaling to up-regulation of heme oxygenase-1. J Ethnopharmacol. 2007;113:240–247. doi: 10.1016/j.jep.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Choi YH, Yan GH, Chai OH, Lim JM, Sung SY, Zhang X, Kim JH, Choi SH, Lee MS, Han EH, Kim HT, Song CH. Inhibition of anaphylaxis-like reaction and mast cell activation by water extract from the fruiting body of Phellinus linteus. Biol Pharm Bull. 2006;29:1360–1365. doi: 10.1248/bpb.29.1360. [DOI] [PubMed] [Google Scholar]
- 18.Steel RG, Torrie JH, Dickey DA. Principles and Procedures of Statistics: A Biometrical Approach. New York: McGraw-Hill; 1996. [Google Scholar]
- 19.Noh JR, Lee IK, Ly SY, Yang KJ, Gang GT, Kim YH, Hwang JH, Yun BS, Lee CH. A Phellinus baumii extract reduces obesity in high-fat diet-fed mice and absorption of triglyceride in lipid-loaded mice. J Med Food. 2011;14:209–218. doi: 10.1089/jmf.2010.1152. [DOI] [PubMed] [Google Scholar]
- 20.Choi JS, Kim H, Jung MH, Hong S, Song J. Consumption of barley beta-glucan ameliorates fatty liver and insulin resistance in mice fed a high-fat diet. Mol Nutr Food Res. 2010;54:1004–1013. doi: 10.1002/mnfr.200900127. [DOI] [PubMed] [Google Scholar]
- 21.Song YS, Kim SH, Sa JH, Jin C, Lim CJ, Park EH. Antiangiogenic, antioxidant and xanthine oxidase inhibition activities of the mushroom Phellinus linteus. J Ethnopharmacol. 2003;88:113–116. doi: 10.1016/s0378-8741(03)00178-8. [DOI] [PubMed] [Google Scholar]
- 22.Jeon TI, Hwang SG, Lim BO, Park DK. Extracts of Phellinus linteus grown on germinated brown rice suppress liver damage induced by carbon tetrachloride in rats. Biotechnol Lett. 2003;25:2093–2096. doi: 10.1023/b:bile.0000007071.28105.c1. [DOI] [PubMed] [Google Scholar]
- 23.Liang CH, Syu JL, Mau JL. Antioxidant properties of solid-state fermented adlay and rice by Phellinus linteus. Food Chem. 2009;116:841–845. [Google Scholar]
- 24.Zhu T, Guo J, Collins L, Kelly J, Xiao ZJ, Kim SH, Chen CY. Phellinus linteus activates different pathways to induce apoptosis in prostate cancer cells. Br J Cancer. 2007;96:583–590. doi: 10.1038/sj.bjc.6603595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho SH, Han YH, Kim YS. Effects of bee venom herbal acupuncture on experimental rat model of benign prostatic hyperplasia. J Korean Orient Intern Med. 2010;31:166–176. [Google Scholar]